Synthesis and Application of Levofloxacin–Tin Complexes as New Photostabilizers for Polyvinyl Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
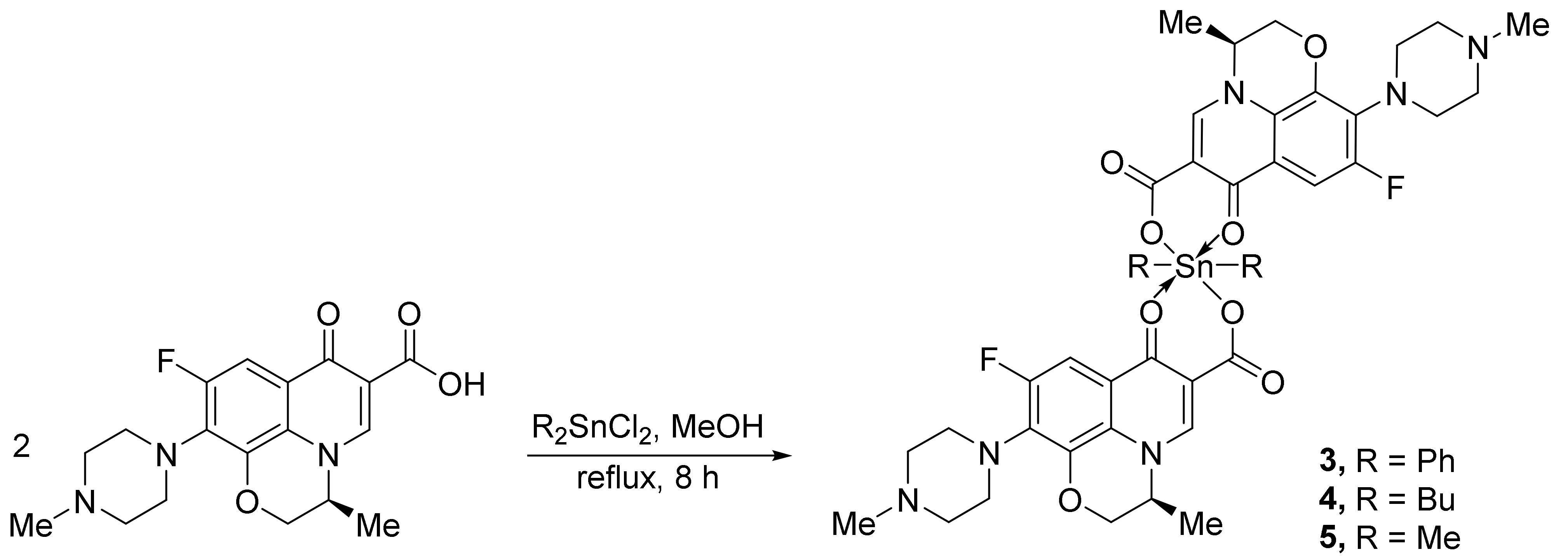
2.2. Synthesis of Tin Complexes 1 and 2
2.3. Synthesis of Tin Complexes 3–5
2.4. PVC Films Preparation
2.5. Determination of the Weight Loss of PVC
2.6. Determination of the Average Molecular Weight (Mv) of PVC
2.7. FTIR Spectrophotometry of PVC
3. Results and Discussion
3.1. Synthesis of Tin Complexes 1–5
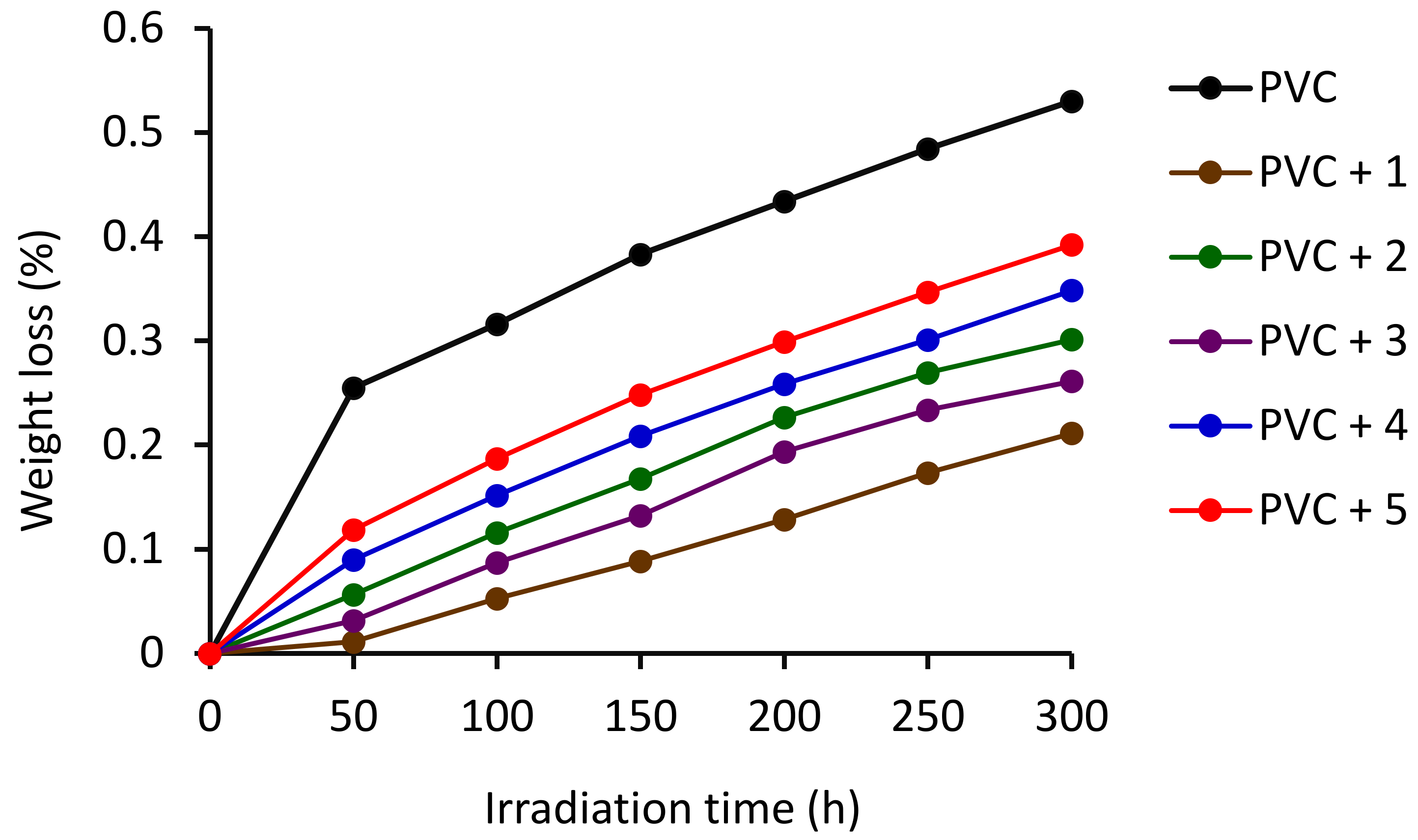
3.2. Weight Loss on Irradiation
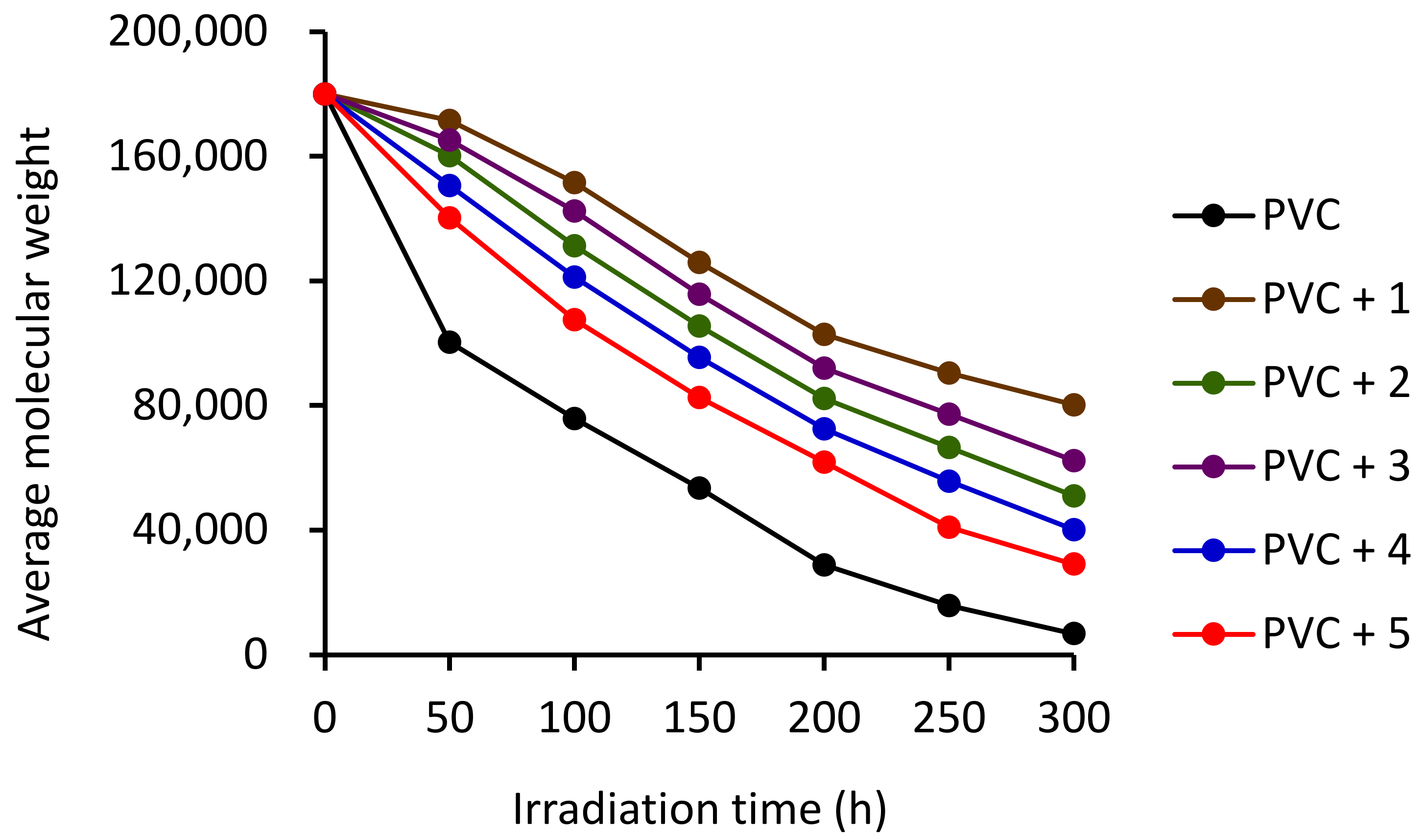
3.3. Average Molecular Weight (Mv) on Irradiation
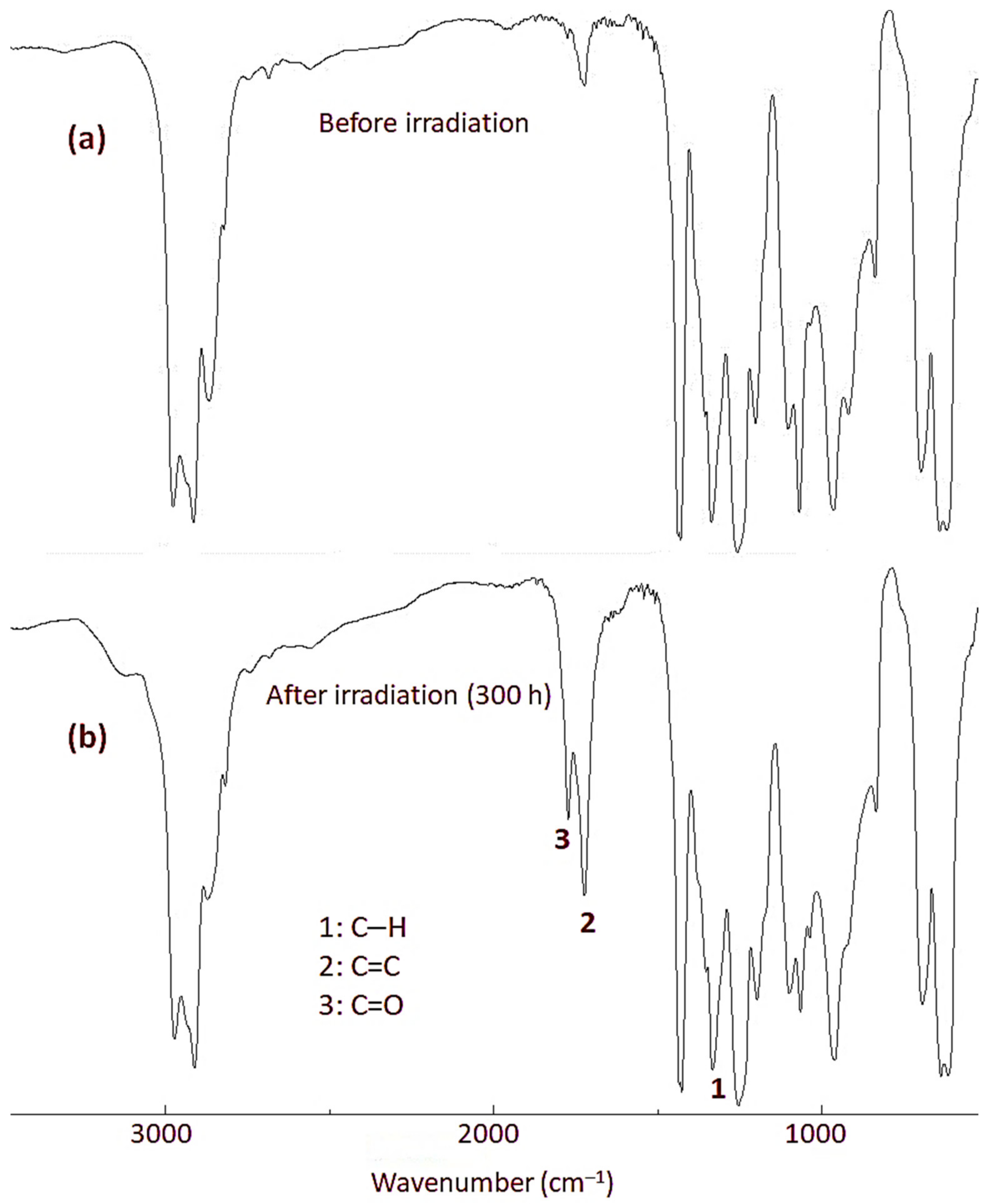
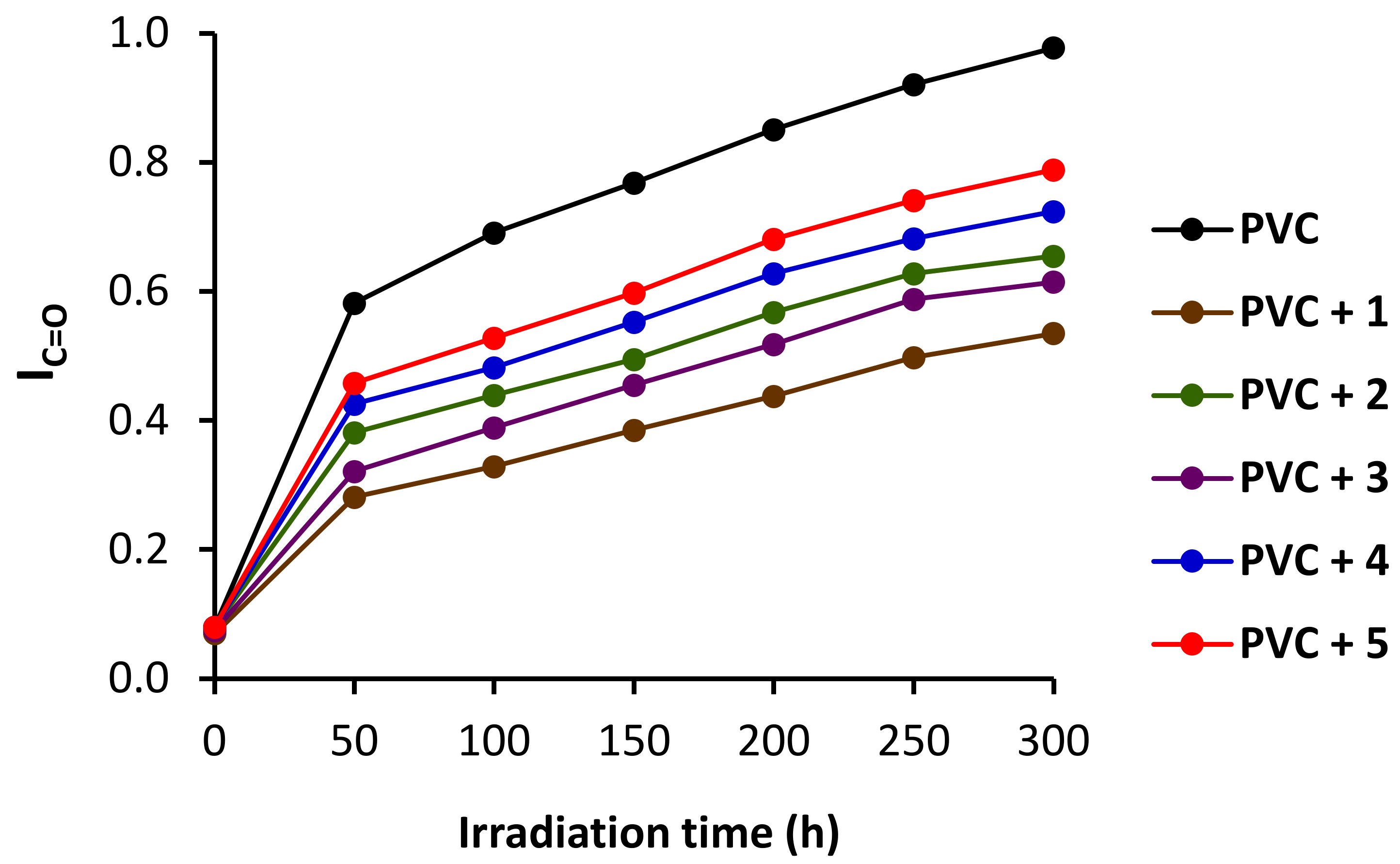
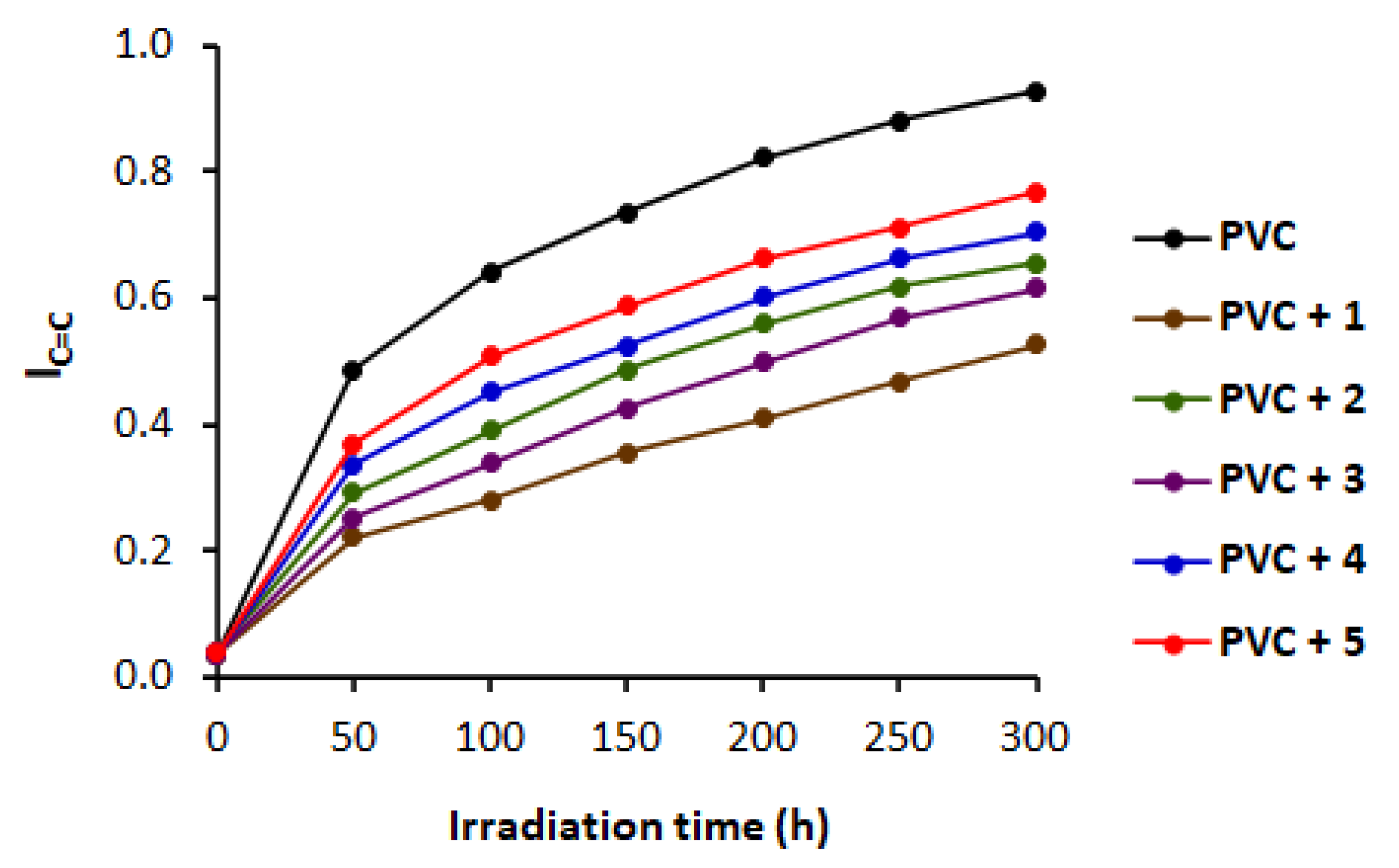
3.4. FTIR Spectrophotometry on Irradiation
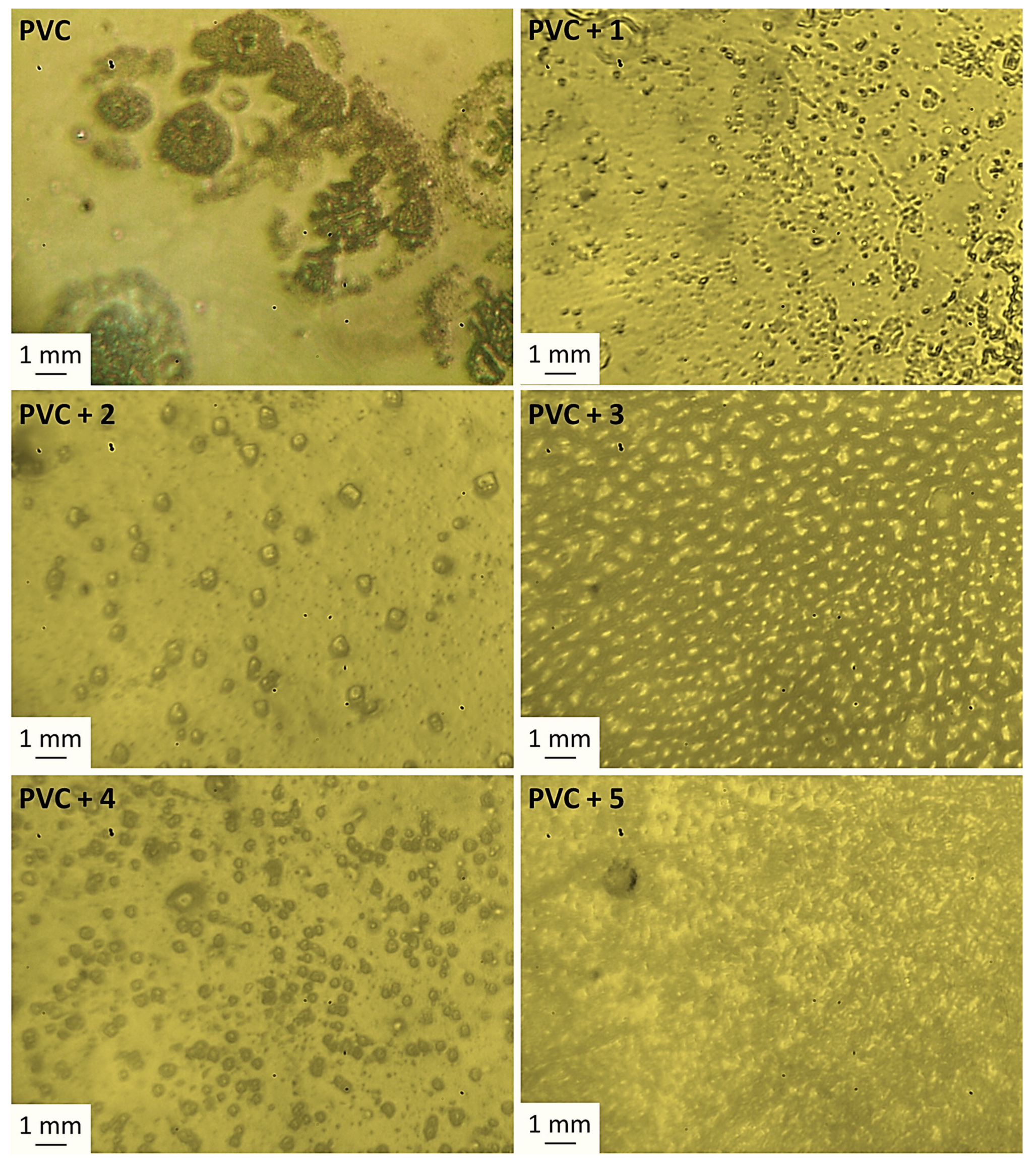
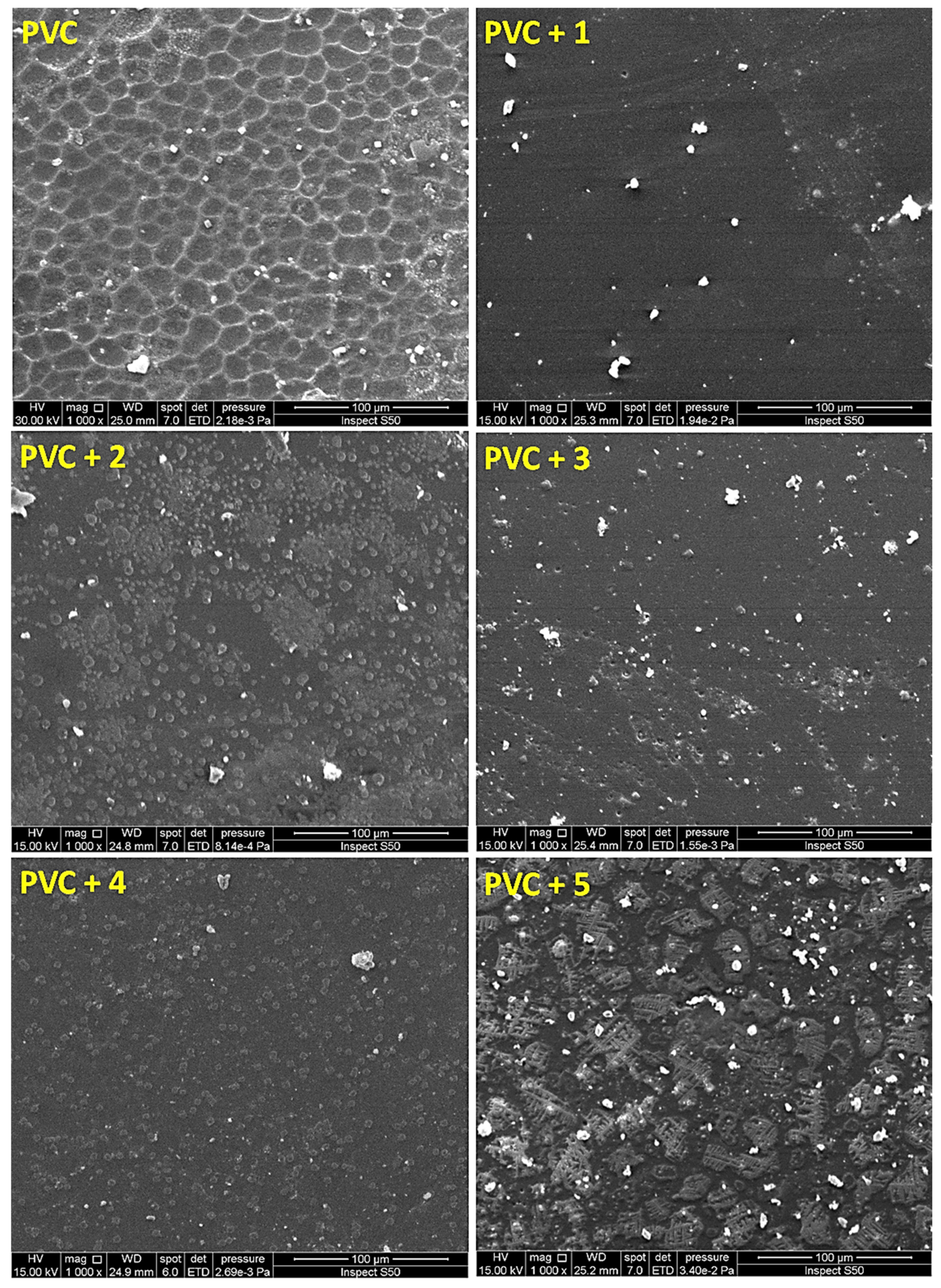
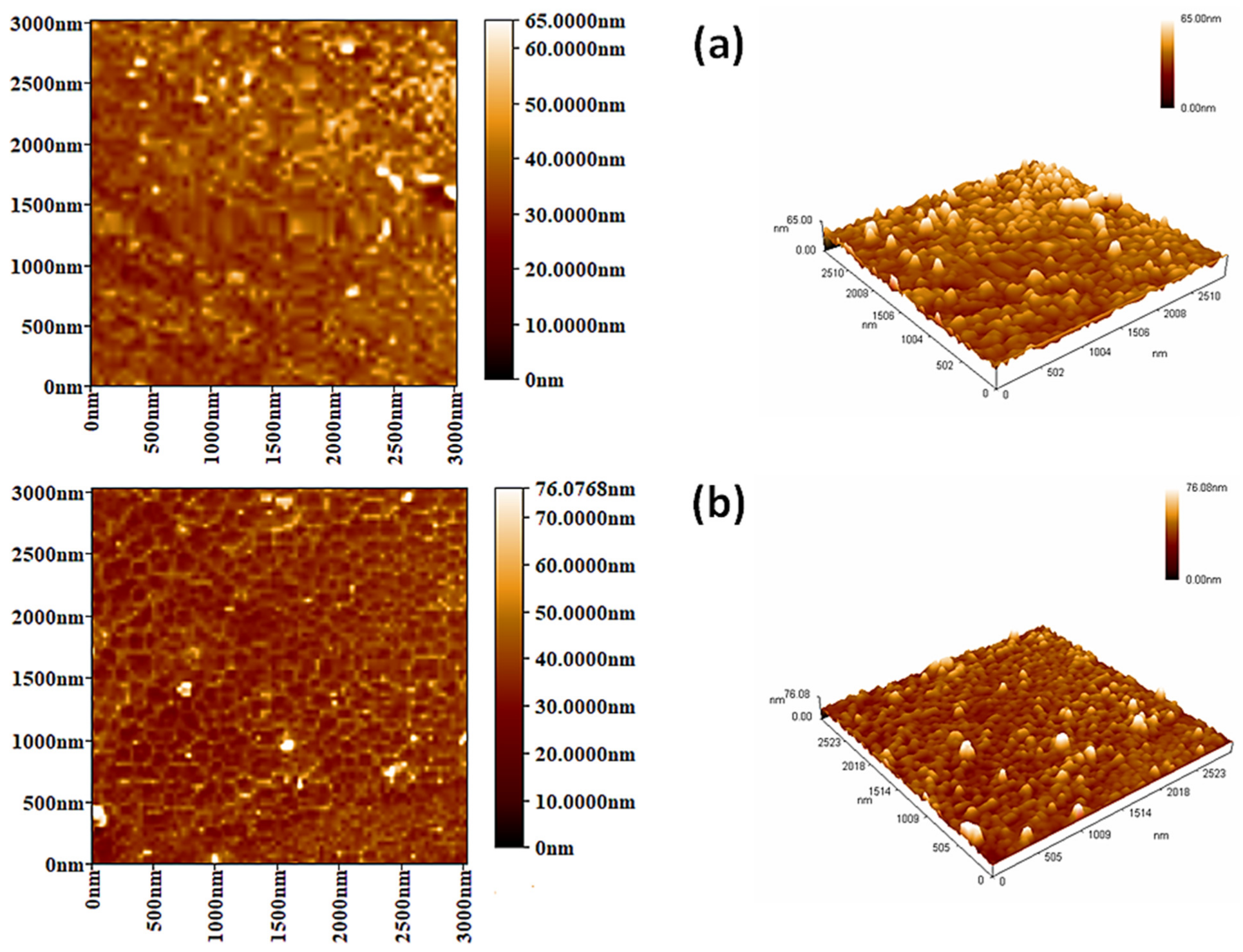
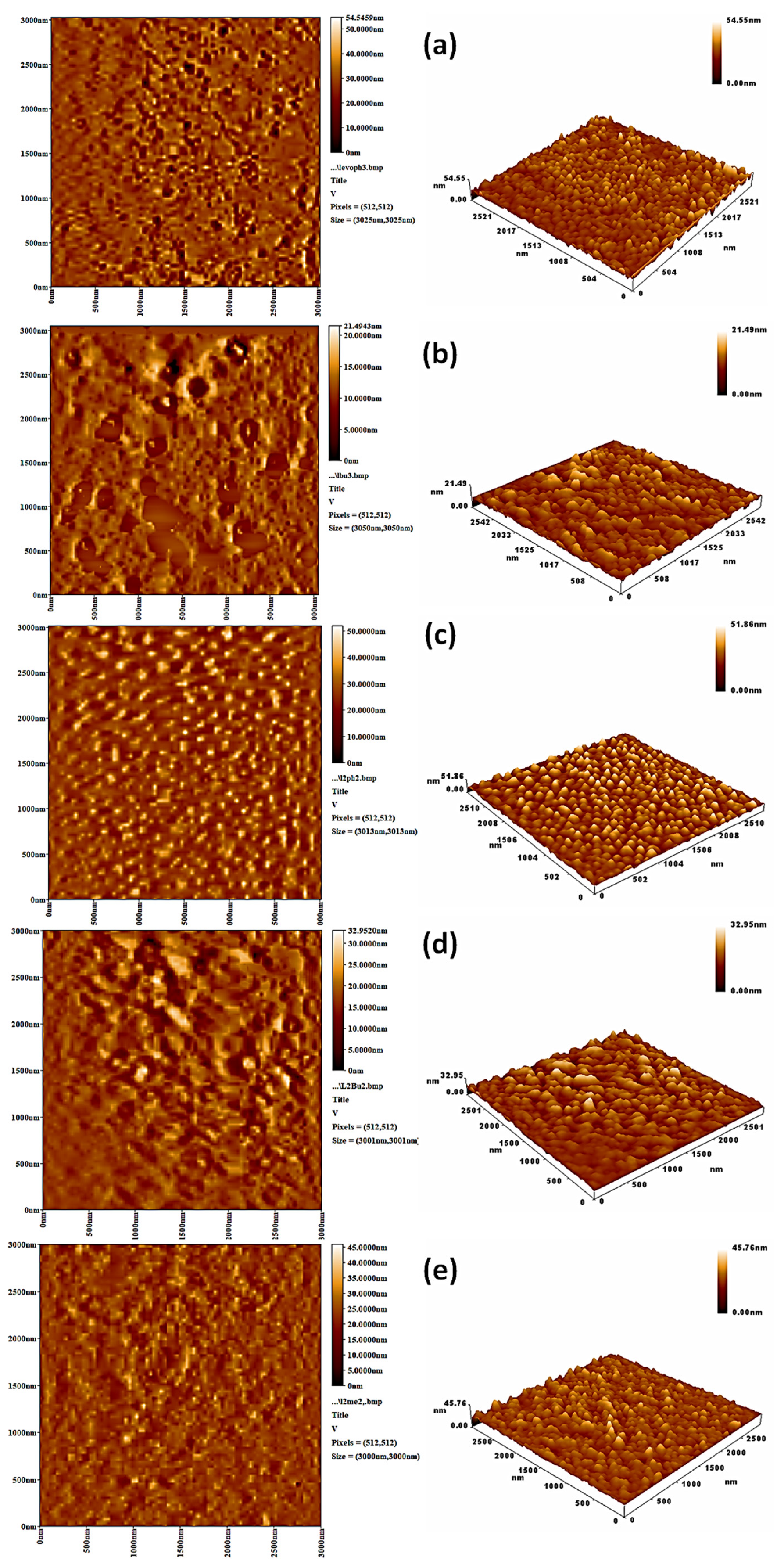
3.5. Surface Analysis on Irradiation
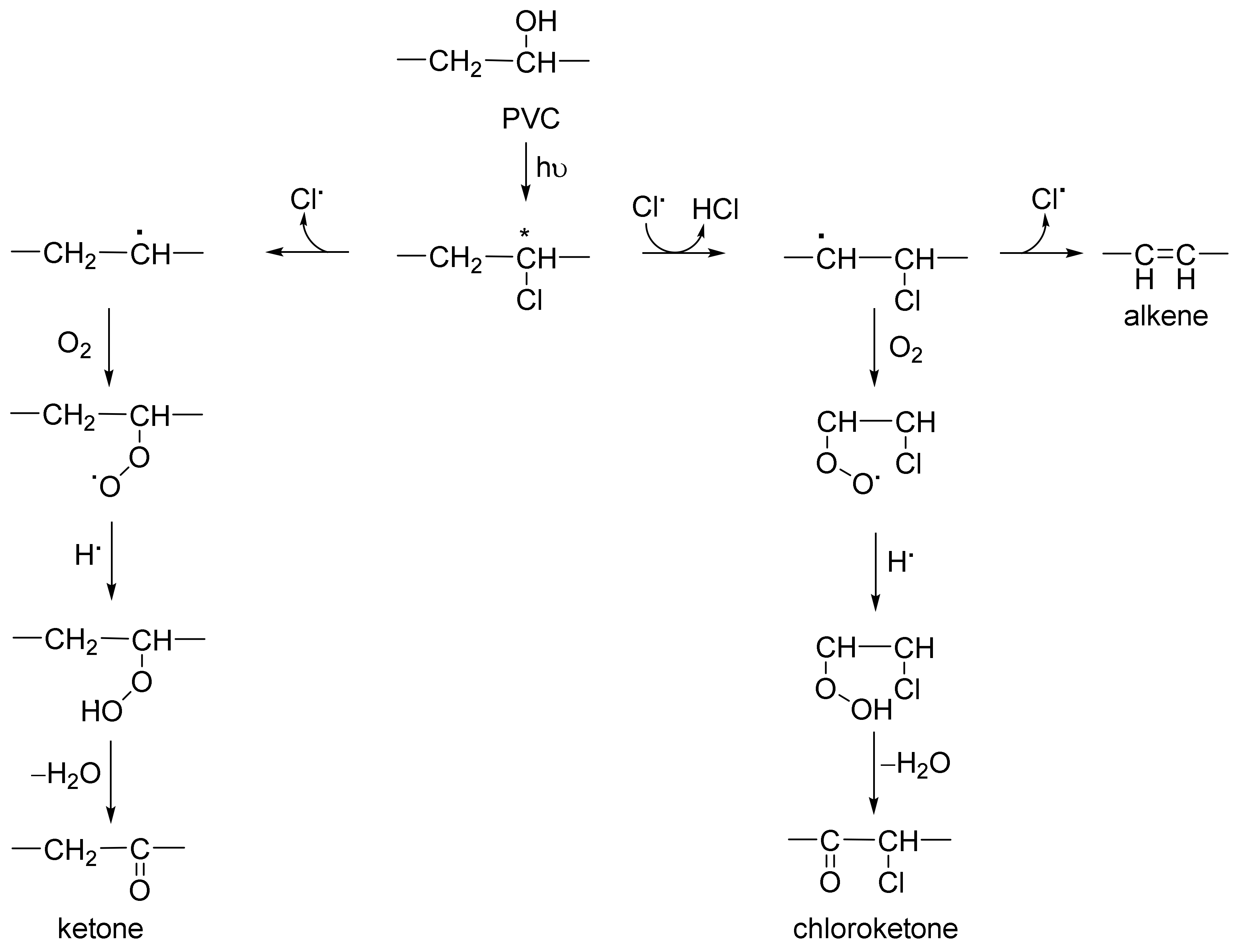
3.6. Photostabilization Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Neuba, L.D.M.; Junio, R.F.P.; Ribeiro, M.P.; Souza, A.T.; Lima, E.D.S.; Filho, F.D.C.G.; Figueiredo, A.B.-H.D.S.; Braga, F.D.O.; De Azevedo, A.R.G.; Monteiro, S.N. Promising mechanical, thermal, and ballistic properties of novel epoxy composites reinforced with Cyperus malaccensis sedge fiber. Polymers 2020, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the application of synthetic polymer materials in contemporary public art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 76. