Preparation and Properties of Asymmetric Polyvinyl Pyrroli-Done/Polycaprolactone Composite Nanofiber Loaded with Tea Tree Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Water Extraction
2.3. Electrospinning and Electro-Spraying
2.4. Characterizations
2.5. Antioxidative Capacity Evaluation
2.6. Antibacterial Assay
3. Results and Discussion
3.1. Characterization
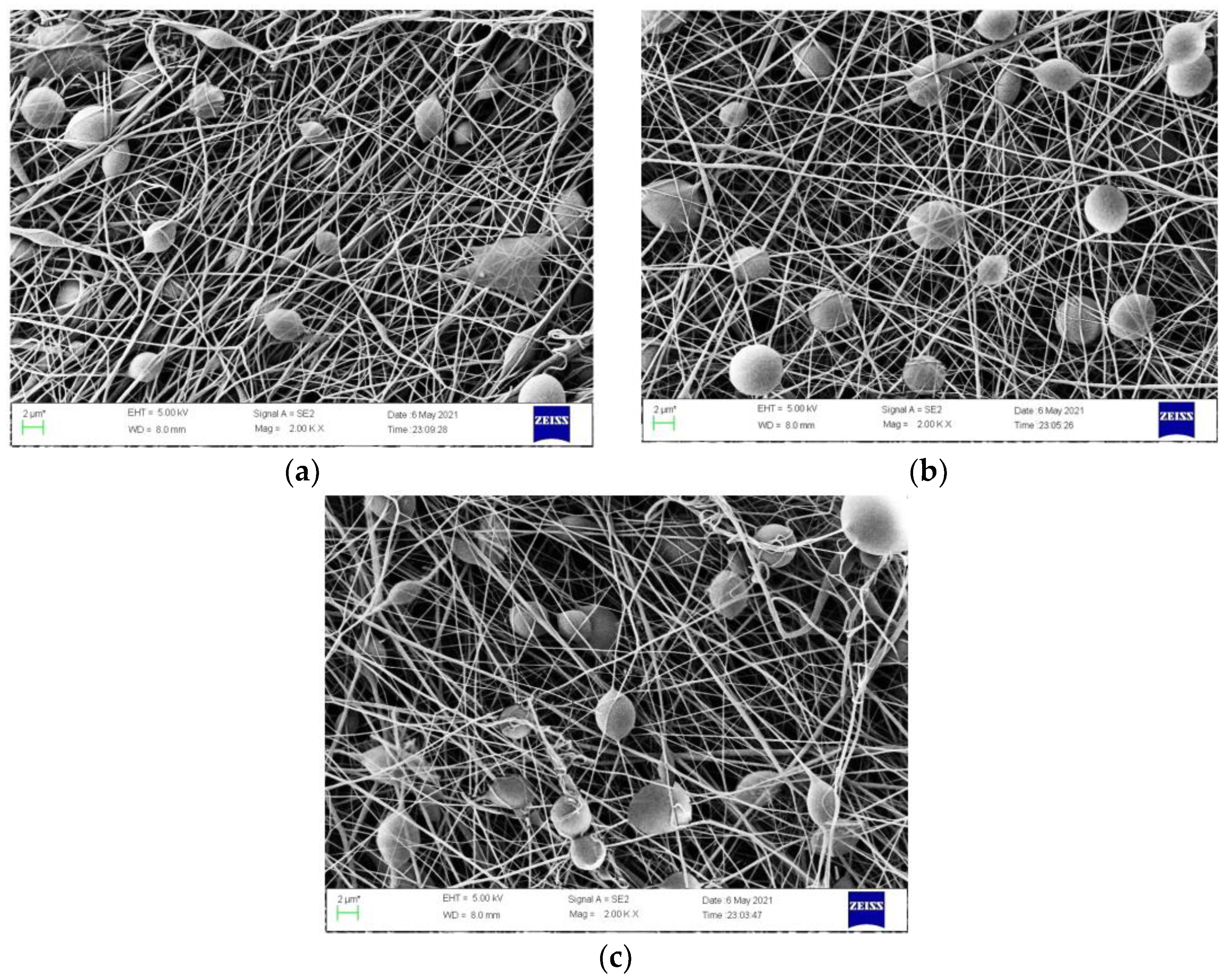
3.1.1. Morphology
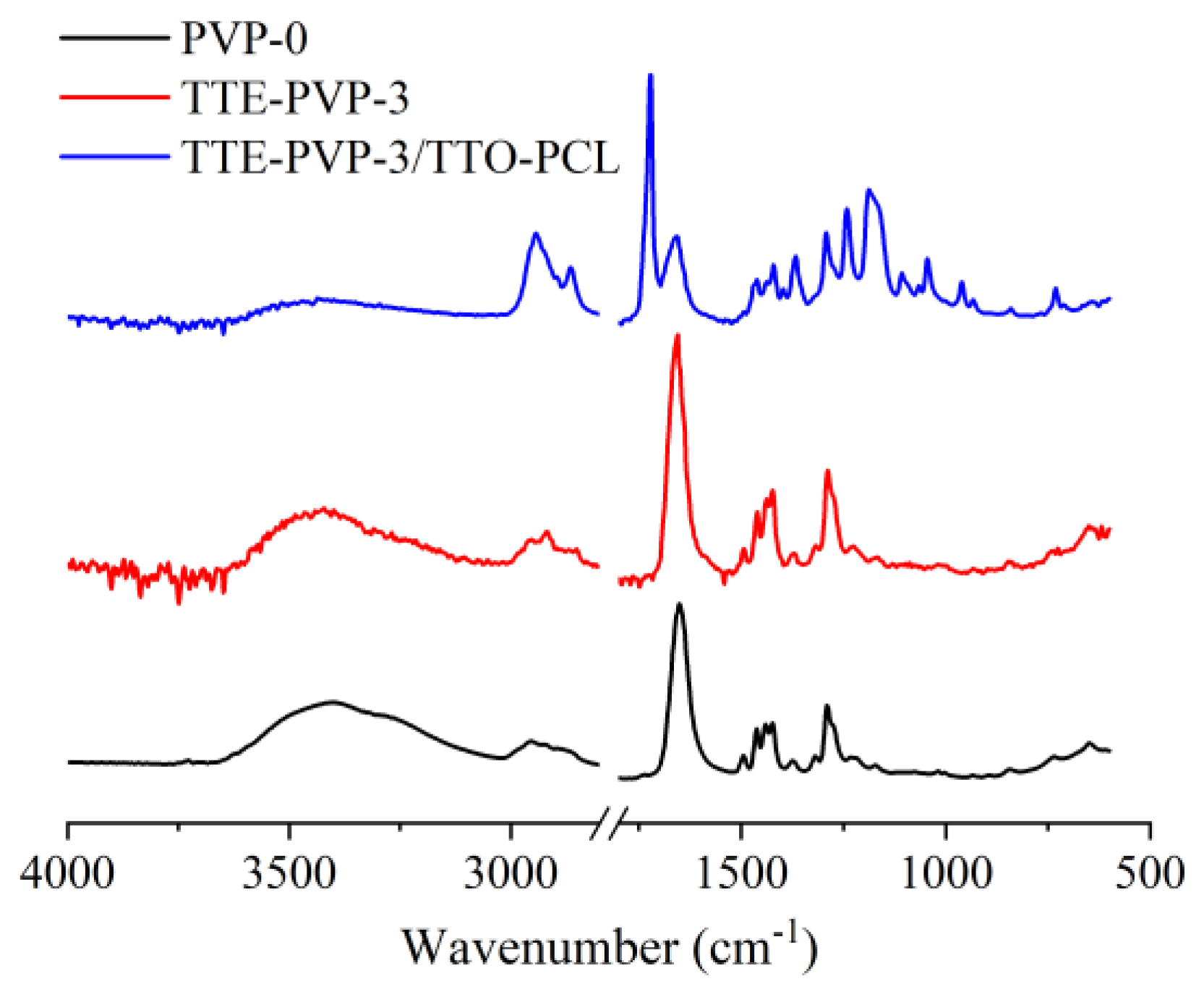
3.1.2. Chemical Structure
3.2. Antioxidant Properties
3.3. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Redondo, A.; Jang, D.; Korley, L.T.J.; Gunkel, I.; Steiner, U. Electrospinning of Cellulose Nanocrystal-Reinforced Polyurethane Fibrous Mats. Polymers 2020, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Li, C.; Luo, X.; Li, L.; Cai, Y.; Kang, X.; Li, P. Carboxymethyl chitosan-based electrospun nanofibers with high citral-loading for potential anti-infection wound dressings. Int. J. Biol. Macromol. 2022, 209, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, C.; Hou, T.; Cai, Y.; Liang, L.; Chen, L.; Li, M. Polyhexamethylene guanidine functionalized chitosan nanofiber membrane with superior adsorption and antibacterial performances. React. Funct. Polym. 2019, 145, 104379. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Li, T.Y.; Han, Y.F.; Li, F.J.; Liu, Y. Recent development of electrospun wound dressing. Curr. Opin. Biomed. Eng. 2021, 17, 100247. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. 2018, 14, 130–143. [Google Scholar] [CrossRef]
- Yan, T.; Kong, S.; Ouyang, Q.; Li, C.; Hou, T.; Chen, Y.; Li, S. Chitosan-Gentamicin Conjugate Hydrogel Promoting Skin Scald Repair. Mar. Drugs 2020, 18, 233. [Google Scholar] [CrossRef]
- Liang, L.; Liu, T.; Ouyang, Q.; Li, S.; Li, C. Solid phase synthesis of oxidized sodium alginate-tobramycin conjugate and its application for infected wound healing. Carbohydr. Polym. 2022, 295, 119843. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2022, 8, 341–354. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Sadri, M.; Arab-Sorkhi, S.; Vatani, H.; Bagheri-Pebdeni, A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015, 16, 1742–1750. [Google Scholar] [CrossRef]
