Preparation and Photodegradation Properties of Carbon-Nanofiber-Based Catalysts
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of Carbon Nanofiber Membrane
2.3. Preparation of Fe2O3/CNFs Composites
- (1)
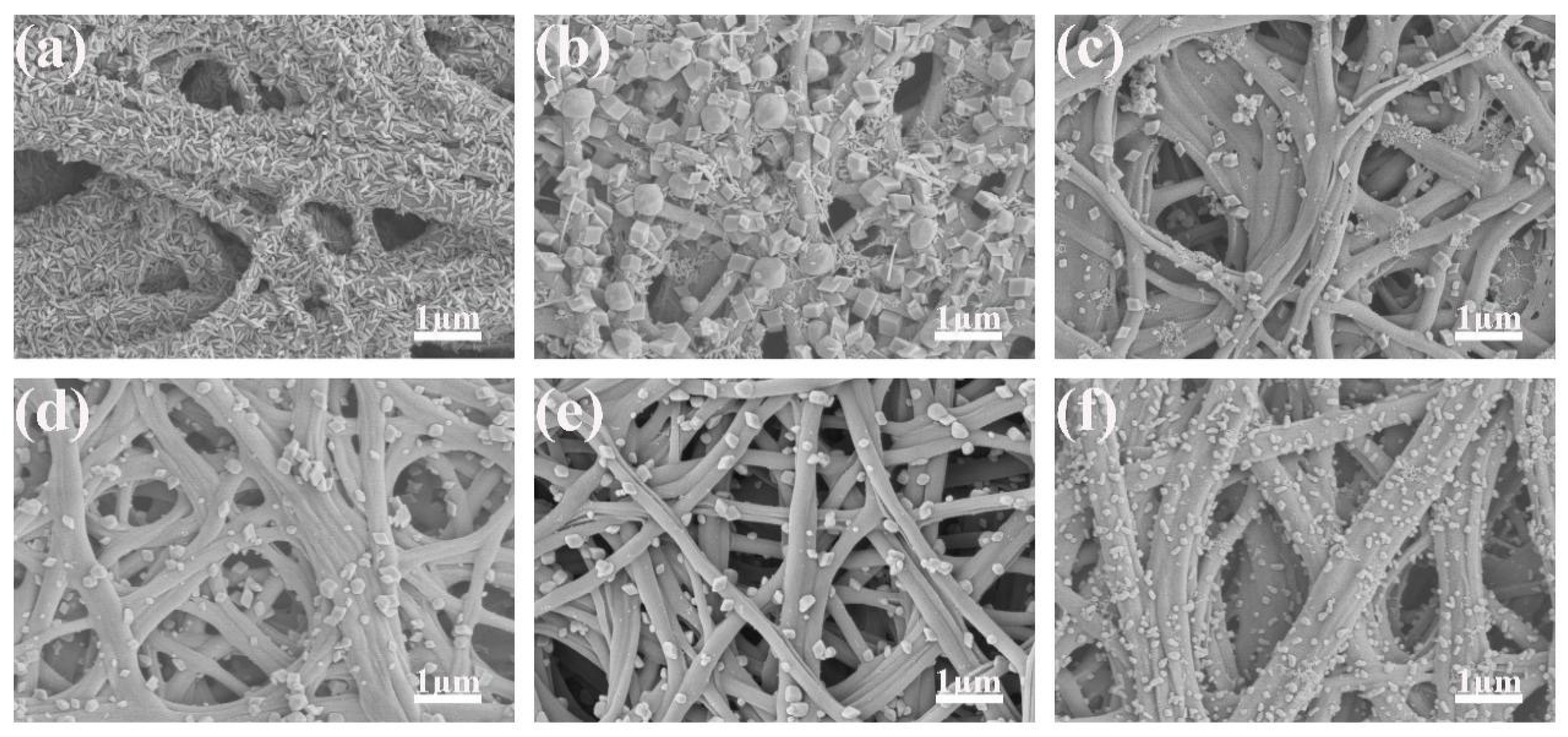
- The hydrothermal reaction time was 1 h, and the reaction temperatures were 130 °C, 140 °C, 150 °C, 160 °C, 170 °C and 180 °C, respectively.