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Al-Khazrajy, O.S.A.; Abdallh, M.; Alanazi, S.A. Modifications of polymers through the addition of ultraviolet absorbers to reduce the aging effect of accelerated and natural irradiation. Polymers 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Noor, N.H.M.; Serrà, A.; Mohamad Ibrahim, M.N. Advances and challenges in developing efficient graphene oxide-based ZnO photocatalysts for dye photo-oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.A. Catalytic conversion of waste plastics: Focus on waste PVC. J. Chem. Technol. Biotechnol. 2007, 82, 787–795. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Liao, S.-L.; Li, Q.-G.; Guan, Q.; Jia, P.-Y.; Zhou, Y.-H. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration—Still on the run. React. Funct. Polym. 2019, 147, 104458. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant. Int. J. Min. Sci. Technol. 2021, 31, 1117–1128. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Yang, B.; Feng, Q.; Liu, D. Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper–ammonium species. Miner. Eng. 2022, 187, 107796. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Feng, Q.; Wang, H. Enhanced sulfidization of azurite surfaces by ammonium phosphate and its effect on flotation. Int. J. Miner. Metall. Mater. 2022, 29, 1150–1160. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.B.; Kumosa, M. Particle removal mechanisms in synergistic aging of polymers and glass reinforced polymer composites under combined UV and water. Compos. Sci. Technol. 2017, 153, 273–281. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Valko, L.; Klein, E.; Kovařík, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1132. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Luo, Z.; Wang, H.; Wei, Z. Molecular chain model construction, thermo-stability, and thermo-oxidative degradation mechanism of poly(vinyl chloride). RSC Adv. 2016, 6, 31898–31905. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]
