Vibrational Emission Study of the CN and C2 in Nylon and ZnO/Nylon Polymer Using Laser-Induced Breakdown Spectroscopy (LIBS)
Abstract
:1. Introduction
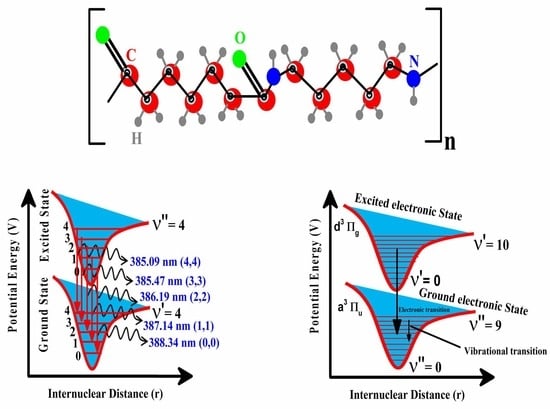
2. Materials and Methods
Experimental Details
3. Results and Discussion
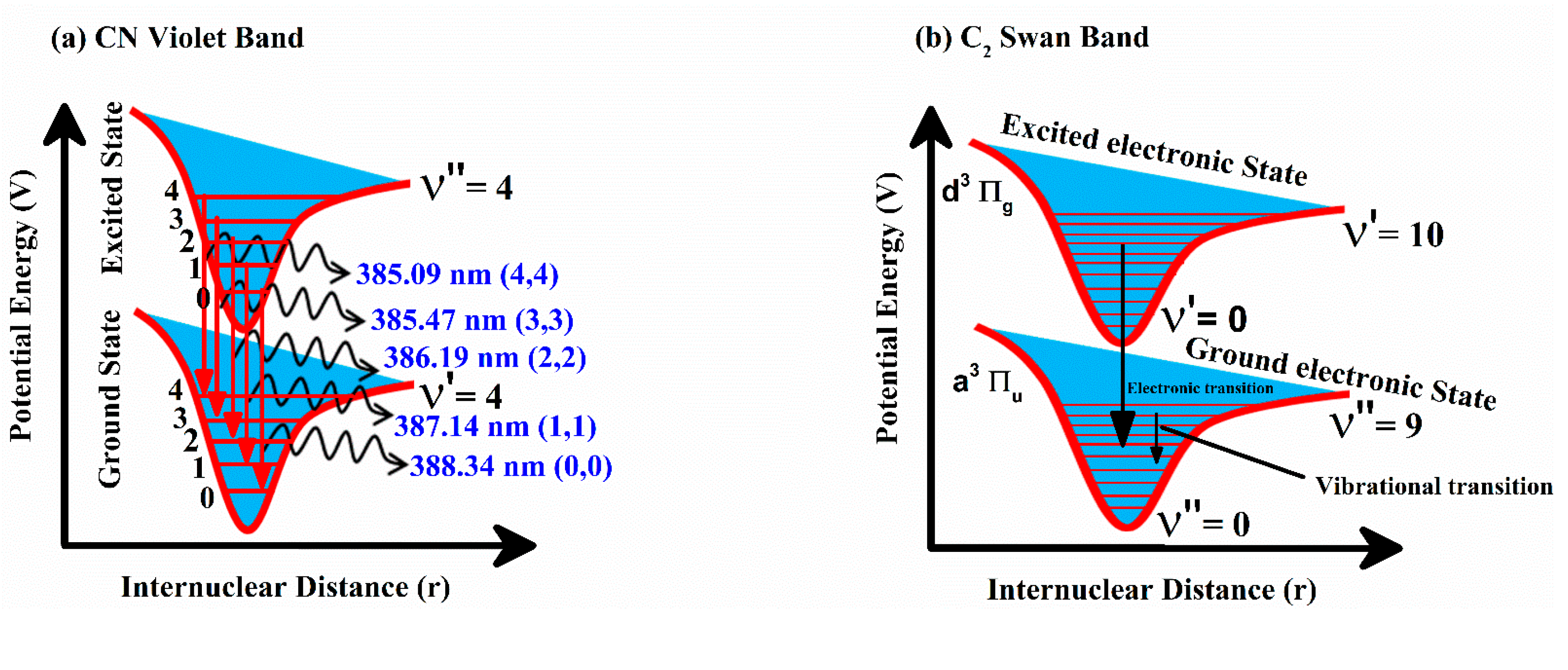
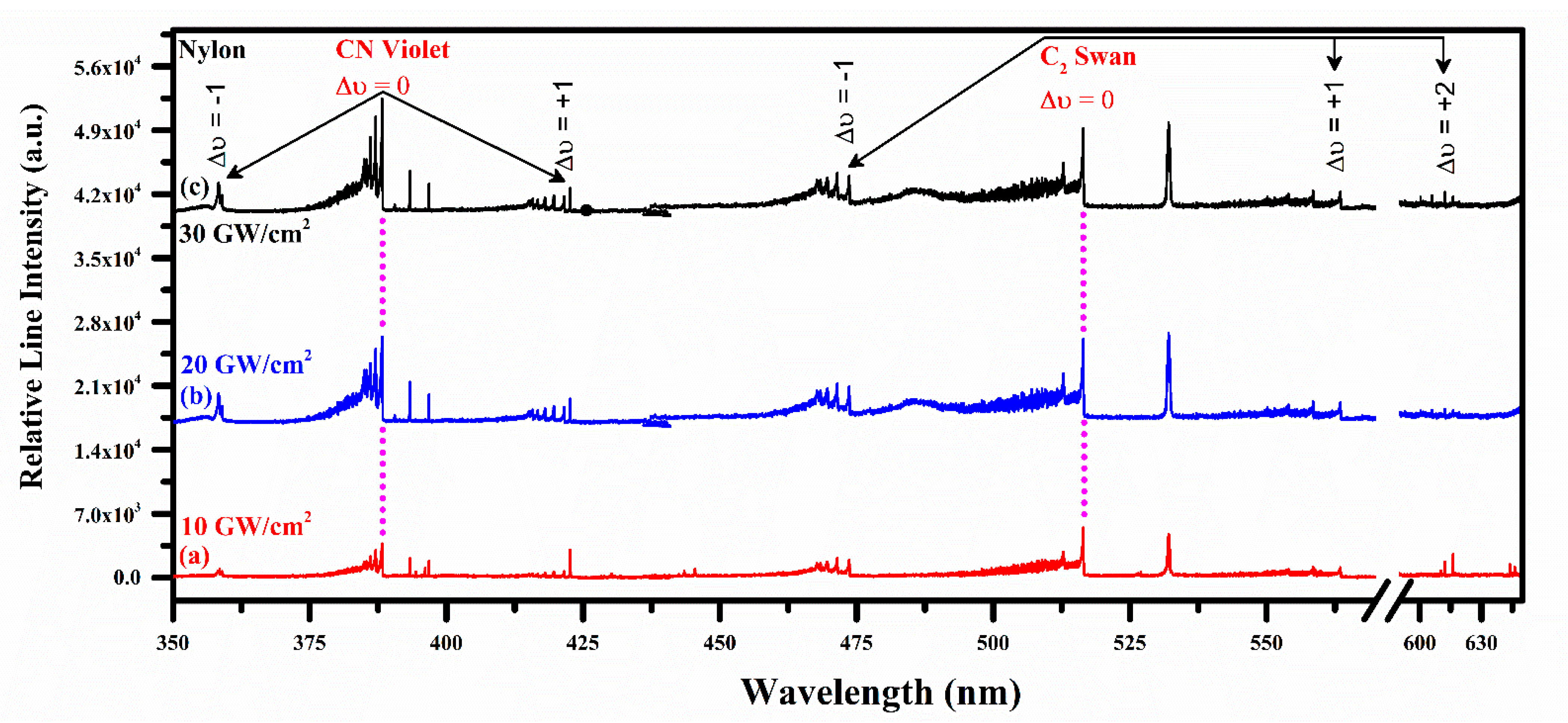
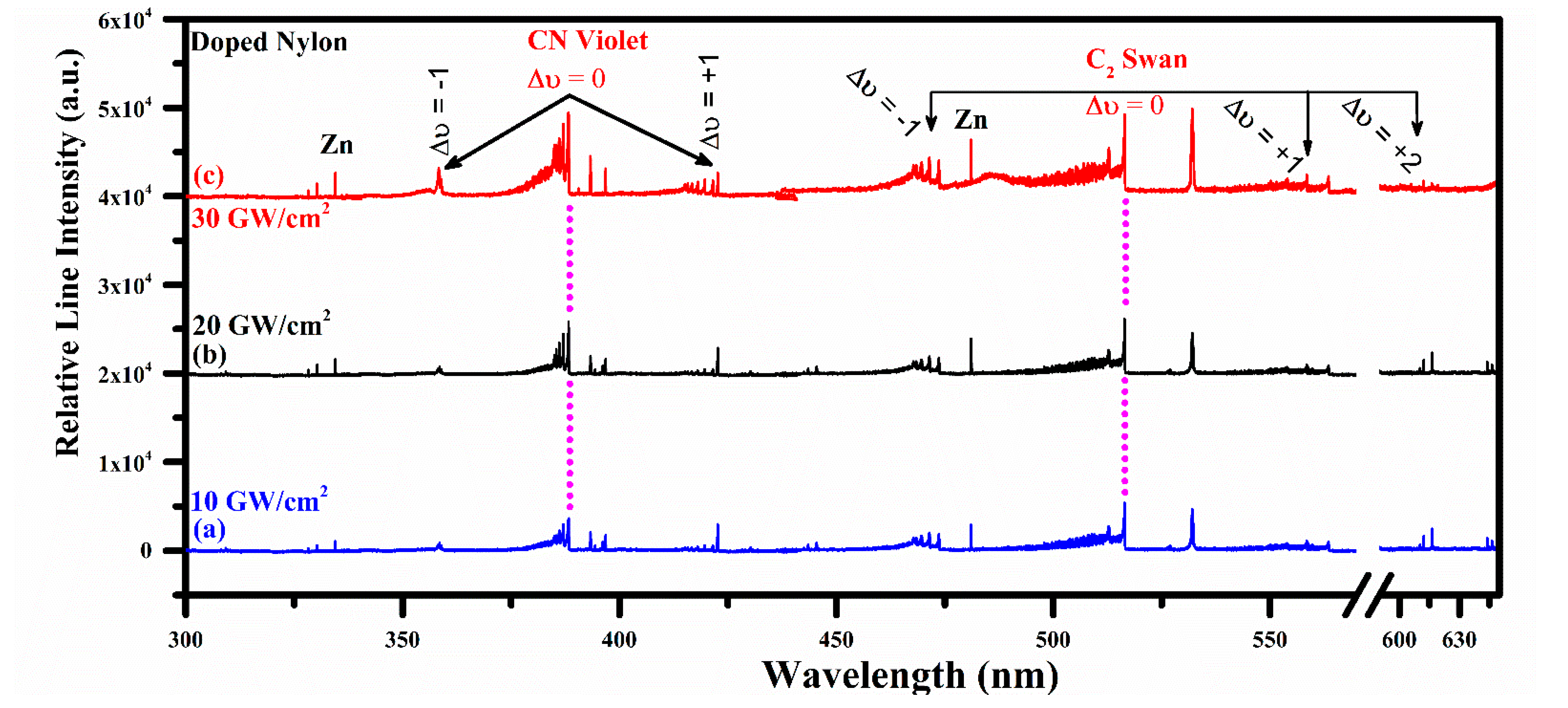
Emission Studies of the CN Violet and C2 Swan System
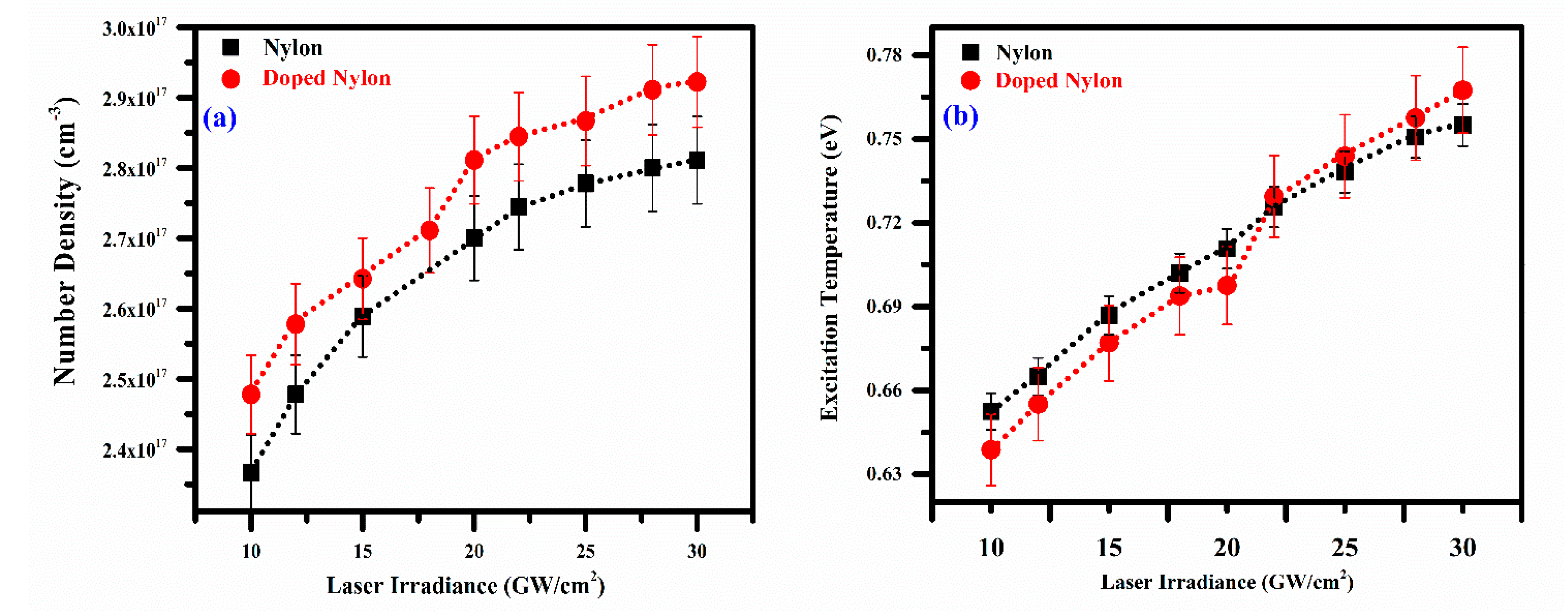
4. Plasma Characterization
4.1. Plasma Excitation Temperature
4.2. Electron Number Density
4.3. Local Thermodynamical Equilibrium (LTE) Condition
4.4. Effect of Laser Irradiance on the Plasma Parameters
4.5. Effect of Laser Irradiance on CN and C2 Vibrational Emission
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, S.; Wang, Y.; Wu, X.; Zhang, X.; Zhu, Y.; Yu, N.; Zhang, Y.; Wu, Y. Nylon-Based Composite Gel Membrane Fabricated via Sequential Layer-by-Layer Electrospinning for Rechargeable Lithium Batteries with High Performance. Polymers 2020, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.; Lee, S. Study of shape memory and tensile property of 3D printed sinusoidal sample/nylon composite focused on various thicknesses and shape memory cycles. Polymers 2020, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Hammond, W.B.; Goddard, W.A. Crystal structures and properties of nylon polymers from theory. J. Am. Chem. Soc. 1996, 118, 12291–12301. [Google Scholar] [CrossRef]
- Rai, S.; Rai, A.K. Characterization of organic materials by LIBS for exploration of correlation between molecular and elemental LIBS signals. AIP Adv. 2011, 1, 042103. [Google Scholar] [CrossRef]