- (2)
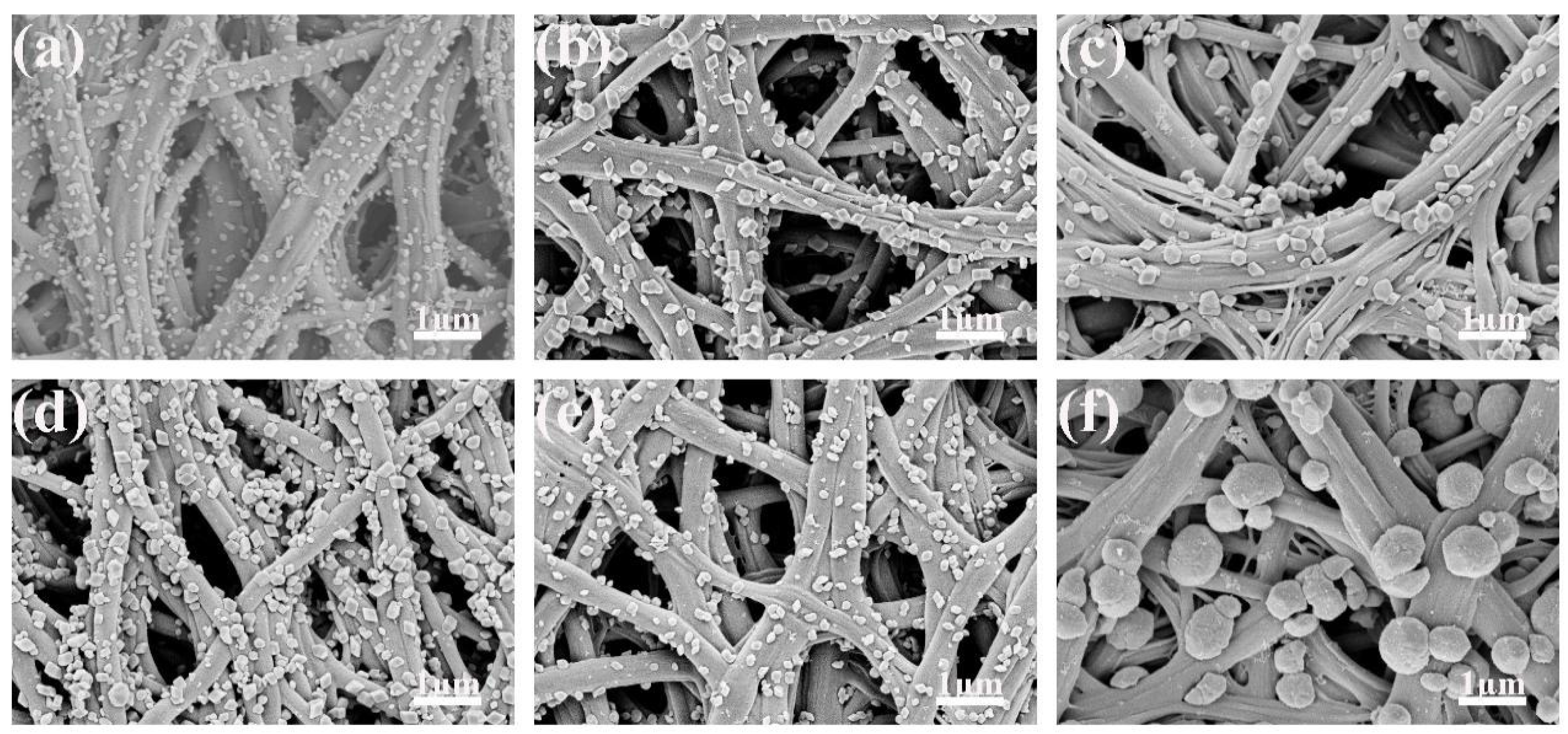
- The hydrothermal reaction temperature was 180 °C, and the reaction time was 1 h, 2 h, 3 h, 4 h, 5 h and 6 h, respectively.
