Chitosan–Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy
Abstract
:1. Introduction
2. Methodology
3. Polymeric Nanoparticles
4. Hyaluronic Acid Nanoparticles as an Active Targeting Drug Delivery System
5. Chitosan–Hyaluronic Acid Nanoparticles for Cancer
5.1. Breast Cancer
5.2. Lung Cancer
5.3. Liver Cancer
5.4. Oral Cavity Squamous Cancer
5.5. Colon Cancer
5.6. Bladder Cancer
5.7. Others
6. Author Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Indonesia-Global Cancer Observatory. Globocan 2020, 858, 1–2. [Google Scholar]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Martini, S.; Di Muzio, J.; Cavallin, C.; Arcadipane, F.; Rampino, M.; Ostellino, O.; Pecorari, G.; Garzino Demo, P.; Fasolis, M.; et al. Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck cancer. Med. Oncol. 2017, 34, 81. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Naik, J. Nano Based Drug Delivery; IAPC: Zagreb, Croatia, 2015. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Atyabi, F.; Dinarvand, R.; Ostad, S.N. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomed. 2012, 7, 1851–1863. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. SPJ 2018, 26, 64. [Google Scholar] [CrossRef]
- Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The use of megamolecular polysaccharide Sacran in food and biomedical applications. Molecules 2021, 26, 3362. [Google Scholar] [CrossRef]
- Wathoni, N.; Meylina, L.; Rusdin, A.; Abdelwahab Mohammed, A.F.; Tirtamie, D.; Herdiana, Y.; Motoyama, K.; Panatarani, C.; Joni, I.M.; Lesmana, R.; et al. The potential cytotoxic activity enhancement of α-mangostin in chitosan-kappa carrageenan-loaded nanoparticle against mcf-7 cell line. Polymers 2021, 13, 1681. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Kashanian, S.; Lucia, L.A.; Khazaei, M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J. Control. Release 2017, 260, 213–225. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Kashanian, S.; Taherpour, A. (Arman) Hydrophobic amino acids grafted onto chitosan: A novel amphiphilic chitosan nanocarrier for hydrophobic drugs. Drug Dev. Ind. Pharm. 2017, 43, 1–11. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Azandaryani, A.H.; Kashanian, S.; Shahlaei, M.; Derakhshandeh, K.; Motiei, M.; Moradi, S. A Comprehensive Physicochemical, In Vitro and Molecular Characterization of Letrozole Incorporated Chitosan-Lipid Nanocomplex. Pharm. Res. 2019, 36, 62. [Google Scholar] [CrossRef]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Zhaleh, H.; Cruz, L.J. Enhanced Intracellular Delivery of Curcumin by Chitosan-Lipoic Acid as Reduction-Responsive Nanoparticles. Curr. Pharm. Biotechnol. 2020, 22, 622–635. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zheng, Y.; Feng, L.; Liu, Z.; Guo, R.; Zhang, Y. Novel hyaluronic acid coated hydrophobically modified chitosan polyelectrolyte complex for the delivery of doxorubicin. Int. J. Biol. Macromol. 2019, 126, 254–261. