A Highly Selective and Sensitive Nano-Silver sol Sensor for Hg2+ and Fe3+: Green Preparation and Mechanism
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Reagents and Apparatus
2.2. Synthesis of CMS/PVP-Ag NPs Colloidal Solution
2.3. Characterization of CMS/PVP-Ag NPs Colloidal Solution
2.4. Adsorption and Detection of Hg2+ and Fe3+
3. Results and Discussion
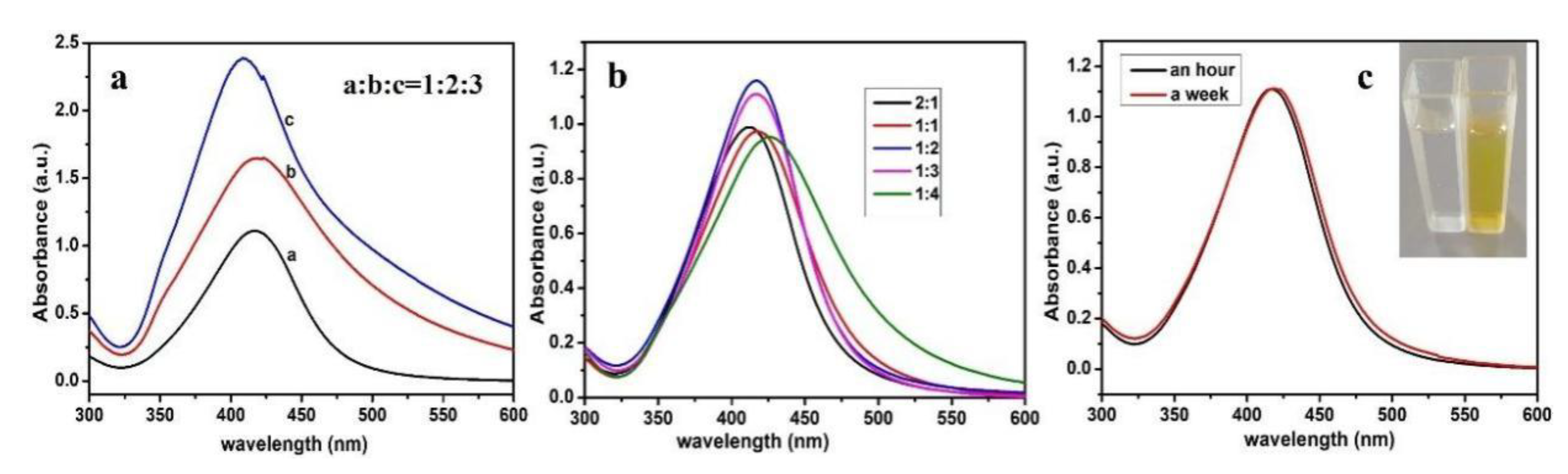
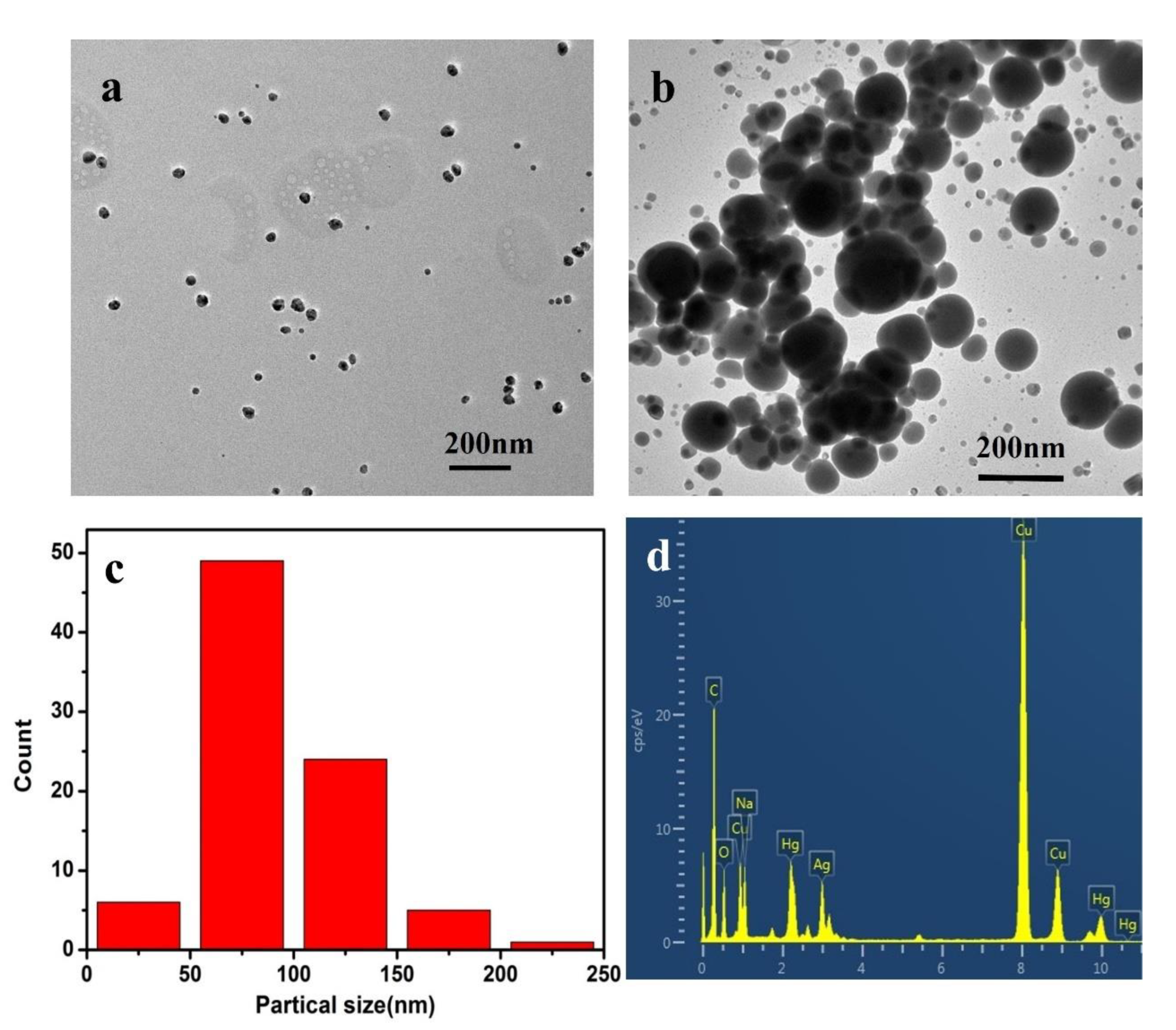
3.1. Characterization Studies of CMS/PVP-Ag NPs Colloidal Solution
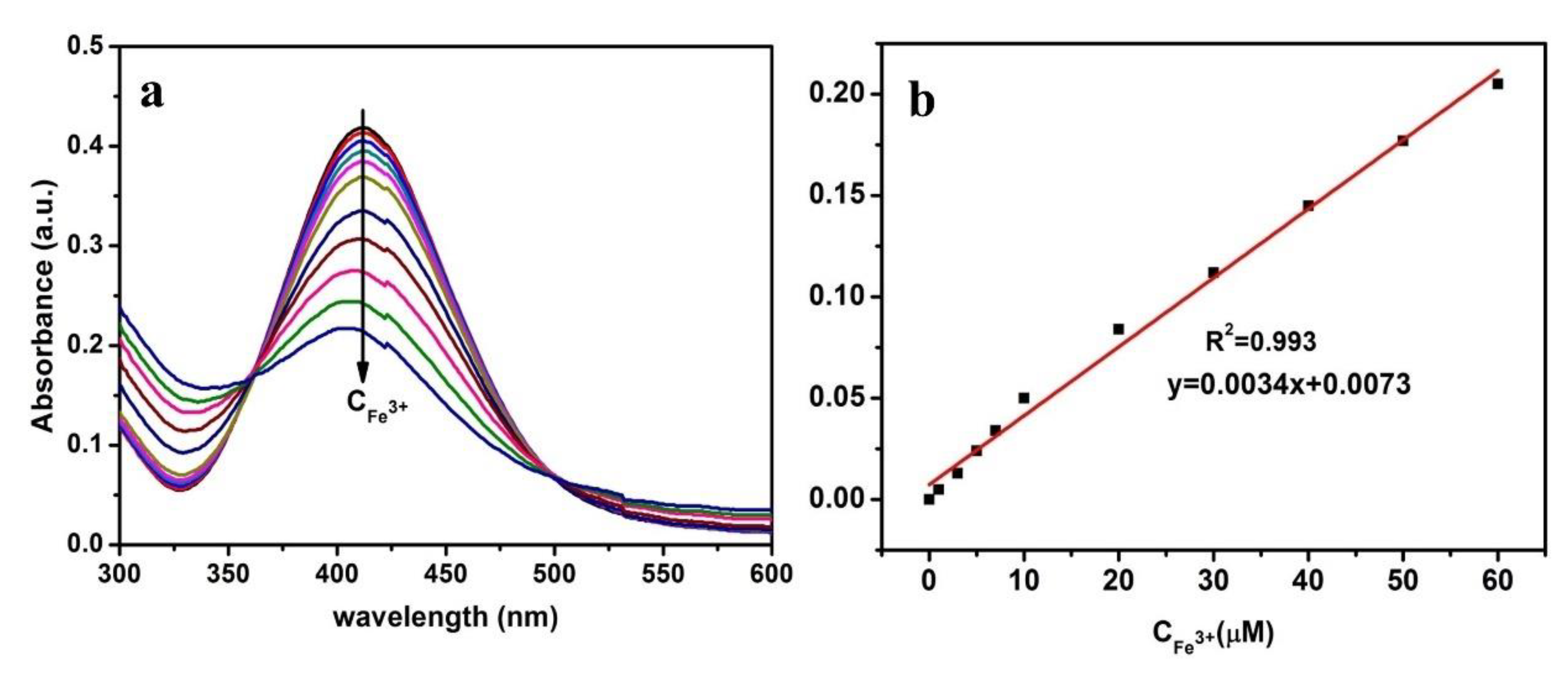
3.2. Detection Selectivity and Sensitivity of Hg2+ and Fe3+
3.3. Mechanism Study for the CMS/PVP-Ag NPs Colloidal Solution and the Detection of Hg2+ and Fe3+
3.4. Detection of Hg2+ and Fe3+ in Tap Water and Seawater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Gui, L.; Li, W. A colorimetric silver nanoparticle-based assay for Hg(II) using lysine as a particle-linking reagent, Microchim. Acta 2015, 182, 1977–1981. [Google Scholar]
- Ahmed, F.; Ali, I.; Ali, H.S.; Yasmeen, S.; Ullah, S.; Burki, S.; Adil, M.; Nisar, J.; Shah, M.R. Synthesis and characterization of a plant growth regulator based silver nanoparticles for the ultrasensitive detection of environmentally toxic Hg2+ ions in tap water. New J. Chem. 2021, 45, 18039–18047. [Google Scholar] [CrossRef]
- Mohammadi, A.; Yaghoubi, S. A new dual colorimetric chemosensor based on quinazolinone for CN−, AcO− and Cu2+ ions. Sens. Actuators B Chem. 2017, 241, 1069–1075. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Zhao, L.; Xue, Y.; Zhao, X.; Li, Q.; Xia, Y. Facile and Green Fabrication of Carrageenan-Silver Nanoparticles for Colorimetric Determination of Cu2+ and S2-. Nanomaterials 2020, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wang, J.; Xu, D. Synthesis of a novel hyperbranched polymer and its application in multi-channel sensing Fe3+. Res. Chem. Intermed. 2019, 46, 1425–1435. [Google Scholar] [CrossRef]
- Chanajaree, R.; Ratanatawanate, C.; Ruangchaithaweesuk, S.; Lee, V.S.; Wittayanarakul, K. Colorimetric detection of Pb2+ ions using curcumin silver nanoparticles. J. Mol. Liq. 2021, 343, 117629. [Google Scholar] [CrossRef]
- Mohammadi, A.; Yaghoubi, S. Development of a highly selective and colorimetric probe for simultaneous detection of Cu2+ and CN− based on an azo chromophore. Sens. Actuators B Chem. 2017, 251, 264–271. [Google Scholar] [CrossRef]