- Moros, J.; Laserna, J. Laser-induced breakdown spectroscopy (LIBS) of organic compounds: A review. Appl. Spectrosc. 2019, 73, 963–1011. [Google Scholar] [CrossRef]
- De Lucia, F.C.; Harmon, R.S.; McNesby, K.L.; Winkel, R.J.; Miziolek, A.W. Laser-induced breakdown spectroscopy analysis of energetic materials. Appl. Opt. 2003, 42, 6148–6152. [Google Scholar] [CrossRef]
- Baudelet, M.; Boueri, M.; Yu, J.; Mao, X.; Mao, S.S.; Russo, R. Laser ablation of organic materials for discrimination of bacteria in an inorganic background. In Ultrafast Phenomena in Semiconductors and Nanostructure Materials XIII; SPIE: Bellingham, WA, USA, 2009; Volume 7214, pp. 97–106. [Google Scholar]
- Moros, J.; Lorenzo, J.A.; Lucena, P.; Miguel Tobaria, L.; Laserna, J.J. Simultaneous Raman Spectroscopy− Laser-Induced Breakdown Spectroscopy for instant standoff analysis of explosives using a mobile integrated sensor platform. Anal. Chem. 2010, 82, 1389–1400. [Google Scholar] [CrossRef]
- Carbone, E.; D’Isa, F.; Hecimovic, A.; Fantz, U. Analysis of the C2 Swan bands as a thermometric probe in CO2 microwave plasmas. Plasma Sources Sci. Technol. 2020, 29, 055003. [Google Scholar] [CrossRef]
- Gottfried, J.L.; De Lucia Jr, F.C.; Munson, C.A.; Miziolek, A.W. Double-pulse standoff laser-induced breakdown spectroscopy for versatile hazardous materials detection. Spectrochim. Acta Part B Acta Spectrosc. 2007, 62, 1405–1411. [Google Scholar] [CrossRef]
- Jihua, G.; Ali, A.; Dagdigian, P.J. State-to-state collisional interelectronic and intraelectronic energy transfer involving CN A 2Π v= 3 and X 2Σ+ v= 7 rotational levels. J. Chem. Phys. 1986, 85, 7098–7105. [Google Scholar] [CrossRef]
- Sauder, D.G.; Patel-Misra, D.; Dagdigian, P.J. The vibronic state distribution of the NCO 2 Π) product from the CN+O2 reaction. J. Chem. Phys. 1991, 95, 1696–1707. [Google Scholar] [CrossRef]