- Pusporini, P.; Edikresnha, D.; Sriyanti, I.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Electrospun polyvinylpyrrolidone (PVP)/green tea extract composite nanofiber mats and their antioxidant activities. Mater. Res. Express 2018, 5, 054001. [Google Scholar] [CrossRef]
- Ravin, H.A.; Seligman, A.M.; Fine, J. Polyvinyl pyrrolidone as a plasma expander; Studies on its excretion, distribution and metabolism. N. Engl. J. Med. 1952, 247, 921–929. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; Fakhry, H.; Ajarem, J.; Maodaa, S.N.; Allam, A.A.; Hafez, E.E. Wound dressing of chitosan-based-crosslinked gelatin/polyvinyl pyrrolidone embedded silver nanoparticles, for targeting multidrug resistance microbes. Carbohydr. Polym. 2021, 255, 117484. [Google Scholar] [CrossRef]
- Sailer, R.; Berger, T.; Reichling, J.; Harkenthal, M. Pharmaceutical and medicinal aspects of Australian tea tree oil. Phytomedicine 1998, 5, 489–495. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wu, C.Q.; Wang, X.; Chung, H.Y.; Jin, Z.Y. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.; Ko, S.W.; Son, B.C.; Lee, J.H.; Kim, C.S.; Park, C.H. Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications. Polymers 2020, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Puvaca, N.; Cabarkapa, I.; Petrovic, A.; Bursic, V.; Prodanovic, R.; Solesa, D.; Levic, J. Tea tree (Melaleuca alternifolia) and its essential oil: Antimicrobial, antioxidant and acaricidal effects in poultry production. World Poult. Sci. J. 2019, 75, 235–246. [Google Scholar] [CrossRef]
- Koseki, Y.; Tanaka, R.; Murata, H. Development of antibacterial denture cleaner for brushing containing tea tree and lemongrass essential oils. Dent. Mater. J. 2018, 37, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zou, L.; Liu, Q.; Geng, Y.; Xu, G.; Chen, L.; Pan, P.; Chen, J. Construction of chitosan-based asymmetric antioxidant and anti-inflammatory repair film for acceleration of wound healing. Int. J. Biol. Macromol. 2022, 215, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Cui, H.Y.; Bai, M.; Lin, L. Plasma-treated poly(ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef]