- Alshawi, J.M.S.; Mohammed, M.Q.; Alesary, H.F.; Ismail, H.K.; Barton, S. Voltammetric Determination of Hg(2+), Zn(2+), and Pb(2+) Ions Using a PEDOT/NTA-Modified Electrode. ACS Omega 2022, 7, 20405–20419. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.Q.; Ismail, H.K.; Alesary, H.F.; Barton, S. Use of a Schiff base-modified conducting polymer electrode for electrochemical assay of Cd(II) and Pb(II) ions by square wave voltammetry. Chem. Pap. 2021, 76, 715–729. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhao, L.; Sun, L.; Zhao, X.; Xia, Y. κ-Carrageenan-derived carbon dots for highly selective and sensitive detection of Fe3+ and oxytetracycline. J. Mater. Sci. 2020, 56, 1272–1285. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Akhtar, K.; Seo, J.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Phytosynthesis of silver nanoparticles; naked eye cellulose filter paper dual mechanism sensor for mercury ions and ammonia in aqueous solution. J. Mater. Sci. Mater. Electron. 2019, 30, 7367–7383. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Sun, L.; Qi, Q.; Zhao, X. Chitosan and kappa-carrageenan-derived nitrogen and sulfur co-doped carbon dots "on-off-on" fluorescent probe for sequential detection of Fe3+ and ascorbic acid. Int. J. Biol. Macromol. 2021, 191, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, X.; Ma, Y.; Qian, T.; Wang, C.; Chu, F. Facile and cost-effective preparation of carbon quantum dots for Fe3+ ion and ascorbic acid detection in living cells based on the “on-off-on” fluorescence principle. Appl. Surf. Sci. 2019, 469, 911–916. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, W.; Wang, J.; He, H.; Hou, X.; Zhou, S.; Wang, S. Tuneable design of a pulp fibre-based colorimetric sensor and its visual recognition mechanism for ppb levels of Ag+. Cellulose 2019, 26, 9149–9161. [Google Scholar] [CrossRef]
- Jain, A.; Wadhawan, S.; Kumar, V.; Mehta, S.K. Colorimetric sensing of Fe3+ ions in aqueous solution using magnesium oxide nanoparticles synthesized using green approach. Chem. Physic Lett. 2018, 706, 53–61. [Google Scholar] [CrossRef]
- Separovic, L.; Lourenço, F.R. Measurement uncertainty evaluation of an analytical procedure for determination of terbinafine hydrochloride in creams by HPLC and optimization strategies using Analytical Quality by Design. Microchem. J. 2022, 178, 107386. [Google Scholar] [CrossRef]