- Fernández-Bravo, Á.; Delgado, T.; Lucena, P.; Laserna, J.J. Vibrational emission analysis of the CN molecules in laser-induced breakdown spectroscopy of organic compounds. Spectrochim. Acta Part B Acta Spectrosc. 2013, 89, 77–83. [Google Scholar] [CrossRef]
- Dong, M.; Chan, G.C.Y.; Mao, X.; Gonzalez, J.J.; Lu, J.; Russo, R.E. Elucidation of C2 and CN formation mechanisms in laser-induced plasmas through correlation analysis of carbon isotopic ratio. Spectrochim. Acta Part B Acta Spectrosc. 2014, 100, 62–69. [Google Scholar] [CrossRef]
- Dong, M.; Mao, X.; Gonzalez, J.J.; Lu, J.; Russo, R.E. Carbon isotope separation and molecular formation in laser-induced plasmas by laser ablation molecular isotopic spectrometry. Anal. Chem. 2013, 85, 2899–2906. [Google Scholar] [CrossRef]
- Boueri, M.; Motto-Ros, V.; Lei, W.Q.; Ma, Q.L.; Zheng, L.J.; Zeng, H.P.; Yu, J. Identification of polymer materials using laser-induced breakdown spectroscopy combined with artificial neural networks. Appl. Spectrosc. 2011, 65, 307–314. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Farsani, M.H.; Darbani, S.M.R.; Asadorian, N.; Soltanolkotabi, M.; Majd, A.E. Identification of atomic lines and molecular bands of benzene and carbon disulfide liquids by using LIBS. Appl. Opt. 2015, 54, 1713–1720. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Hemati Farsani, M.; Darbani, S.M.R.; Mousaviazar, A.; Soltanolkotabi, M.; Eslami Majd, A. CN and C2 vibrational spectra analysis in molecular LIBS of organic materials. Appl. Phys. B 2016, 122, 106. [Google Scholar] [CrossRef]