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, K.; Banstola, A.; Gautam, M.; Soe, Z.C.; Pham, L.M.; Jeong, J.H.; Choi, H.G.; Ku, S.K.; Yong, C.S.; Tran, T.H.; et al. Redox/photo dual-responsive, self-targeted, and photosensitizer-laden bismuth sulfide nanourchins for combination therapy in cancer. Nanoscale 2021, 13, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Qin, A.; Lin, X.; Yang, L.; Wang, Q.; Wang, Z.; Shan, Z.; Li, S.; Wang, J.; Fan, S.; et al. Biodegradable and biocompatible high elastic chitosan scaffold is cell-friendly both in vitro and in vivo. Oncotarget 2017, 8, 35583–35591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snetkov, P.; Morozkina, S.; Uspenskaya, M.; Olekhnovich, R. Hyaluronan-based nanofibers: Fabrication, characterization and application. Polymers 2019, 11, 2036. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Wang, F.; Pan, S.; Yuan, S.; Liu, Y.; Xu, Y. Redox/ph-responsive biodegradable thiol-hyaluronic acid/chitosan charge-reversal nanocarriers for triggered drug release. Polymers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Steinhauser, I.; Spänkuch, B.; Strebhardt, K.; Langer, K. Trastuzumab-modified nanoparticles: Optimisation of preparation and uptake in cancer cells. Biomaterials 2006, 27, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sze, R.; Zhang, M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J. Biomed. Mater. Res.-Part A 2006, 78, 550–557. [Google Scholar] [CrossRef]
- Bernela, M.; Ahuja, M.; Thakur, R. Enhancement of anti-inflammatory activity of glycyrrhizic acid by encapsulation in chitosan-katira gum nanoparticles. Eur. J. Pharm. Biopharm. 2016, 105, 141–147. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Tsung, J.; Burgess, D.J. Biodegradable polymers in drug delivery systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Boston, MA, USA, 2012. [Google Scholar]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Han, J.H.; Choi, S.H.; Jung, H.Y.; Park, J.D.; An, H.J.; Kim, S.E.; Kim, D.H.; Doh, J.; Han, D.K.; et al. Cationic Nanoparticle-Mediated Activation of Natural Killer Cells for Effective Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 56731–56740. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Kashanian, S. Preparation of amphiphilic chitosan nanoparticles for controlled release of hydrophobic drugs. J. Nanosci. Nanotechnol. 2017, 17, 5226–5232. [Google Scholar] [CrossRef]
- Li, G.-Y.; Jiang, Y.-R.; Huang, K.-L.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Kim, J.H.; Nam, Y.S.; Lee, S.; Nam, H.Y.; Kim, K.; Park, J.H.; Kim, I.S.; Choi, K.; Kim, S.Y.; et al. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J. Control. Release 2007, 122, 305–314. [Google Scholar] [CrossRef]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic acid-coated chitosan nanoparticles: Molecular weight-dependent effects on morphology and hyaluronic acid presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Varaprasad, K.; Akbari-Fakhrabadi, A.; Hameed, A.S.H.; Sadiku, R. Biomolecule chitosan, curcumin and ZnO-based antibacterial nanomaterial, via a one-pot process. Carbohydr. Polym. 2020, 249, 116825. [Google Scholar] [CrossRef] [PubMed]
- Salahpour Anarjan, F. Active targeting drug delivery nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Kumar, R.; Saladi, S.; Rafeeqi, T.A.; Khan, W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf. B Biointerfaces 2016, 143, 532–546. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139. [Google Scholar] [CrossRef]