- Braun, D. Recycling of PVC. Prog. Polym. Sci. 2002, 27, 2171–2195. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Porta, M.; Zumeta, E. Implementing the Stockholm treaty on persistent organic pollutants. Occup. Environ. Med. 2002, 59, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.F. Mixed metal vinyl stabilizer synergism. II: Reactions with zinc replacing cadmium. J. Vinyl Addit. Technol. 1990, 12, 142–145. [Google Scholar] [CrossRef]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Star, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly(vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M. PVC Additives: Performance, Chemistry, Developments, and Sustainability; Carl Hanser Verlag: Munich, Germany, 2015. [Google Scholar]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide particles on the surface morphology and the mechanical properties of PVC composites during QUV accelerated weathering. Polym. Compos. 2016, 37, 3391–3397. [Google Scholar] [CrossRef]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering. Polym. Degrad. Stab. 2014, 104, 33–39. [Google Scholar] [CrossRef]
- Annuar, S.N.S.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Cellular basis of organotin(IV) derivatives as anticancer metallodrugs: A review. Front. Chem. 2021, 9, 657599. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Y.; Li, Q. Medicinal properties of organotin compounds and their limitations caused by toxicity. Inorg. Chim. Acta 2014, 423, 2–13. [Google Scholar] [CrossRef]
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological activity studies on organotin (IV)n+ complexes and parent compounds. J. Organomet. Chem. 2006, 691, 1733–1747. [Google Scholar] [CrossRef]