- Naik, V.M.; Gunjal, D.B.; Gore, A.H.; Pawar, S.P.; Mahanwar, S.T.; Anbhule, P.V.; Kolekar, G.B. Quick and low cost synthesis of sulphur doped carbon dots by simple acidic carbonization of sucrose for the detection of Fe3+ ions in highly acidic environment. Diam. Relat. Mater. 2018, 88, 262–268. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, C.; Qian, W.; Teng, C. Flower-like MnO2 on layered carbon derived from sisal hemp for asymmetric supercapacitor with enhanced energy density. J. Alloy. Compd. 2020, 826, 154133. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Z.; Gong, A.; Zhang, Y.; Li, Q. Synthesis of highly photoluminescent carbon dots via citric acid and Tris for iron(III) ions sensors and bioimaging. Talanta 2015, 143, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, S.Y.; Jung, J.M.; Kim, M.S.; Kim, C. Selective detection of Cu2+ and S2− by a colorimetric chemosensor: Experimental and theoretical calculations. Inorg. Chim. Acta 2018, 471, 709–717. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Shi, M.; Li, H.; Ma, L.; Niu, H. Morphology controllable synthesis of heteroatoms-doped carbon materials for high-performance flexible supercapacitor. Dye. Pigment. 2022, 199, 109968. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A High-Sensitive Dopamine Electrochemical Sensor Based on Multilayer Ti3C2 MXene, Graphitized Multi-Walled Carbon Nanotubes and ZnO Nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Pouzesh, M.; Nekouei, S.; Zadeh, M.A.F.; Keshtpour, F.; Wang, S.; Nekouei, F. Fabrication of stable copper nanoparticles embedded in nanocellulose film as a bionanocomposite plasmonic sensor and thereof for optical sensing of cyanide ion in water samples. Cellulose 2019, 26, 4945–4956. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Yang, Q.; Dong, J.; Ma, L.; Jiang, Z.; Huang, Y. 3D hierarchical oxygen-deficient AlCoNi-(oxy)hydroxides/N-doped carbon hybrids enable efficient battery-type asymmetric supercapacitor. J. Energy Chem. 2022, 72, 416–423. [Google Scholar] [CrossRef]
- Song, J.; Zhao, L.; Wang, Y.; Xue, Y.; Deng, Y.; Zhao, X.; Li, Q. Carbon Quantum Dots Prepared with Chitosan for Synthesis of CQDs/AuNPs for Iodine Ions Detection. Nanomaterials 2018, 8, 1043. [Google Scholar] [CrossRef]
- Sun, L.; Wei, W.; Zhang, H.; Xu, J.; Zhao, X. A simple colorimetric and fluorescent “on-off-on” dual-mode sensor based on cyan fluorescent carbon dots/AuNPs for the detection of L-cysteine and Zinc thiazole. Microchem. J. 2022, 174, 107079. [Google Scholar] [CrossRef]