2.4. Characterization
2.5. Degradation Experiments
- (1)
- Effect of reaction temperature: Fe2O3/CNFs composite is 0.8 g/L, the concentration of hydrogen peroxide is 0.194 mol/L, and the reaction time is 0~120 min. The reaction temperatures are 50 °C, 60 °C, 70 °C and 80 °C, respectively.
- (2)
- Effect of the amount of Fe2O3/CNFs composite: The initial concentration of hydrogen peroxide is 0.194 mol/L, the reaction temperature is 80 °C, and the reaction time is 0~120 min. The amounts of the Fe2O3/CNFs composite are 0.4 g/L, 0.6 g/L, 0.8 g/L and 1.0 g/L, respectively.
- (3)
- Effect of initial concentration of hydrogen peroxide: Fe2O3/CNFs composite is 0.8 g/L, the reaction temperature is 80 °C and the reaction time is 0~240 min. The initial concentrations of hydrogen peroxide are 0.097 mol/L, 0.146 mol/L, 0.194 mol/L, and 0.243 mol/L, respectively.
2.6. Degradation Performance of Fe2O3/CNFs
3. Results and Discussion
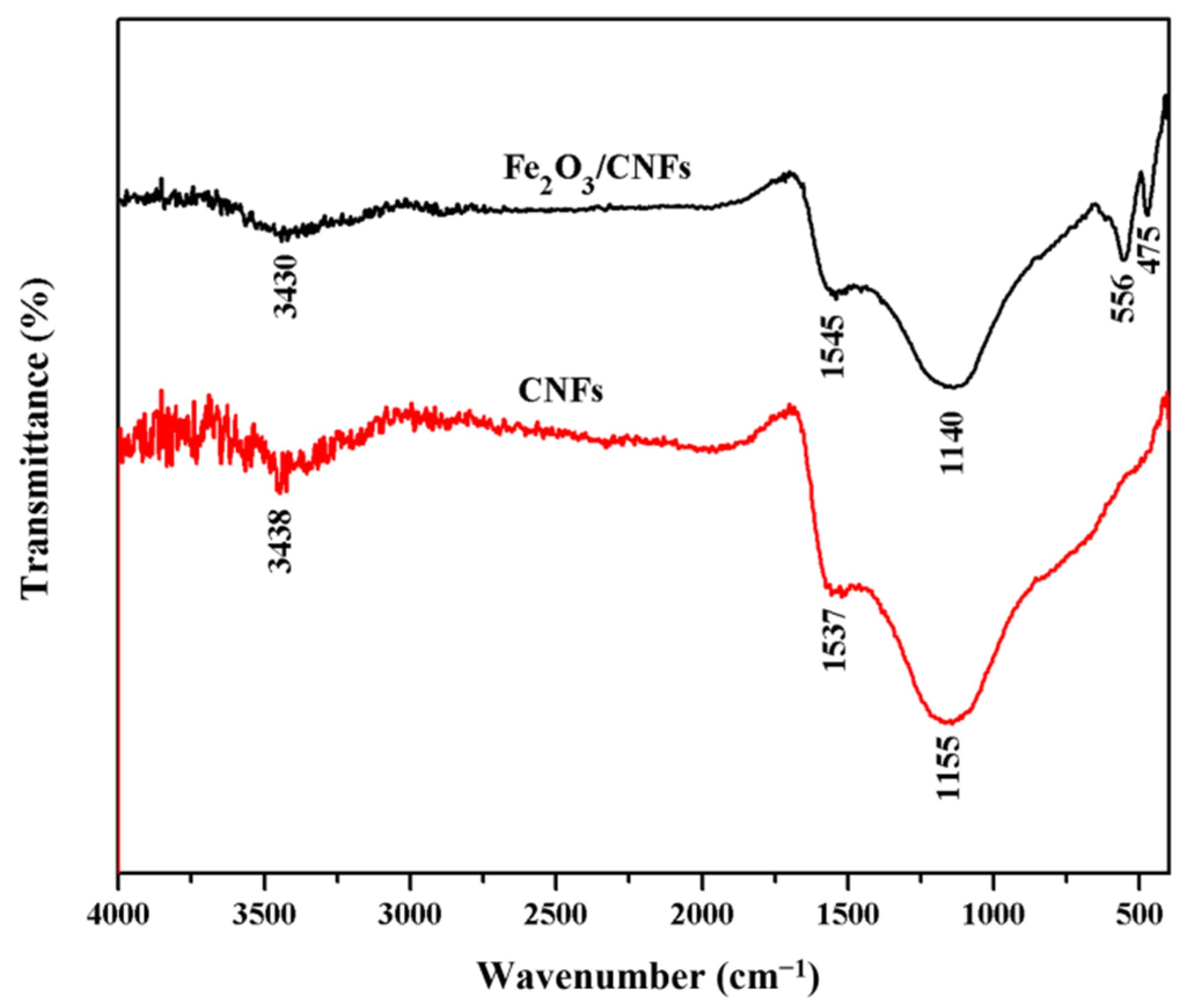
3.1. Characterization of Fe2O3/CNFs
3.2. Heterogeneous Fenton Degradation of MO by Fe2O3/CNFs

3.3. Degradation Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Leon, O.; Munoz-Bonilla, A.; Soto, D.; Perez, D.; Rangel, M.; Colina, M.; Fernandez-Garcia, M. Removal of Anionic and Cationic Dyes with Bioadsorbent Oxidized Chitosans. Carbohydr. Polym. 2018, 194, 375–383. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Punzi, M.; Anbalagan, A.; Borner, R.A.; Svensson, B.-M.; Jonstrup, M.; Mattiasson, B. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Li, H.; An, N.; Liu, G.; Li, J.; Liu, N.; Jia, M.; Zhang, W.; Yuan, X. Adsorption behaviors of methyl orange dye on nitrogen-doped mesoporous carbon materials. J. Colloid Interface Sci. 2016, 466, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Garba, Z.N.; Sun, S.; Lawan, L.; Wang, L.; Lin, M.; Yuan, Z. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020, 253, 119989. [Google Scholar] [CrossRef]
- Mercante, L.A.; Facure, M.H.M.; Locilento, D.A.; Sanfelice, R.C.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Solution blow spun PMMA nanofibers wrapped with reduced graphene oxide as an efficient dye adsorbent. New J. Chem. 2017, 41, 9087–9094. [Google Scholar] [CrossRef]
- Bu, J.; Yuan, L.; Zhang, N.; Liu, D.; Meng, Y.; Peng, X. High-efficiency adsorption of methylene blue dye from wastewater by a thiosemicarbazide functionalized graphene oxide composite. Diam. Relat. Mater. 2020, 101, 107604. [Google Scholar] [CrossRef]
- Xie, X.H.; Liu, N.; Yang, F.; Zhang, Q.Y.; Zheng, X.L.; Wang, Y.Q.; Liu, J.S. Comparative study of antiestrogenic activity of two dyes after Fenton oxidation and biological degradation. Ecotoxicol. Environ. Saf. 2018, 164, 416–424. [Google Scholar] [CrossRef]