- Batool, A.; Arshad, R.; Razzaq, S.; Nousheen, K.; Kiani, M.H.; Shahnaz, G. Formulation and evaluation of hyaluronic acid-based mucoadhesive self nanoemulsifying drug delivery system (SNEDDS) of tamoxifen for targeting breast cancer. Int. J. Biol. Macromol. 2020, 152, 503–515. [Google Scholar] [CrossRef]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef]
- Aragona, P.; Simmons, P.A.; Wang, H.; Wang, T. Physicochemical properties of hyaluronic acid–based lubricant eye drops. Transl. Vis. Sci. Technol. 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for in vitro synergistic combination chemotherapy of breast cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef]
- Shabani Ravari, N.; Goodarzi, N.; Alvandifar, F.; Amini, M.; Souri, E.; Khoshayand, M.R.; Hadavand Mirzaie, Z.; Atyabi, F.; Dinarvand, R. Fabrication and biological evaluation of chitosan coated hyaluronic acid-docetaxel conjugate nanoparticles in CD44+ cancer cells. DARU J. Pharm. Sci. 2016, 24, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-sensitive and hyaluronic acid-functionalized nanoparticles for improving breast cancer treatment by cytoplasmic 17α-methyltestosterone delivery. Molecules 2020, 25, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, R.; Abdelkader, A.; Fathi, H.A.; Elsabahy, M.; Fetih, G.; El-Badry, M. Development and Evaluation of Letrozole-Loaded Hyaluronic Acid/Chitosan-Coated Poly(d,l-lactide-co-glycolide) Nanoparticles. J. Pharm. Innov. 2021, 17, 572–583. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.H.; Geneidi, A.S.; Mansour, S. Surface functionalization of methotrexate-loaded chitosan nanoparticles with hyaluronic acid/human serum albumin: Comparative characterization and in vitro cytotoxicity. Int. J. Pharm. 2017, 522, 128–136. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, S.; Williams, G.R.; Wu, J.; Chen, X.; Zheng, H.; Zhu, L.M. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr. Polym. 2019, 221, 84–93. [Google Scholar] [CrossRef]
- Arafa, K.K.; Smyth, H.D.C.; El-Sherbiny, I.M. Mitotropic triphenylphosphonium doxorubicin-loaded core-shell nanoparticles for cellular and mitochondrial sequential targeting of breast cancer. Int. J. Pharm. 2021, 606, 120936. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, S.; Yang, Y.; Zhou, F.; Rong, J.; Zhao, J. ATP/Hyals dually responsive core-shell hyaluronan/chitosan-based drug nanocarrier for potential application in breast cancer therapy. Int. J. Biol. Macromol. 2021, 183, 839–851. [Google Scholar] [CrossRef]
- Al-jubori, A.A.; Sulaiman, G.M.; Tawfeeq, A.T.; Mohammed, H.A.; Khan, R.A.; Mohammed, S.A.A. Layer-by-Layer Nanoparticles of Tamoxifen and Resveratrol for Dual Drug Delivery System and Potential Triple-Negative Breast Cancer Treatment. Pharmaceutics 2021, 13, 1098. [Google Scholar] [CrossRef]