- Davies, A.G. Organotin Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany; John Wiley: Chichester, UK, 2004. [Google Scholar]
- Arkış, E.; Balköse, D. Thermal stabilisation of poly(vinyl chloride) by organotin compounds. Polym. Degrad. Stab. 2005, 88, 46–51. [Google Scholar] [CrossRef]
- Croom, K.F.; Goa, K.L. Levofloxacin: A review of its use in the treatment of bacterial infections in the United States. Drugs 2003, 63, 2769–2802. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Karayıldırım, T.; Yanık, J.; Yüksel, M.; Saglam, M.; Haussmann, M. Degradation of PVC containing mixtures in the presence of HCl fixators. J. Polym. Environ. 2005, 13, 365–379. [Google Scholar] [CrossRef]
- Nief, O.A. Photostabilization of polyvinyl chloride by some new thiadiazole derivatives. Eur. J. Chem. 2015, 6, 242–247. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Alcock, N.W.; Culver, J.; Roe, S.M. Secondary bonding. Part 15. Influence of lone pairs on coordination: Comparison of diphenyl-tin (IV) and –tellurium (IV) carboxylates and dithiocarbamates. J. Chem. Soc. Dalton Trans. 1992, 9, 1477–1484. [Google Scholar] [CrossRef]
- Pejchal, V.; Holeček, J.; Nádvorník, M.; Lyčka, A. 13C and 119Sn NMR spectra of some mono-n-butyltin (IV) compounds. Collect. Czechoslov. Chem. Commun. 1995, 60, 1492–1501. [Google Scholar] [CrossRef]
- Shahid, K.; Ali, S.; Shahzadi, S.; Badshah, A.; Khan, K.M.; Maharvi, G.M. Organotin (IV) complexes of aniline derivatives. I. Synthesis, spectral and antibacterial studies of di- and triorganotin (IV) derivatives of 4-bromomaleanilic acid. Synth. React. Inorg. Met. Org. Chem. 2003, 33, 1221–1235. [Google Scholar] [CrossRef]