- Esteves, B.M.; Rodrigues, C.S.D.; Boaventura, R.A.R.; Maldonado-Hodar, F.J.; Madeira, L.M. Coupling of acrylic dyeing wastewater treatment by heterogeneous Fenton oxidation in a continuous stirred tank reactor with biological degradation in a sequential batch reactor. J. Environ. Manag. 2016, 166, 193–203. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Titus, A.; Gnanamani, A.; Mandal, A.B.; Sekaran, G. Treatment of textile wastewater by homogeneous and heterogeneous Fenton oxidation processes. Desalination 2011, 281, 438–445. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gomez, L.; Gomez-Garcia, M.A. Coagulation-flocculation sequential with Fenton or Photo-Fenton processes as an alternative for the industrial textile wastewater treatment. J. Environ. Manag. 2017, 203, 615. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Torrades, F.; Varga, M.D.L.; Garcia-Montano, J. Fenton and biological-Fenton coupled processes for textile wastewater treatment and reuse. Desalination 2012, 286, 394–399. [Google Scholar] [CrossRef]
- Solomon, D.; Kiflie, Z.; Hulle, S.V. Integration of sequencing batch reactor and homo-catalytic advanced oxidation processes for the treatment of textile wastewater. Nanotechnol. Environ. Eng. 2020, 5, 7. [Google Scholar] [CrossRef]
- Asgari, G.; Faradmal, J.; Nasab, H.Z.; Ehsani, H. Catalytic ozonation of industrial textile wastewater using modified C-doped MgO eggshell membrane powder. Adv. Powder Technol. 2019, 30, 1297–1311. [Google Scholar] [CrossRef]
- Sathya, U.; Keerthi Nithya, M.; Balasubramanian, N. Evaluation of advanced oxidation processes (AOPs) integrated membrane bioreactor (MBR) for the real textile wastewater treatment. J. Environ. Manag. 2019, 246, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.M.; Tao, F.R.; Cui, Y.Z. Evaluation of nitriloacetic acid modified cellulose film on adsorption of methylene blue. Int. J. Biol. Macromol. 2018, 114, 400–407. [Google Scholar] [CrossRef]
- Lade, H.; Kadam, A.; Paul, D.; Govindwar, S. Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J. 2015, 14, 158–174. [Google Scholar]
- Guo, G.; Hao, J.X.; Tian, F.; Liu, C.; Ding, K.Q.; Xu, J.; Zhou, W.; Guan, Z.B. Decolorization and detoxification of azo dye by halo-alkaliphilic bacterial consortium: Systematic investigations of performance, pathway and metagenome. Ecotoxicol. Environ. Saf. 2020, 204, 111073. [Google Scholar] [CrossRef]
- He, X.L.; Song, C.; Li, Y.Y.; Wang, N.; Xu, L.; Han, X.; Wei, D.S. Efficient degradation of Azo dyes by a newly isolated fungus Trichoderma tomentosum under non-sterile conditions. Ecotoxicol. Environ. Saf. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Kumar, R.; Negi, S.; Sharma, P.; Prasher, I.B.; Chaudhary, S.; Dhau, J.S.; Umar, A. Wastewater cleanup using Phlebia acerina fungi: An insight into mycoremediation. J. Environ. Manag. 2018, 228, 130–139. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ. Sci. Pollut. Res. 2019, 26, 22704–22722. [Google Scholar] [CrossRef] [PubMed]