- Sang, M.; Han, L.; Luo, R.; Qu, W.; Zheng, F.; Zhang, K.; Liu, F.; Xue, J.; Liu, W.; Feng, F. CD44 targeted redox-triggered self-assembly with magnetic enhanced EPR effects for effective amplification of gambogic acid to treat triple-negative breast cancer. Biomater. Sci. 2020, 8, 212–223. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Int. J. Nanomed. 2019, 14, 5287–5301. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Choi, Y.J.; Jeong, M.S.; Park, Y.I.; Motoyama, K.; Kim, M.W.; Kwon, S.H.; Choi, J.H. Hyaluronic Acid-Decorated Glycol Chitosan Nanoparticles for pH-Sensitive Controlled Release of Doxorubicin and Celecoxib in Nonsmall Cell Lung Cancer. Bioconjug. Chem. 2020, 31, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, F.M.; Abd-Rabou, A.A.; Mohamed, M.S. Raloxifene-encapsulated hyaluronic acid-decorated chitosan nanoparticles selectively induce apoptosis in lung cancer cells. Bioorg. Med. Chem. 2019, 27, 1629–1638. [Google Scholar] [CrossRef]
- Taghipour-Sabzevar, V.; Sharifi, T.; Bagheri-Khoulenjani, S.; Goodarzi, V.; Kooshki, H.; Halabian, R.; Moosazadeh Moghaddam, M. Targeted delivery of a short antimicrobial peptide against CD44-overexpressing tumor cells using hyaluronic acid-coated chitosan nanoparticles: An in vitro study. J. Nanoparticle Res. 2020, 22, 99. [Google Scholar] [CrossRef]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic acid decorated naringenin nanoparticles: Appraisal of chemopreventive and curative potential for lung cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, J.; Huang, P.; Chang, L.; Long, X.; Dong, A.; Liu, J.; Chu, L.; Hu, F.; Deng, L. Tumor targeting and pH-responsive polyelectrolyte complex nanoparticles based on hyaluronic acid-paclitaxel conjugates and Chitosan for oral delivery of paclitaxel. Macromol. Res. 2013, 21, 1331–1337. [Google Scholar] [CrossRef]
- Sato, T.; Nakata, M.; Yang, Z.; Torizuka, Y.; Kishimoto, S.; Ishihara, M. In vitro and in vivo gene delivery using chitosan/hyaluronic acid nanoparticles: Influences of molecular mass of hyaluronic acid and lyophilization on transfection efficiency. J. Gene Med. 2017, 19, e2926. [Google Scholar] [CrossRef]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Jahangiri, M.; Ebrahimnejad, P. Synthesis of novel polymeric nanoparticles (methoxy-polyethylene glycol-chitosan/hyaluronic acid) containing 7-ethyl-10-hydroxycamptothecin for colon cancer therapy: In vitro, ex vivo and in vivo investigation. Artif. Cells Nanomed. Biotechnol. 2021, 49, 367–380. [Google Scholar] [CrossRef]
- Budi, H.S.; Izadi, S.; Timoshin, A.; Asl, S.H.; Beyzai, B.; Ghaderpour, A.; Alian, F.; Eshaghi, F.S.; Mousavi, S.M.; Rafiee, B.; et al. Blockade of HIF-1α and STAT3 by hyaluronate-conjugated TAT-chitosan-SPION nanoparticles loaded with siRNA molecules prevents tumor growth. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102373. [Google Scholar] [CrossRef] [PubMed]
- Salimifard, S.; Karoon Kiani, F.; Sadat Eshaghi, F.; Izadi, S.; Shahdadnejad, K.; Masjedi, A.; Heydari, M.; Ahmadi, A.; Hojjat-Farsangi, M.; Hassannia, H.; et al. Codelivery of BV6 and anti-IL6 siRNA by hyaluronate-conjugated PEG-chitosan-lactate nanoparticles inhibits tumor progression. Life Sci. 2020, 260, 118423. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; Moslehi, A.; Kheiry, H.; Karoon Kiani, F.; Ahmadi, A.; Masjedi, A.; Ghani, S.; Rafiee, B.; Karpisheh, V.; Hajizadeh, F.; et al. Codelivery of HIF-1α siRNA and Dinaciclib by Carboxylated Graphene Oxide-Trimethyl Chitosan-Hyaluronate Nanoparticles Significantly Suppresses Cancer Cell Progression. Pharm. Res. 2020, 37, 196. [Google Scholar] [CrossRef]