- Rehman, W.; Baloch, M.K.; Badshah, A.; Ali, S. Synthesis and characterization of biologically potent di-organotin (IV) complexes of mono-methyl glutarate. J. Chin. Chem. Soc. 2005, 52, 231–236. [Google Scholar] [CrossRef]
- Chaochanchaikul, K.; Rosarpitak, V.; Sombatsompop, N. Photodegradation profiles of PVC compound and wood/PVC composites under UV weathering. Express Polym. Lett. 2013, 7, 146–160. [Google Scholar] [CrossRef]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Skillicorn, D.E.; Perkins, G.G.A.; Slark, A.; Dawkins, J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(vinyl chloride). 1. A reexamination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Pi, H.; Xiong, Y.; Guo, S. The kinetic studies of elimination of HCl during thermal decomposition of PVC in the presence of transition metal oxides. Polym. Plast. Technol. Eng. 2005, 44, 275–288. [Google Scholar] [CrossRef]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. J. Appl. Polym. Sci. 2003, 89, 36–46. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly(vinyl chloride) films containing naproxen diorganotin(IV) complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef]
- Mousa, O.G.; El-Hiti, G.A.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Synthesis of carvedilol-organotin complexes and their effects on reducing photodegradation of poly(vinyl chloride). Polymers 2021, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly(vinyl chloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, A.A.; Yousif, E.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Kariuki, B.M.; Ahmed, D.S.; Bufaroosha, M. FTIR, weight, and surface morphology of poly(vinyl chloride) doped with tin complexes containing aromatic and heterocyclic moieties. Polymers 2021, 13, 3264. [Google Scholar] [CrossRef]