- Erkanli, M.; Yilmaz, L.; Culfaz-Emecen, P.Z.; Yetis, U. Brackish water recovery from reactive dyeing wastewater via ultrafiltration. J. Clean. Prod. 2017, 165, 1204–1214. [Google Scholar] [CrossRef]
- Othmani, A.; Kesraoui, A.; Akrout, H.; Elaissaoui, I.; Seffen, M. Coupling anodic oxidation, biosorption and alternating current as alternative for wastewater purification. Chemosphere 2020, 249, 126480. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Pascu, L.F.; Demba, A.; Nita-Lazar, M.; Badea, I.A.; Aboul-Enein, H.Y. Removal of the Acid Orange 10 by ion exchange and microbiological methods. Int. J. Environ. Sci. Technol. 2019, 16, 6357–6366. [Google Scholar] [CrossRef]
- Waly, A.I.; Khedr, M.A.; Ali, H.M.; Riad, B.Y.; Ahmed, I.M. Synthesis and Characterization of Ion Exchanger based on Waste Cotton for Dye Removal from Wastewater. Egypt. J. Chem. 2018, 62, 451–468. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Kunduzcu, G.; Arica, M.Y. Preparation and characterization of strong cation exchange terpolymer resin as effective adsorbent for removal of disperse dyes. Polym. Eng. Sci. 2020, 60, 192–201. [Google Scholar] [CrossRef]
- Kaykioglu, G.; Coban, A.; Debik, E.; Kayacan, B.B.; Koyuncu, I. Mass transport coefficients of different nanofiltration membranes for biologically pre-treated textile wastewaters. Desalination 2011, 269, 254–259. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Ali, M.B.S.; Dhahbi, M. Treatment of textile wastewater by a hybrid electrocoagulation/nanofiltration process. J. Hazard. Mater. 2009, 168, 868–874. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Uzal, N.; Yetis, U.; Dilek, F.B. Biological treatment and nanofiltration of denim textile wastewater for reuse. J. Hazard. Mater. 2008, 153, 1142–1148. [Google Scholar] [CrossRef]
- Lin, L.; Wan, H.; Mia, R.; Jiang, H.Y.; Liu, H.H.; Mahmud, S. Bioreduction and Stabilization of Antibacterial Nanosilver Using Radix Lithospermi Phytonutrients for Azo-contaminated Wastewater Treatment: Synthesis, Optimization and Characterization. J. Clust. Sci. 2022. [Google Scholar] [CrossRef]
- Zhang, G.B.; Wan, H.; Mia, R.; Huang, Q.L.; Liu, H.H.; Mahmud, S. Fabrication and stabilization of nanosilver using Houttugniae for antibacterial and catalytic application. Int. J. Environ. Anal. Chem. 2022, 1–21. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Feng, M.L.; Yu, S.C.; Wu, P.C.; Wang, Z.W.; Liu, S.H.; Fu, J.W. Rapid, high-efficient and selective removal of cationic dyes from wastewater using hollow polydopamine microcapsules: Isotherm, kinetics, thermodynamics and mechanism. Appl. Surf. Sci. 2021, 542, 148633. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jin, W.; Zhao, Y.P.; Zhang, G.S.; Zhang, W. Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl. Catal. B Environ. 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.J.; Yang, W.; Fida, H.; You, L.M.; Zhou, K. Scalable synthesis of Ca-doped α-Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl. Catal. B Environ. 2020, 262, 118520. [Google Scholar] [CrossRef]
- Ding, M.M.; Chen, W.; Xu, H.; Shen, Z.; Lin, T.; Hu, K.; Lu, C.H.; Xie, Z.L. Novel α-Fe2O3/MXene nanocomposite as heterogeneous activator of peroxymonosulfate for the degradation of salicylic acid. J. Hazard. Mater. 2020, 382, 121064. [Google Scholar] [CrossRef]
- Niu, L.J.; Zhang, G.M.; Xian, G.; Ren, Z.J.; Wei, T.; Li, Q.G.; Zhang, Y.; Zou, Z.G. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Wang, W.L.; Zhao, W.L.; Zhang, H.C.; Dou, X.C.; Shi, H.F. 2D/2D step-scheme α-Fe2O3/Bi2WO6 photocatalyst with efficient charge transfer for enhanced photo-Fenton catalytic activity. Chin. J. Catal. 2021, 42, 97–106. [Google Scholar] [CrossRef]
- Park, H.; Lee, Y.C.; Choi, B.G.; Choi, Y.S.; Yang, J.W.; Hong, W.H. Green one-pot assembly of iron-based nanomaterials for the rational design of structure. Chem. Commun. 2009, 27, 4058–4060. [Google Scholar] [CrossRef]
- Liang, H.-W.; Cao, X.; Zhang, W.-J.; Lin, H.-T.; Zhou, F.; Chen, L.-F.; Yu, S.-H. Robust and Highly Efficient Free-Standing Carbonaceous Nanofiber Membranes for Water Purification. Adv. Funct. Mater. 2011, 21, 3851–3858. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yang, F.; Gan, J.; Zhao, W.Y.; Wu, Y. A flower-like waterborne coating with self-cleaning, self-repairing properties for superhydrophobic applications. J. Mater. Res. Technol. 2021, 14, 1820–1829. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (alpha-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, Y.; Song, L.; Wang, Y.X.; He, C.; Wang, Z.; Cui, L.F. High and stable catalytic activity of Ag/Fe2O3 catalysts derived from MOFs for CO oxidation. Mol. Catal. 2018, 447, 80–89. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, X.; Xu, D.M.; Kong, L.X.; Lv, L.F.; Yang, F.; Wang, F.L.; Liu, W.; Liu, J.R. Design and synthesis of TiO2/Co/carbon nanofibers with tunable and efficient electromagnetic absorption. Chem. Eng. J. 2020, 380, 122591. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized gamma-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, C.A.; Murad, E. Iron oxide catalysts: Fenton and Fenton-like reactions a review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Jia, L.D.; Zhang, Q.R. Heterogeneous Fenton catalytic oxidation for water treatment. Prog. Chem. 2020, 32, 978–988. [Google Scholar]