- Huang, P.; Yang, C.; Liiu, J.; Wang, W.; Guo, S.; Li, J.; Sun, Y.; Dong, H.; Deng, L.; Zhang, J.; et al. Improving the oral delivery efficiency of anticancer drugs by chitosan coated polycaprolactone-grafted hyaluronic acid nanoparticles. J. Mater. Chem. B 2014, 2, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B Biointerfaces 2020, 196, 111279. [Google Scholar] [CrossRef]
- Salehi Khesht, A.M.; Karpisheh, V.; Sahami Gilan, P.; Melnikova, L.A.; Olegovna Zekiy, A.; Mohammadi, M.; Hojjat-Farsangi, M.; Majidi Zolbanin, N.; Mahmoodpoor, A.; Hassannia, H.; et al. Blockade of CD73 using siRNA loaded chitosan lactate nanoparticles functionalized with TAT-hyaluronate enhances doxorubicin mediated cytotoxicity in cancer cells both in vitro and in vivo. Int. J. Biol. Macromol. 2021, 186, 849–863. [Google Scholar] [CrossRef]
- Karpisheh, V.; Fakkari Afjadi, J.; Nabi Afjadi, M.; Haeri, M.S.; Abdpoor Sough, T.S.; Heydarzadeh Asl, S.; Edalati, M.; Atyabi, F.; Masjedi, A.; Hajizadeh, F.; et al. Inhibition of HIF-1α/EP4 axis by hyaluronate-trimethyl chitosan-SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int. J. Biol. Macromol. 2021, 167, 1006–1019. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Z.; Yan, J.; Wang, P. Irinotecan and 5-fluorouracil-co-loaded, hyaluronic acid-modified layer-by-layer nanoparticles for targeted gastric carcinoma therapy. Drug Des. Devel. Ther. 2017, 11, 2595–2604. [Google Scholar] [CrossRef] [Green Version]
- Pelegrino, M.T.; Baldi, C.; Souza, A.C.S.; Seabra, A.B. Cytotoxicity of hyaluronic acid coated chitosan nanoparticles containing nitric oxide donor against cancer cell lines. J. Phys. Conf. Ser. 2019, 1323, 12019. [Google Scholar] [CrossRef]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Akentieva, N.P.; Gizatullin, A.R.; Silvestre, O.; Savchuk, O.; Shkondina, N.I.; Prichodchenko, T.P.; Mitschenko, D.V.; Zhilenkov, A.V.; Troshin, P.A.; Sanina, N.A.; et al. Development of chitosan-hyaluronic acid nanoparticles and study of their physico-chemical properties for targeted delivery of anticancer drugs. IOP Conf. Ser. Mater. Sci. Eng. 2020, 848, 12002. [Google Scholar] [CrossRef]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef]
- Parashar, P.; Tripathi, C.B.; Arya, M.; Kanoujia, J.; Singh, M.; Yadav, A.; Saraf, S.A. A facile approach for fabricating CD44-targeted delivery of hyaluronic acid-functionalized PCL nanoparticles in urethane-induced lung cancer: Bcl-2, MMP-9, caspase-9, and BAX as potential markers. Drug Deliv. Transl. Res. 2019, 9, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Hsu, H.S.; Tyan, S.W.; Li, F.Y.; Shew, J.Y.; Lee, W.H.; Chen, J.Y. Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene 2017, 36, 2457–2471. [Google Scholar] [CrossRef] [Green Version]