- Farooq, W.A.; Al-Johani, A.S.; Alsalhi, M.S.; Tawfik, W.; Qindeel, R. Analysis of polystyrene and polycarbonate used in manufacturing of water and food containers using laser induced breakdown spectroscopy. J. Mol. Struct. 2020, 1201, 127152. [Google Scholar] [CrossRef]
- Lasheras, R.J.; Bello-Gálvez, C.; Anzano, J. Identification of polymers by libs using methods of correlation and normalized coordinates. Polym. Test. 2010, 29, 1057–1064. [Google Scholar] [CrossRef]
- Costa, V.C.; Aquino, F.W.B.; Paranhos, C.M.; Pereira-Filho, E.R. Identification and classification of polymer e-waste using laser-induced breakdown spectroscopy (LIBS) and chemometric tools. Polym. Test. 2017, 59, 390–395. [Google Scholar] [CrossRef]
- Grégoire, S.; Motto-Ros, V.; Ma, Q.L.; Lei, W.Q.; Wang, X.C.; Pelascini, F.; Surma, F.; Detalle, V.; Yu, J. Correlation between native bonds in a polymeric material and molecular emissions from the laser-induced plasma observed with space and time resolved imaging. Spectrochim. Acta Part B Acta Spectrosc. 2012, 74, 31–37. [Google Scholar] [CrossRef]
- Aquino, F.W.; Pereira-Filho, E.R. Analysis of the polymeric fractions of scrap from mobile phones using laser-induced breakdown spectroscopy: Chemometric applications for better data interpretation. Talanta 2015, 134, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, X.; Sun, R.; Cao, Z.; Yuan, M.; Sun, L.; Yu, X.; Wu, J. Investigation of the CN and C2 emission characteristics and microstructural evolution of coal to char using laser-induced breakdown spectroscopy and Raman spectroscopy. Energy 2022, 240, 122827. [Google Scholar] [CrossRef]
- Aga, A.; Mu, M. Doping of Polymers with ZnO Nanostructures for Optoelectronic and Sensor Applications. In Nanowires Science and Technology; Lupu, N., Ed.; IntechOpen: London, UK, 2010. [Google Scholar]