- Hieu, V.Q.; Phung, T.K.; Nguyen, T.-Q.; Khan, A.; Doan, V.D.; Tran, V.A.; Le, V.T. Photocatalytic degradation of methyl orange dye by Ti3C2–TiO2 heterojunction under solar light. Chemosphere 2021, 276, 130154. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and Characterization of Co-ZnO and Evaluation of Its Photocatalytic Activity for Photodegradation of Methyl Orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Shahzad, K.; Najam, T.; Bashir, M.S.; Nazir, M.A.; Rehman, A.U.; Bashir, M.A.; Shah, S.S.A. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorg. Chem. Commun. 2021, 123, 108357. [Google Scholar] [CrossRef]
- Zeng, Q.Q.; Liu, Y.; Shen, L.G.; Lin, H.J.; Yu, W.M.; Xu, Y.C.; Li, R.J.; Huang, L.L. Facile preparation of recyclable magnetic Ni@filter paper composite materials for efficient photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2021, 582, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Znad, H.; Abbas, K.; Hena, S.; Awual, M.R. Synthesis a novel multilamellar mesoporous TiO2/ZSM-5 for photo-catalytic degradation of methyl orange dye in aqueous media. J. Environ. Chem. Eng. 2018, 6, 218–227. [Google Scholar] [CrossRef]
- Saravanan, R.; Sacari, E.; Gracia, F.; Khan, M.M.; Mosquera, E.; Gupta, V.K. Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 2016, 221, 1029–1033. [Google Scholar] [CrossRef]








| Catalysts | Initial Concentration | Decolorization Rate | Reaction Time | References |
|---|---|---|---|---|
| Fe2O3/CNFs | 100 mg/L | More than 99% | 60 min | This work |
| Ti3C2-TiO2 | 40 mg/L | 99% | 40 min | [48] |
| 10% Co-ZnO | 100 mg/L | 100% | 120 min | [49] |
| Ag-PMOS | 20 mg/L | 81% & 48% | 60 min | [50] |
| ZnO-PMOS | 47% & 57% | |||
| Ni@FP | 15 mg/L | 93.40% | 5 min | [51] |
| TiO2/ZSM-5 | 20 mg/L | 99.55% | 180 min | [52] |
| PANI(1.5 mol)/ZnO | - | 98.3% | 180 min | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, F.; Shi, X.; Wei, J.; Yan, W.; Dong, Y.; Hu, H.; Wei, K. Preparation and Photodegradation Properties of Carbon-Nanofiber-Based Catalysts. Polymers 2022, 14, 3584. https://doi.org/10.3390/polym14173584
Zhang M, Wang F, Shi X, Wei J, Yan W, Dong Y, Hu H, Wei K. Preparation and Photodegradation Properties of Carbon-Nanofiber-Based Catalysts. Polymers. 2022; 14(17):3584. https://doi.org/10.3390/polym14173584
Chicago/Turabian StyleZhang, Mingpan, Fuli Wang, Xinran Shi, Jing Wei, Weixia Yan, Yihang Dong, Huiqiang Hu, and Kai Wei. 2022. "Preparation and Photodegradation Properties of Carbon-Nanofiber-Based Catalysts" Polymers 14, no. 17: 3584. https://doi.org/10.3390/polym14173584
APA StyleZhang, M., Wang, F., Shi, X., Wei, J., Yan, W., Dong, Y., Hu, H., & Wei, K. (2022). Preparation and Photodegradation Properties of Carbon-Nanofiber-Based Catalysts. Polymers, 14(17), 3584. https://doi.org/10.3390/polym14173584