- Song, J.M.; Molla, K.; Anandharaj, A.; Cornax, I.; Gerard O’Sullivan, M.; Kirtane, A.R.; Panyam, J.; Kassie, F. Triptolide suppresses the in vitro and in vivo growth of lung cancer cells by targeting hyaluronan-CD44/RHAMM signaling. Oncotarget 2017, 8, 26927–26940. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Zhang, H.; Wu, X.; Zhang, Y.; Li, J.; Shen, J.; Zhao, Y.; Xiao, Z.; Lu, L.; Huang, C.; et al. CD44 inhibition attenuates EGFR signaling and enhances cisplatin sensitivity in human EGFR wild-type non-small-cell lung cancer cells. Int. J. Mol. Med. 2020, 45, 1783–1792. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Choong, P.F.; Poon, L.F.; Zhou, J.; Khng, J.; Jasinghe, V.J.; Palaniyandi, S.; Chen, C.S. Inhibition of CD44 expression in hepatocellular carcinoma cells enhances apoptosis, chemosensitivity, and reduces tumorigenesis and invasion. Cancer Chemother. Pharmacol. 2008, 62, 949–957. [Google Scholar] [CrossRef]
- Phillips, R.J.; Helbig, K.J.; Van der Hoek, K.H.; Seth, D.; Beard, M.R. Osteopontin increases hepatocellular carcinoma cell growth in a CD44 dependant manner. World J. Gastroenterol. 2012, 18, 3389–3399. [Google Scholar] [CrossRef]
- Henry, J.C.; Park, J.K.; Jiang, J.; Kim, J.H.; Nagorney, D.M.; Roberts, L.R.; Banerjee, S.; Schmittgen, T.D. miR-199a-3p targets CD44 and reduces proliferation of CD44 positive hepatocellular carcinoma cell lines. Biochem. Biophys. Res. Commun. 2010, 403, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Kaneko, M.K.; Kato, Y. Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C44Mab-5. Biochem. Biophys. Rep. 2018, 14, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Boxberg, M.; Götz, C.; Haidari, S.; Dorfner, C.; Jesinghaus, M.; Drecoll, E.; Boskov, M.; Wolff, K.D.; Weichert, W.; Haller, B.; et al. Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphological parameters and clinicopathological factors. Histopathology 2018, 73, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.L.H.; Fiscus, R.R.; Tung, J.W.; Tin, V.P.C.; Cheng, L.C.; Sihoe, A.D.L.; Fink, L.M.; Ma, Y.; Wong, M.P. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS ONE 2010, 5, e14062. [Google Scholar] [CrossRef] [Green Version]
- Jeannot, V.; Mazzaferro, S.; Lavaud, J.; Vanwonterghem, L.; Henry, M.; Arboléas, M.; Vollaire, J.; Josserand, V.; Coll, J.L.; Lecommandoux, S.; et al. Targeting CD44 receptor-positive lung tumors using polysaccharide-based nanocarriers: Influence of nanoparticle size and administration route. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 921–932. [Google Scholar] [CrossRef]
- Song, J.M.; Im, J.; Nho, R.S.; Han, Y.H.; Upadhyaya, P.; Kassie, F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol. Carcinog. 2019, 58, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Li, K.; Lin, T.; Xue, W.; Mu, X.; Xu, E.; Yang, X.; Chen, F.; Li, G.; Ma, L.; Wang, G.; et al. Current status of diagnosis and treatment of bladder cancer in China e Analyses of Chinese Bladder Cancer Consortium database. Asian J. Urol. 2015, 2, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, Y.; Gao, J.; Lian, X.; Wang, Y. The clinicopathological and prognostic value of CD44 expression in bladder cancer: A study based on meta-analysis and TCGA data. Bioengineered 2020, 11, 572–581. [Google Scholar] [CrossRef]
- Morera, D.S.; Hennig, M.S.; Talukder, A.; Lokeshwar, S.D.; Wang, J.; Garcia-Roig, M.; Ortiz, N.; Yates, T.J.; Lopez, L.E.; Kallifatidis, G.; et al. Hyaluronic acid family in bladder cancer: Potential prognostic biomarkers and therapeutic targets. Br. J. Cancer 2017, 117, 1507–1517. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, Z.; Hua, X.; Huang, M.; Xu, J.; Li, J.; Huang, H.; Cohen, M.; Huang, C. Isorhapontigenin (ISO) inhibits stem cell-like properties and invasion of bladder cancer cell by attenuating CD44 expression. Cell. Mol. Life Sci. 2020, 77, 351–363. [Google Scholar] [CrossRef]