- Fadhil, M.; Yousif, E.; Ahmed, D.S.; Mohammed, A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of new norfloxacin–tin complexes to mitigate the effect of ultraviolet-visible irradiation in polyvinyl chloride films. Polymers 2022, 14, 2812. [Google Scholar] [CrossRef]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef]
- Ghani, H.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Tin complexes of 4-(benzylideneamino)benzenesulfonamide: Synthesis, structure elucidation and their efficiency as PVC photostabilizers. Polymers 2021, 13, 2434. [Google Scholar] [CrossRef]
- Hadi, A.G.; Baqir, S.J.; Ahmed, D.S.; El-Hiti, G.A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; Yousif, E. Substituted organotin complexes of 4-methoxybenzoic acid for reduction of poly(vinyl chloride) photodegradation. Polymers 2021, 13, 3946. [Google Scholar] [CrossRef]
- Scott, G. Mechanism of Polymer Degradation and Stabilization; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Larché, J.F.; Bussière, P.O.; Therias, S.; Gardette, J.L. Photooxidation of polymers: Relating material properties to chemical changes. Polym. Degrad. Stab. 2012, 97, 25–34. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 48–49. [Google Scholar]


| Complex | M.P. (°C) | Yield (%) | Found (Calculated; %) | |||
|---|---|---|---|---|---|---|
| C | H | N | Sn | |||
| 1 | 110–113 | 79 | 60.78 (60.87) | 4.96 (4.82) | 5.91 (5.92) | 16.69 (16.71) |
| 2 | 128–130 | 81 | 55.31 (55.40) | 7.17 (7.13) | 6.45 (6.46) | 18.22 (18.25) |
| 3 | 222–225 | 85 | 57.90 (58.02) | 5.06 (4.87) | 8.44 (8.46) | 11.92 (11.95) |
| 4 | 194–196 | 83 | 55.30 (55.42) | 6.12 (5.92) | 8.79 (8.81) | 12.42 (12.45) |
| 5 | 175–178 | 78 | 52.37 (52.49) | 5.12 (5.10) | 9.64 (9.67) | 13.62 (13.65) |