- Sun, B.; Gou, Y.; Ma, Y.; Zheng, X.; Bai, R.; Abdelmoaty, A.A.A.; Hu, F. Investigate electrochemical immunosensor of cortisol based on gold nanoparticles/magnetic functionalized reduced graphene oxide. Biosens. Bioelectron. 2017, 88, 55–62. [Google Scholar] [CrossRef]
- Akhondi, M.; Jafari, A.H.; Jamalizadeh, E. Selective colorimetric detection of HgII using silver nanoparticles modified with Apple and Nigella Sativa seed extracts and β-Cyclodextrin. J. Environ. Chem. Eng. 2020, 8, 103566. [Google Scholar] [CrossRef]
- Joshi, P.; Nemiwal, M.; Al-Kahtani, A.A.; Ubaidullah, M.; Kumar, D. Biogenic AgNPs for the non-cross-linking detection of aluminum in aqueous systems. J. King Saud Univ. Sci. 2021, 33, 101527. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.X.; Liu, S.G.; Li, N.; Lin, S.M.; Fan, Y.Z.; Lei, J.L.; Luo, H.Q.; Li, N.B. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg(2+) ions detection. J. Hazard. Mater. 2017, 322, 430–436. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Park, W.H. Green Synthesis of Silver Nanoparticles Stabilized with Mussel-Inspired Protein and Colorimetric Sensing of Lead(II) and Copper(II) Ions. Int. J. Mol. Sci. 2016, 17, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Hu, C.; Guan, L.; Zhang, W.; Gu, J. Green Synthesis of Cellulose Nanofibrils Decorated with Ag Nanoparticles and Their Application in Colorimetric Detection of l-Cysteine. ACS Sustain. Chem. Eng. 2020, 8, 12713–12721. [Google Scholar] [CrossRef]
- Sharma, N.; Selvam, S.P.; Yun, K. Electrochemical detection of amikacin sulphate using reduced graphene oxide and silver nanoparticles nanocomposite. Appl. Surf. Sci. 2020, 512, 145742. [Google Scholar] [CrossRef]
- Gan, T.; Lv, Z.; Sun, J.; Shi, Z.; Liu, Y. Preparation of graphene oxide-wrapped carbon sphere@silver spheres for high performance chlorinated phenols sensor. J. Hazard. Mater. 2016, 302, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-M. The Preparation of Ag Nanoparticles/Graphene Nanocomposites with Polydopamine as Coupling Agent for Enhanced Detection of H2O2. Int. J. Electrochem. Sci. 2019, 14, 6840–6854. [Google Scholar] [CrossRef]
- Ali, S.; Shah, M.R.; Hussain, S.; Khan, S.; Latif, A.; Ahmad, M.; Ali, M. A Facile Approach Based on Functionalized Silver Nanoparticles as a Chemosensor for the Detection of Paraquat. J. Clust. Sci. 2021, 33, 413–420. [Google Scholar] [CrossRef]