- Yanilmaz, M.; Zhu, J.; Lu, Y.; Ge, Y.; Zhang, X. High-strength, thermally stable nylon 6, 6 composite nanofiber separators for lithium-ion batteries. J. Mater. Sci. 2017, 52, 5232–5241. [Google Scholar] [CrossRef]
- Xu, Z.; Mahalingam, S.; Rohn, J.L.; Ren, G.; Edirisinghe, M. Physio-chemical and antibacterial characteristics of pressure spun nylon nanofibres embedded with functional silver nanoparticles. Mater. Sci. Eng. C 2015, 56, 195–204. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Moon, J.; Kim, J.J. Microstructure and mechanical properties of hardened cement paste including Nylon 66 nanofibers. Constr. Build. Mater. 2020, 232, 117134. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Sambudi, N.S.; Mohd Yusoff, A.R. Improving performance of electrospun nylon 6, 6 nanofiber membrane for produced water filtration via solvent vapor treatment. Polymers 2019, 11, 2117. [Google Scholar] [CrossRef]
- Hu, S.; Yuan, D.; Liu, Y.; Zhao, L.; Guo, H.; Niu, Q.; Zong, W.; Liu, R. The toxic effects of alizarin red S on catalase at the molecular level. RSC Adv. 2019, 9, 33368–33377. [Google Scholar] [CrossRef]
- Alrebdi, T.A.; Fayyaz, A.; Asghar, H.; Zaman, A.; Asghar, M.; Alkallas, F.H.; Hussain, A.; Iqbal, J.; Khan, W. Quantification of Aluminum Gallium Arsenide (AlGaAs) Wafer Plasma Using Calibration-Free Laser-Induced Breakdown Spectroscopy (CF-LIBS). Molecules 2022, 27, 3754. [Google Scholar] [CrossRef]
- Iqbal, J.; Asghar, H.; Shah, S.K.H.; Naeem, M.; Abbasi, S.A.; Ali, R. Elemental analysis of sage (herb) using calibration-free laser-induced breakdown spectroscopy. Appl. Opt. 2020, 59, 4927–4932. [Google Scholar] [CrossRef]