| Complex | Wave Number (ν; cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| C=O | COO | C=C | Sn–C | Sn–O | |||
| Asym | Sym | Δν | |||||
| 1 | 1618 | 1714 | 1386 | 328 | 1588 | 540 | 450 |
| 2 | 1617 | 1718 | 1399 | 315 | 1575 | 571 | 495 |
| 3 | 1614 | 1714 | 1383 | 331 | 1588 | 556 | 451 |
| 4 | 1618 | 1707 | 1395 | 312 | 1586 | 563 | 491 |
| 5 | 1617 | 1707 | 1397 | 310 | 1587 | 568 | 461 |
| Complex | NMR (DMSO-d6), δ (ppm), and J (Hz) | |
|---|---|---|
| 1H | 119Sn | |
| 1 | 9.13 (s, 1H, Ar), 7.95 (d, J = 12.0 Hz, 1H, Ar), 7.57–7.32 (m, 15H, 3 Ph), 4.95 (m, 1H, CH), 4.58 (dd, J = 4.0 & 12.0 Hz, 1H, 1H of CH2), 4.37 dd, J = 4.0 & 12.0 Hz, 1H, 1H of CH2), 3.31 (m, 4H, CH2CH2), 2.45 (br, 4H, CH2CH2), 2.28 (s, 3H, Me), 1.45 (d, J = 7.0 Hz, 3H, Me) | −170.6 |
| 2 | 9.05 (s, 1H, Ar), 7.62 (d, J = 12.1 Hz, 1H, Ar), 5.00 (m, 1H, CH), 4.65 (dd, J = 4.1 & 12.0 Hz, 1H, 1H of CH2), 4.43 dd, J = 4.1 & 12.0 Hz, 1H, 1H of CH2), 3.43 (m, 4H, CH2CH2), 2.78 (br, 4H, CH2CH2), 2.50 (s, 3H, Me), 1.74 (m, 6H, 3 CH2), 1.62 (m, 6H, 3 CH2), 1.39 (d, J = 7.0 Hz, 3H, Me), 1.15 (m, 6H, 3 CH2), 0.95 (t, J = 7.1 Hz, 9H, 3 Me) | −175.9 |
| 3 | 8.94 (s, 2H, Ar), 7.92 (d, J = 12.0 Hz, 2H, Ar), 7.54–7.32 (m, 10H, 2 Ph), 5.02 (m, 2H, CH), 4.60 (dd, J = 4.0 & 12.0 Hz, 2H, 1H of 2 CH2), 4.40 (dd, J = 4.0 & 12.0 Hz, 2H, 1H of 2 CH2), 3.38 (m, 8H, 2 CH2CH2), 2.75 (br, 8H, 2 CH2CH2), 2.42 (s, 6H, 2 Me), 1.46 (d, J = 7.1 Hz, 6H, 2 Me) | −502.8 |
| 4 | 9.01 (s, 2H, Ar), 7.52 (d, J = 12.0 Hz, 2H, Ar), 4.92 (m, 2H, 2 CH), 4.54 (dd, J = 4.0 & 12.0 Hz, 2H, 1H of 2 CH2), 4.40 dd, J = 4.1 & 12.0 Hz, 2H, 1H of 2 CH2), 3.44 (m, 8H, 2 CH2CH2), 2.75 (br, 8H, 2 CH2CH2), 2.51 (s, 6H, 2 Me), 1.60 (m, 4H, 2 CH2), 1.52 (m, 4H, 2 CH2), 1.36 (d, J = 7.0 Hz, 6H, Me), 1.20 (m, 4H, 2 CH2), 0.83 (t, J = 7.2 Hz, 6H, 2 Me) | −313.5 |