- Ma, L.; Bi, Z.; Zhang, W.; Zhang, Z.; Xiao, Y.; Niu, H.; Huang, Y. Synthesis of a Three-Dimensional Interconnected Oxygen-, Boron-, Nitrogen-, and Phosphorus Tetratomic-Doped Porous Carbon Network as Electrode Material for the Construction of a Superior Flexible Supercapacitor. ACS Appl. Mater. Interfaces 2020, 12, 46170–46180. [Google Scholar] [CrossRef]
- Ullah, A.; Ali, I.; Ahmed, F.; Khan, S.; Shah, M.R.; Shaheen, F. Synthesis and characterization of peptide-conjugated silver nanoparticle for selective detection of Hg2+ in human blood plasma and tap water. J. Mol. Liq. 2019, 296, 112095. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Dinh, N.X.; Trang, N.L.N.; Khi, N.T.; Quy, N.V.; Tuan, P.A.; Tri, D.Q.; Thang, L.H.; Huy, T.Q.; Le, A.-T. Functionalized silver nanoparticles-based efficient colorimetric platform: Effects of surface capping agents on the sensing response of thiram pesticide in environmental water samples. Mater. Res. Bull. 2021, 139, 111278. [Google Scholar] [CrossRef]
- Eswaran, S.G.; Ashkar, M.A.; Mamat, M.H.; Sahila, S.; Mahalingam, V.; Koppisetti, H.V.S.R.M.; Vasimalai, N. Preparation of a portable calorimetry kit and one-step spectrophotometric nanomolar level detection of l-Histidine in serum and urine samples using sebacic acid capped silver nanoparticles. J. Sci. Adv. Mater. Dev. 2021, 6, 100–107. [Google Scholar]
- Rajamanikandan, R.; Ilanchelian, M. β-cyclodextrin functionalised silver nanoparticles as a duel colorimetric probe for ultrasensitive detection of Hg2+ and S2− ions in environmental water samples. Mater. Today Commun. 2018, 15, 61–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Gui, L.; Chen, Z. Colorimetric detection of Hg2+ based on target-mediated growth of gold nanoparticles. Sens. Actuators B Chem. 2017, 241, 262–267. [Google Scholar] [CrossRef]
- Deng, L.; Ouyang, X.; Jin, J.; Ma, C.; Jiang, Y.; Zheng, J.; Li, J.; Li, Y.; Tan, W.; Yang, R. Exploiting the higher specificity of silver amalgamation: Selective detection of mercury(II) by forming Ag/Hg amalgam. Anal. Chem. 2013, 85, 8594–8600. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Nirmalkar, N.; Deb, M.K.; Dewangan, K.; Nirmalkar, J.; Kumar, S. Application of functionalized silver nanoparticles as a biochemical sensor for selective detection of lysozyme protein in milk sample. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2019, 213, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Diamai, S.; Negi, D.P.S. Cysteine-stabilized silver nanoparticles as a colorimetric probe for the selective detection of cysteamine. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2019, 215, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Aktara, M.N.; Sahoo, N.K.; Jha, P.K.; Hossain, M. Sensitive and robust colorimetric assay of Hg2+ and S2− in aqueous solution directed by 5-sulfosalicylic acid-stabilized silver nanoparticles for wide range application in real samples. J. Environ. Chem. Eng. 2017, 5, 5645–5654. [Google Scholar] [CrossRef]
- Chen, J.L.; Yang, P.C.; Wu, T.; Lin, Y.W. Determination of mercury (II) ions based on silver-nanoparticles-assisted growth of gold nanostructures: UV-Vis and surface enhanced Raman scattering approaches. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2018, 199, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Eksin, E.; Erdem, A.; Fafal, T.; Kıvçak, B. Eco-friendly Sensors Developed by Herbal Based Silver Nanoparticles for Electrochemical Detection of Mercury (II) Ion. Electroanalysis 2019, 31, 1075–1082. [Google Scholar] [CrossRef]
- Ertürk, A.S. Biosynthesis of Silver Nanoparticles Using Epilobium parviflorum Green Tea Extract: Analytical Applications to Colorimetric Detection of Hg2+ Ions and Reduction of Hazardous Organic Dyes. J. Clust. Sci. 2019, 30, 1363–1373. [Google Scholar] [CrossRef]
- Faghiri, F.; Ghorbani, F. Colorimetric and naked eye detection of trace Hg2+ ions in the environmental water samples based on plasmonic response of sodium alginate impregnated by silver nanoparticles. J. Hazard. Mater. 2019, 374, 329–340. [Google Scholar] [CrossRef]
- Guo, L.; Song, Y.; Cai, K.; Wang, L. “On-off” ratiometric fluorescent detection of Hg2+ based on N-doped carbon dots-rhodamine B@TAPT-DHTA-COF. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 227, 117703. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, F.; Li, Y. Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sens. Actuators B Chem. 2018, 255, 1105–1111. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(iii) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Xing, A.; Miao, X.; Liu, T.; Yang, H.; Meng, Y.; Li, X. An intrinsic white-light-emitting hyperbranched polyimide: Synthesis, structure–property and its application as a “turn-off” sensor for iron(iii) ions. J. Mater. Chem. C 2019, 7, 14320–14333. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; He, S.; Li, X.; Chen, W. Colorimetric detection of iron ions (III) based on the highly sensitive plasmonic response of the N-acetyl-L-cysteine-stabilized silver nanoparticles. Anal. Chim. Acta 2015, 879, 118–125. [Google Scholar] [CrossRef]