- Desai, J.; Thakkar, H. Effect of particle size on oral bioavailability of darunavir-loaded solid lipid nanoparticles. J. Microencapsul. 2016, 33, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic acid-coated chitosan nanoparticles as targeted-carrier of tamoxifen against MCF7 and TMX-resistant MCF7 cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Dadashi, F.; Esmaeili, A. Optimization, in-vitro release and in-vivo evaluation of bismuth-hyaluronic acid-melittin-chitosan modified with oleic acid nanoparticles computed imaging-guided radiotherapy of cancer tumor in eye cells. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 270, 115197. [Google Scholar] [CrossRef]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Bose, A.; Gupta, P.; Chattopadhyay, S.; Chattopadhyay, D.; Adhikary, A. Hyaluronic acid engrafted metformin loaded graphene oxide nanoparticle as CD44 targeted anti-cancer therapy for triple negative breast cancer. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 129841. [Google Scholar] [CrossRef]
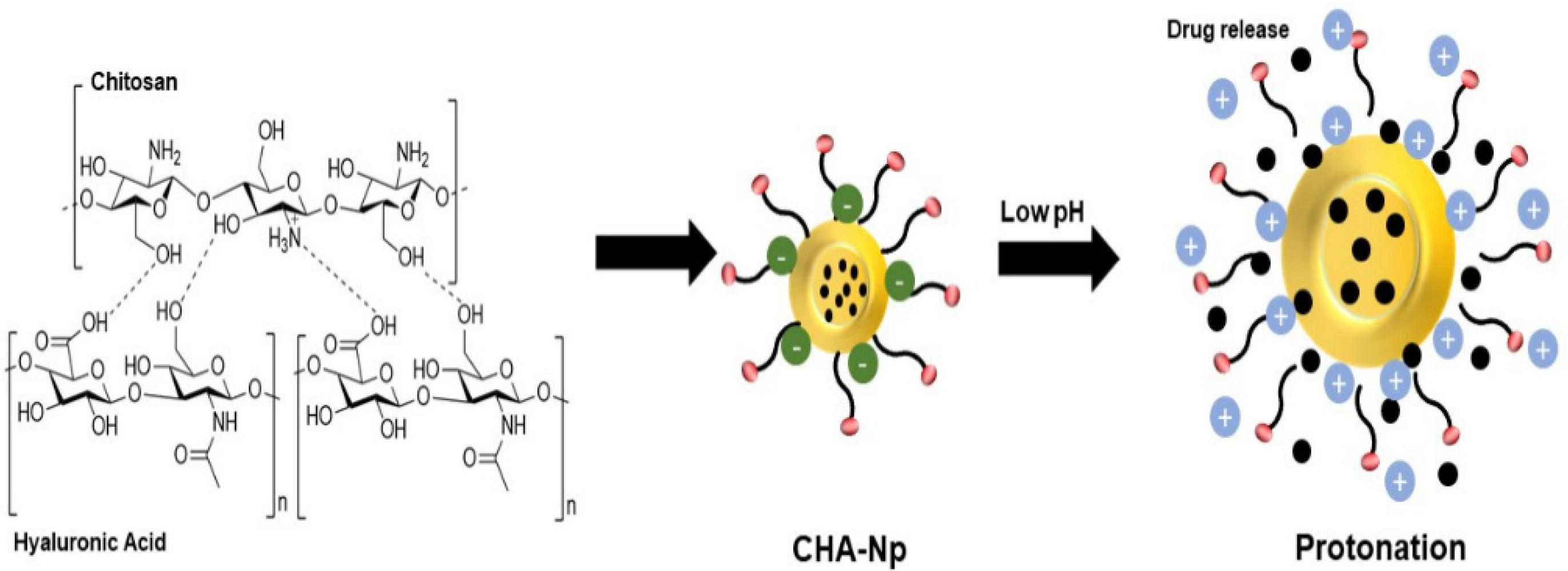


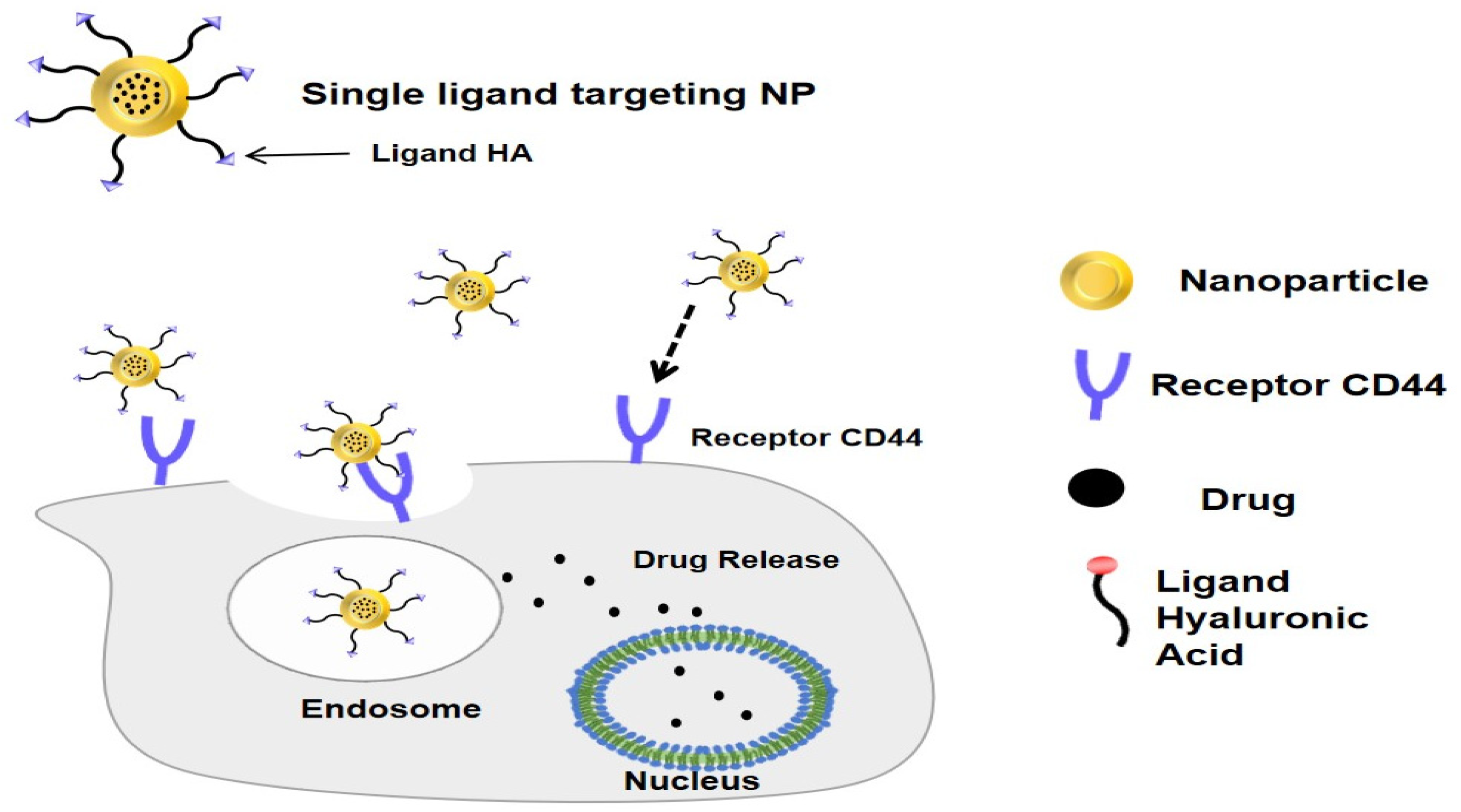

| No | Cancer Types | NDDS | Particle Size (nm) | Zeta Potential (mV) | Cell Line | Testing | Activities In Vitro | Activities In Vivo | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITG | Ap | CU | TV | S | ||||||||
| 1. | Breast cancer | miR-34a, Dox, C, HA | 214 | −33 | MDA-MB-231 | In vitro | √ | √ | - | - | - | [51] |
| 2. | Cisplatin, Dox, C, HA | 160 | −28 | MCF-7 | In vitro | √ | - | √ | - | - | [52] | |
| 3. | DTX, C, HA | 170–210 | 18–24 | MCF-7 and 4T1 | In vitro | √ | - | √ | - | - | [53] | |
| 4. | Methyltestosterone, lipoic acid, C, HA | 280 | 19 | MCF-7 | In vitro | √ | √ | √ | - | - | [54] | |
| 5. | Letrozole, C, HA, PLGA | 464 | −10.5 | MCF-7 | In vitro and in vivo | √ | √ | √ | √ | - | [55] | |
| 6. | Methotrexate, C, HA | 190–300 | −20–(−30) | MCF-7 | In vitro | √ | - | - | - | - | [56] | |
| 7. | Bismuth, oleic acid, C, HA | 10–20 | −30.9 | MCF-7 | In vitro and in vivo | √ | - | - | √ | - | [48] | |
| 8. | Pacitaxel, di(ethylene glycol) methyl ether methacrylate, C, HA | 190 | - | MDA-MB-231 | In vitro and in vivo | √ | √ | √ | √ | √ | [57] | |
| 9. | Dox, TPP, C, HA | 220–280 | - | MCF-7 | In vitro and in vivo | √ | √ | - | √ | - | [58] | |
| 10. | 3-fluoro-4-carboxyphenylboronic acid, PEG, C, HA | 200–330 | −10.8 | MCF-7 and MDA-MB-231 | In vitro | √ | - | √ | - | - | [59] | |
| 11. | PEG, C, HA, hexadecano, Gamboic acid | 220 | 45 | MDA-MB-231 | In vitro | √ | - | √ | - | - | [60] | |
| 12. | Tamoxifen, resveratrol, poloxamer, C, HA. | 217 | 17.5 | MCF-7 | In vitro and in vivo | √ | √ | - | √ | √ | [61] | |
| 13. | Lung cancer | Cyaine3 labeled siRNA, C, HA | 127 | 31 | A549 | In vitro and in vivo | √ | - | √ | √ | √ | [62] |
| 14. | 5-Fluorouracil, C, HA | 119 | 15.6 | A549 | In vitro | √ | √ | √ | - | - | [63] | |