- Xu, H.L.; Wu, Z.Y.; Zhao, D.; Liang, H.Y.; Yuan, H.X.; Wang, C.T. Preparation and characterization of electrospun nanofibers-based facial mask containing hyaluronic acid as a moisturizing component and huangshui polysaccharide as an antioxidant component. Int. J. Biol. Macromol. 2022, 214, 212–219. [Google Scholar] [CrossRef]
- Silva, M.M.C.L.; dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Xie, J.; Lim, L.K.; Phua, Y.; Hua, J.; Wang, C.-H. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Colloid Interface Sci. 2006, 302, 103–112. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.S.; Song, X.L.; Ma, Y.Z.; Huang, Y.F. Effects of added polyvinyl pyrrolidone on morphology and microstructure of multiple-phase mullite nanofibers. Ceram. Int. 2018, 44, 15418–15427. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Zayed, M.A.; El-Dek, S.I.; Hady, M.A.; El Sherbiny, D.H.; Uskokovic, V. Nanofibrous epsilon-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: Physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo. Bioact. Mater. 2021, 6, 2070–2088. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.R.; Barbosa, L.C.A.; Maltha, C.R.A.; Paula, V.F.; Nascimento, E.A. Chemical constituents from Melaleuca alternifolia (Myrtaceae). Quim Nova 2004, 27, 536–539. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xie, L.; Hou, T.; Wang, D.; Zhang, T.; Li, C. Preparation and Properties of Asymmetric Polyvinyl Pyrroli-Done/Polycaprolactone Composite Nanofiber Loaded with Tea Tree Extract. Polymers 2022, 14, 3714. https://doi.org/10.3390/polym14183714
Xu Y, Xie L, Hou T, Wang D, Zhang T, Li C. Preparation and Properties of Asymmetric Polyvinyl Pyrroli-Done/Polycaprolactone Composite Nanofiber Loaded with Tea Tree Extract. Polymers. 2022; 14(18):3714. https://doi.org/10.3390/polym14183714
Chicago/Turabian StyleXu, Yu, Lei Xie, Tingting Hou, Di Wang, Tong Zhang, and Chengpeng Li. 2022. "Preparation and Properties of Asymmetric Polyvinyl Pyrroli-Done/Polycaprolactone Composite Nanofiber Loaded with Tea Tree Extract" Polymers 14, no. 18: 3714. https://doi.org/10.3390/polym14183714
APA StyleXu, Y., Xie, L., Hou, T., Wang, D., Zhang, T., & Li, C. (2022). Preparation and Properties of Asymmetric Polyvinyl Pyrroli-Done/Polycaprolactone Composite Nanofiber Loaded with Tea Tree Extract. Polymers, 14(18), 3714. https://doi.org/10.3390/polym14183714