| 5 | 9.00 (s, 2H, Ar), 7.51 (d, J = 12.1 Hz, 2H, Ar), 4.94 (m, 2H, 2 CH), 4.58 (dd, J = 4.0 & 12.2 Hz, 2H, 1H of 2 CH2), 4.40 (dd, J = 4.0 & 12.2 Hz, 2H, 1H of 2 CH2), 3.43 (m, 8H, 2 CH2CH2), 2.72 (br, 8H, 2 CH2CH2), 2.48 (s, 6H, 2 Me), 1.45 (d, J = 7.0 Hz, 6H, 2 Me), 0.89 (s, 6H, 2 Me) | −227.3 |
| Organic Unit in Tin Complex | Rq Reduction (Fold) | Reference |
|---|---|---|
| Levofloxacin | 15.4 | [this work] |
| Naproxen | 5.2 | [54] |
| Carvedilol | 6.4 | [55] |
| Furosemide | 6.6 | [56] |
| Valsartan | 7.4 | [57] |
| Telmisartan | 9.4 | [58] |
| Trimethoprim | 11.3 | [59] |
| Norfloxacin | 12.9 | [60] |
| Ciprofloxacin | 16.6 | [61] |
| 4-(Benzylideneamino)benzenesulfonamide | 18.4 | [62] |
| 4-Methoxybenzoic acid | 21.2 | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadhil, M.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis and Application of Levofloxacin–Tin Complexes as New Photostabilizers for Polyvinyl Chloride. Polymers 2022, 14, 3720. https://doi.org/10.3390/polym14183720
Fadhil M, Yousif E, Ahmed DS, Kariuki BM, El-Hiti GA. Synthesis and Application of Levofloxacin–Tin Complexes as New Photostabilizers for Polyvinyl Chloride. Polymers. 2022; 14(18):3720. https://doi.org/10.3390/polym14183720
Chicago/Turabian StyleFadhil, Marwa, Emad Yousif, Dina S. Ahmed, Benson M. Kariuki, and Gamal A. El-Hiti. 2022. "Synthesis and Application of Levofloxacin–Tin Complexes as New Photostabilizers for Polyvinyl Chloride" Polymers 14, no. 18: 3720. https://doi.org/10.3390/polym14183720
APA StyleFadhil, M., Yousif, E., Ahmed, D. S., Kariuki, B. M., & El-Hiti, G. A. (2022). Synthesis and Application of Levofloxacin–Tin Complexes as New Photostabilizers for Polyvinyl Chloride. Polymers, 14(18), 3720. https://doi.org/10.3390/polym14183720