- Fayyaz, A.; Liaqat, U.; Adeel Umar, Z.; Ahmed, R.; Aslam Baig, M. Elemental Analysis of Cement by Calibration-Free Laser Induced Breakdown Spectroscopy (CF-LIBS) and Comparison with Laser Ablation–Time-of-Flight–Mass Spectrometry (LA-TOF-MS), Energy Dispersive X-Ray Spectrometry (EDX), X-Ray Fluorescence Spectroscopy (XRF), and Proton Induced X-Ray Emission Spectrometry (PIXE). Anal. Lett. 2019, 52, 1951–1965. [Google Scholar]
- Macrae, R.M. Puzzles in bonding and spectroscopy: The case of dicarbon. Sci. Prog. 2016, 99, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; He, M.; Jiang, Y.; Zhang, B.; Hang, W.; Huang, B. Laser-induced plasma temperature. Spectrochim. Acta Part B Acta Spectrosc. 2014, 97, 13–33. [Google Scholar] [CrossRef]
- Unnikrishnan, V.K.; Alti, K.; Kartha, V.B.; Santhosh, C.; Gupta, G.P.; Suri, B.M. Measurements of plasma temperature and electron density in laser-induced copper plasma by time-resolved spectroscopy of neutral atom and ion emissions. Pramana 2010, 74, 983–993. [Google Scholar] [CrossRef]
- NIST. NIST Standard Reference Database 78; NIST: Gaithersburg, MD, USA, 1995. [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- McWhirter, R.W.P. Plasma Diagnostic Techniques; Academic Press: New York, NY, USA, 1965; Chapter 5. [Google Scholar]
- Gigosos, M.A.; Gonzalez, M.A.; Cardenoso, V. Computer simulated Balmer-alpha,-beta and-gamma Stark line profiles for non-equilibrium plasmas diagnostics. Spectrochim. Acta Part B: Acta Spectrosc. 2003, 58, 1489–1504. [Google Scholar] [CrossRef]
- Praher, B.; Palleschi, V.; Viskup, R.; Heitz, J.; Pedarnig, J.D. Calibration free laser-induced breakdown spectroscopy of oxide materials. Spectrochim. Acta Part B Acta Spectrosc. 2010, 65, 671–679. [Google Scholar] [CrossRef]