| 15. | Dox, Celocoxib, C, HA | 150 | −25 | A549 | In vitro and in vivo | √ | √ | √ | √ | - | [64] | |
| 16. | Raloxifen, C, HA | 142 | −15 | A549 | In vitro | √ | √ | √ | - | - | [65] | |
| 17. | Peptide CM11, C, HA | 140–240 | 51 | A549 | In vitro | √ | √ | - | - | - | [66] | |
| 18. | Naringenin, PCL, C, HA | 251 | −19.5 | A549 | In vitro and in vivo | √ | - | √ | √ | - | [67] | |
| 19. | Gamboic acid, C, HA | 212 | −23 | A549 | In vitro and in vivo | √ | √ | √ | √ | √ | [68] | |
| 20. | Liver cancer | Paclitaxel, C, HA | 100 | −11 | HepG2 | In vitro | √ | - | √ | - | - | [69] |
| 21. | pDNA, C, HA | 203–390 | −37 | Huh7 | In vitro | √ | - | - | - | - | [70] | |
| 22. | Colon cancer | mRNA, C, HA | 265–350 | −40 | HCT-116 | In vitro | √ | - | - | - | - | [71] |
| 23. | 7-ethyl-10-hydroxycamptothecin, PEG, C, HA | 227 | - | HCT-116 | In vitro and in vivo | √ | - | √ | √ | - | [72] | |
| 24. | siRNA, TAT peptide, C, HA | 118 | 20 | CT26 | In vitro | √ | √ | - | - | - | [73] | |
| 25. | anti-IL-6, BV6, PEG, C, HA | 100 | 12 | CT26 | In vitro and in vivo | √ | √ | √ | √ | - | [74] | |
| 26. | siRNA, carboxylate grapheme oxide, trimethyl C, HA | 95 | 27.2 | CT26 | In vitro | √ | √ | √ | - | - | [75] | |
| 27. | Oral squamous cancer | Paclitaxel, PCL, C, HA | 257 | −25 | EC109 | In vitro and in vivo | √ | - | √ | √ | √ | [76] |
| 28. | Cathecol, C, HA | 160 | −12.7 | HN22 | In vitro | √ | √ | √ | - | - | [77] | |
| 29. | Bladder cancer | siRNA, C, HA | 100–120 | 30–40 | T24 | In vitro | √ | - | √ | - | - | [23] |
| 30. | siRNA, Dox, TAT peptide, C, HA | 118 | 9 | T24 | In vitro and in vivo | √ | - | √ | √ | - | [78] | |
| 31. | siRNA and the EP4 antagonist, C, HA | 130 | 27 | T24 | In vitro | √ | - | √ | - | - | [79] | |
| 32. | Others | Irinotecan, 5-fluorouracil, PLGA, C, HA | 153 | −13.7 | MGC803 | In vitro and in vivo | √ | - | √ | √ | - | [80] |
| 33. | Nitric oxide, C, HA | 170 | 15 | PC-3 | In vitro | √ | - | - | - | - | [81] | |
| 34. | Graphene oxide, fluorescein isothiocyanate, C, HA | 200 | −41 | HeLa | In vitro | √ | - | √ | - | - | [82] | |
| 35. | Curcuminoid, C, HA | 210–240 | 25 | C6 | In vitro | √ | - | √ | - | - | [83] | |
| 36. | Dox, nitric oxide, C, HA | 170–200 | −39–(−47) | HeLa | In vitro | √ | - | √ | - | - | [84] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puluhulawa, L.E.; Joni, I.M.; Elamin, K.M.; Mohammed, A.F.A.; Muchtaridi, M.; Wathoni, N. Chitosan–Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers 2022, 14, 3410. https://doi.org/10.3390/polym14163410
Puluhulawa LE, Joni IM, Elamin KM, Mohammed AFA, Muchtaridi M, Wathoni N. Chitosan–Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers. 2022; 14(16):3410. https://doi.org/10.3390/polym14163410
Chicago/Turabian StylePuluhulawa, Lisa Efriani, I Made Joni, Khaled M. Elamin, Ahmed Fouad Abdelwahab Mohammed, Muchtaridi Muchtaridi, and Nasrul Wathoni. 2022. "Chitosan–Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy" Polymers 14, no. 16: 3410. https://doi.org/10.3390/polym14163410
APA StylePuluhulawa, L. E., Joni, I. M., Elamin, K. M., Mohammed, A. F. A., Muchtaridi, M., & Wathoni, N. (2022). Chitosan–Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers, 14(16), 3410. https://doi.org/10.3390/polym14163410