- Kumar, V.; Mohan, S.; Singh, D.K.; Verma, D.K.; Singh, V.K.; Hasan, S.H. Photo-mediated optimized synthesis of silver nanoparticles for the selective detection of Iron(III), antibacterial and antioxidant activity. Mater. Sci. Eng. C 2017, 71, 1004–1019. [Google Scholar] [CrossRef]






| Samples | Concerntration of Hg2+ (μM) | Recovery (%) | RSD (n = 3, %) | ||
|---|---|---|---|---|---|
| Spiked | Measured | ||||
| Tap water | 1 | 5 | 5. 21 | 104.20 | 4.35 |
| 2 | 10 | 10.34 | 103.40 | 4.67 | |
| 3 | 15 | 14.85 | 98.33 | 3.14 | |
| Sea water | 1 | 5 | 4.98 | 99.60 | 3.89 |
| 2 | 10 | 10.22 | 102.20 | 4.20 | |
| 3 | 15 | 15.35 | 102.36 | 4.35 | |
| Samples | Concerntration of Fe3+ (μM) | Recovery (%) | RSD (n = 3, %) | ||
|---|---|---|---|---|---|
| Spiked | Measured | ||||
| Tap water | 1 | 5 | 5.24 | 104.80 | 5.15 |
| 2 | 10 | 10.26 | 102.60 | 4.56 | |
| 3 | 20 | 19.77 | 98.85 | 3.97 | |
| Sea water | 1 | 5 | 4.96 | 99.20 | 3.85 |
| 2 | 10 | 10.21 | 102.10 | 5.25 | |
| 3 | 20 | 20.32 | 101.60 | 4.28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhou, X.; Dong, R.; Wang, Y.; Li, Z.; Xue, Y.; Li, Q. A Highly Selective and Sensitive Nano-Silver sol Sensor for Hg2+ and Fe3+: Green Preparation and Mechanism. Polymers 2022, 14, 3745. https://doi.org/10.3390/polym14183745
Yang Y, Zhou X, Dong R, Wang Y, Li Z, Xue Y, Li Q. A Highly Selective and Sensitive Nano-Silver sol Sensor for Hg2+ and Fe3+: Green Preparation and Mechanism. Polymers. 2022; 14(18):3745. https://doi.org/10.3390/polym14183745
Chicago/Turabian StyleYang, Yining, Xiaodong Zhou, Ruitao Dong, Yanwei Wang, Zichao Li, Yun Xue, and Qun Li. 2022. "A Highly Selective and Sensitive Nano-Silver sol Sensor for Hg2+ and Fe3+: Green Preparation and Mechanism" Polymers 14, no. 18: 3745. https://doi.org/10.3390/polym14183745
APA StyleYang, Y., Zhou, X., Dong, R., Wang, Y., Li, Z., Xue, Y., & Li, Q. (2022). A Highly Selective and Sensitive Nano-Silver sol Sensor for Hg2+ and Fe3+: Green Preparation and Mechanism. Polymers, 14(18), 3745. https://doi.org/10.3390/polym14183745