- Fujimoto, T.; McWhirter, R.W.P. Validity criteria for local thermodynamic equilibrium in plasma spectroscopy. Phys. Rev. A 1990, 42, 6588. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part I: Review of basic diagnostics and plasma–particle interactions: Still-challenging issues within the analytical plasma community. Appl. Spectrosc. 2010, 64, 335A–366A. [Google Scholar] [CrossRef] [PubMed]
- Hey, J.D. Criteria for local thermal equilibrium in non-hydrogenic plasmas. J. Quant. Spectrosc. Radiat. Transf. 1976, 16, 69–75. [Google Scholar] [CrossRef]
- Cristoforetti, G.; De Giacomo, A.; Dell’Aglio, M.; Legnaioli, S.; Tognoni, E.; Palleschi, V.; Omenetto, N. Local thermodynamic equilibrium in laser-induced breakdown spectroscopy: Beyond the McWhirter criterion. Spectrochim. Acta Part B Acta Spectrosc. 2010, 65, 86–95. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Suyanto, H.; Pardede, M.; Karnadi, I.; Kurniawan, K.H.; Chiari, W.; Abdulmadjid, S.N. Cellulose acetate-polyurethane film adsorbent with analyte enrichment for in-situ detection and analysis of aqueous Pb using Laser-Induced Breakdown Spectroscopy (LIBS). Environ. Nanotechnol. Monit. Manag. 2021, 16, 100516. [Google Scholar] [CrossRef]
- Nisah, K.; Ramli, M.; Iqhrammullah, M.; Mitaphonna, R.; Hartadi, B.S.; Abdulmadjid, S.N.; Sani, N.D.M.; Idroes, R.; Safitri, E. Controlling the diffusion of micro-volume Pb solution on hydrophobic polyurethane membrane for quantitative analysis using laser-induced breakdown spectroscopy (LIBS). Arab. J. Chem. 2022, 15, 103812. [Google Scholar] [CrossRef]




| Wavelength λ (nm) | Transition Upper to Lower | Upper-Level Energy Ek (eV) | Transition Probability and Statistical Weight Akgk (108 s−1) |
|---|---|---|---|
| Ca I | |||
| 487.81 | 4s4f 1F3 → 3d4s 1D2 | 5.25 | 1.32 |
| 527.03 | 3d4p 3P2 → 3d4s 3D3 | 4.88 | 2.50 |
| 559.85 | 3d4p 3D1 → 3d4s 3D1 | 4.74 | 1.29 |
| 585.75 | 4p2 1D2 → 4s4p 1P1 | 5.05 | 3.30 |
| 612.22 | 4s5s 3S1 → 4s4p 3P1 | 3.91 | 0.86 |
| 649.97 | 4p 3F2 → 4s 3D2 | 4.43 | 0.41 |
| 671.76 | 4s5p 1P1 → 3d4s 1D2 | 4.55 | 0.36 |
| Zn I | |||
| 328.23 | 4d 3D1 → 4p 3P0 | 7.78 | 2.70 |
| 330.29 | 4d 3D1 → 4p 3P1 | 7.78 | 6.00 |
| 334.50 | 4d 3D3 → 4p 3P2 | 7.78 | 11.9 |
| 481.05 | 5s 3S1 → 4p 3P2 | 6.65 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrebdi, T.A.; Fayyaz, A.; Ben Gouider Trabelsi, A.; Asghar, H.; Alkallas, F.H.; Alshehri, A.M. Vibrational Emission Study of the CN and C2 in Nylon and ZnO/Nylon Polymer Using Laser-Induced Breakdown Spectroscopy (LIBS). Polymers 2022, 14, 3686. https://doi.org/10.3390/polym14173686
Alrebdi TA, Fayyaz A, Ben Gouider Trabelsi A, Asghar H, Alkallas FH, Alshehri AM. Vibrational Emission Study of the CN and C2 in Nylon and ZnO/Nylon Polymer Using Laser-Induced Breakdown Spectroscopy (LIBS). Polymers. 2022; 14(17):3686. https://doi.org/10.3390/polym14173686
Chicago/Turabian StyleAlrebdi, Tahani A., Amir Fayyaz, Amira Ben Gouider Trabelsi, Haroon Asghar, Fatemah H. Alkallas, and Ali M. Alshehri. 2022. "Vibrational Emission Study of the CN and C2 in Nylon and ZnO/Nylon Polymer Using Laser-Induced Breakdown Spectroscopy (LIBS)" Polymers 14, no. 17: 3686. https://doi.org/10.3390/polym14173686
APA StyleAlrebdi, T. A., Fayyaz, A., Ben Gouider Trabelsi, A., Asghar, H., Alkallas, F. H., & Alshehri, A. M. (2022). Vibrational Emission Study of the CN and C2 in Nylon and ZnO/Nylon Polymer Using Laser-Induced Breakdown Spectroscopy (LIBS). Polymers, 14(17), 3686. https://doi.org/10.3390/polym14173686