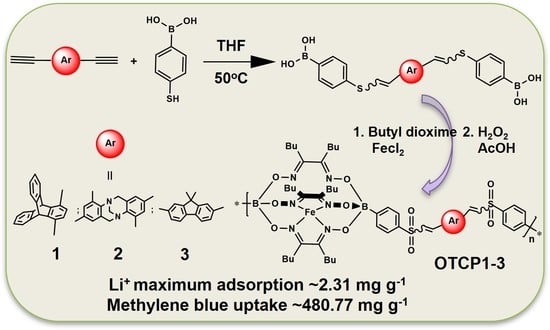
Synthesis of Metalorganic Copolymers Containing Various Contorted Units and Iron(II) Clathrochelates with Lateral Butyl Chains: Conspicuous Adsorbents of Lithium Ions and Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
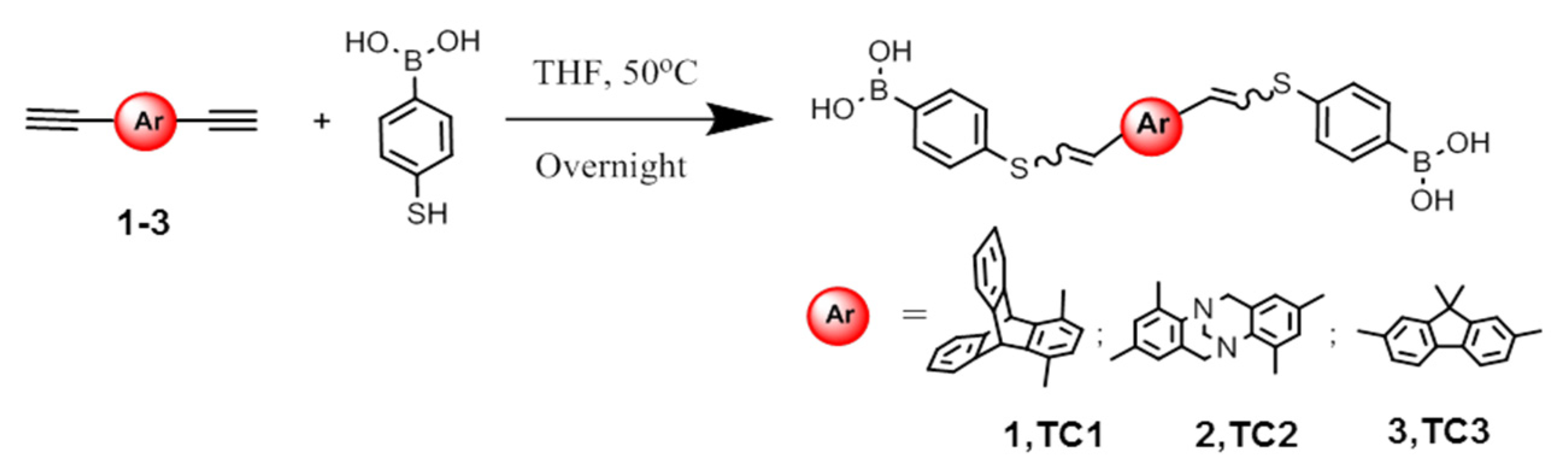
2.1.1. Synthesis of TC1 (Procedure A)
2.1.2. Synthesis of TC2
2.1.3. Synthesis of TC3
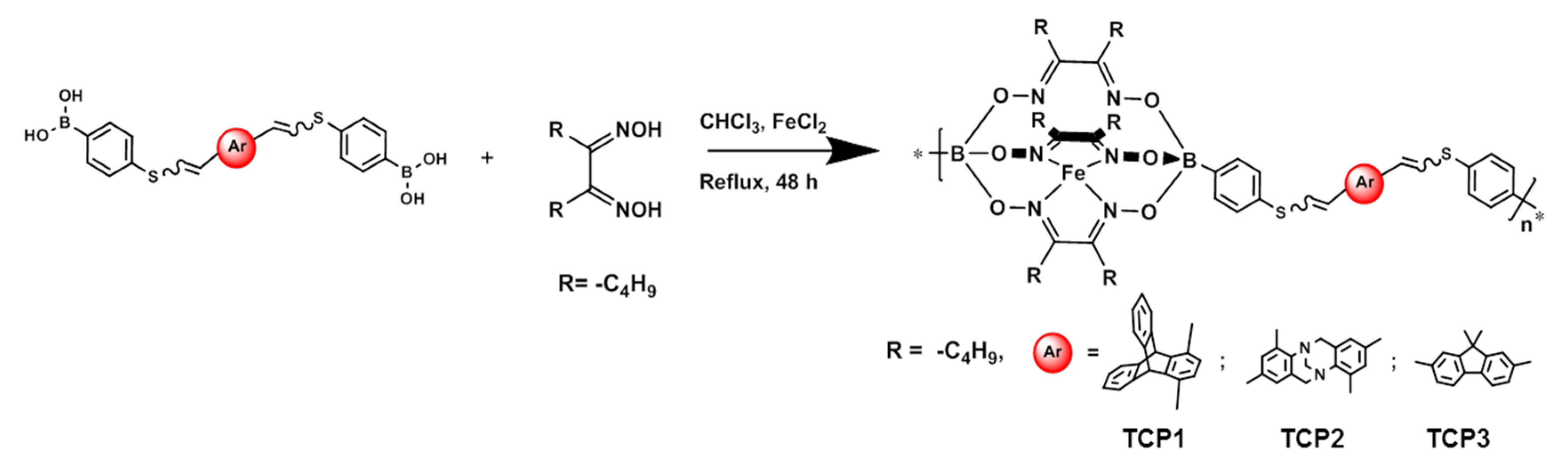
2.1.4. Synthesis of TCP1 (Procedure B)
2.1.5. Synthesis of TCP2
2.1.6. Synthesis of TCP3
2.1.7. Synthesis of OTCP1 (Procedure C)
2.1.8. Synthesis of OTCP2
2.1.9. Synthesis of OTCP3
3. Results
3.1. Synthesis of Synthons TC1–3
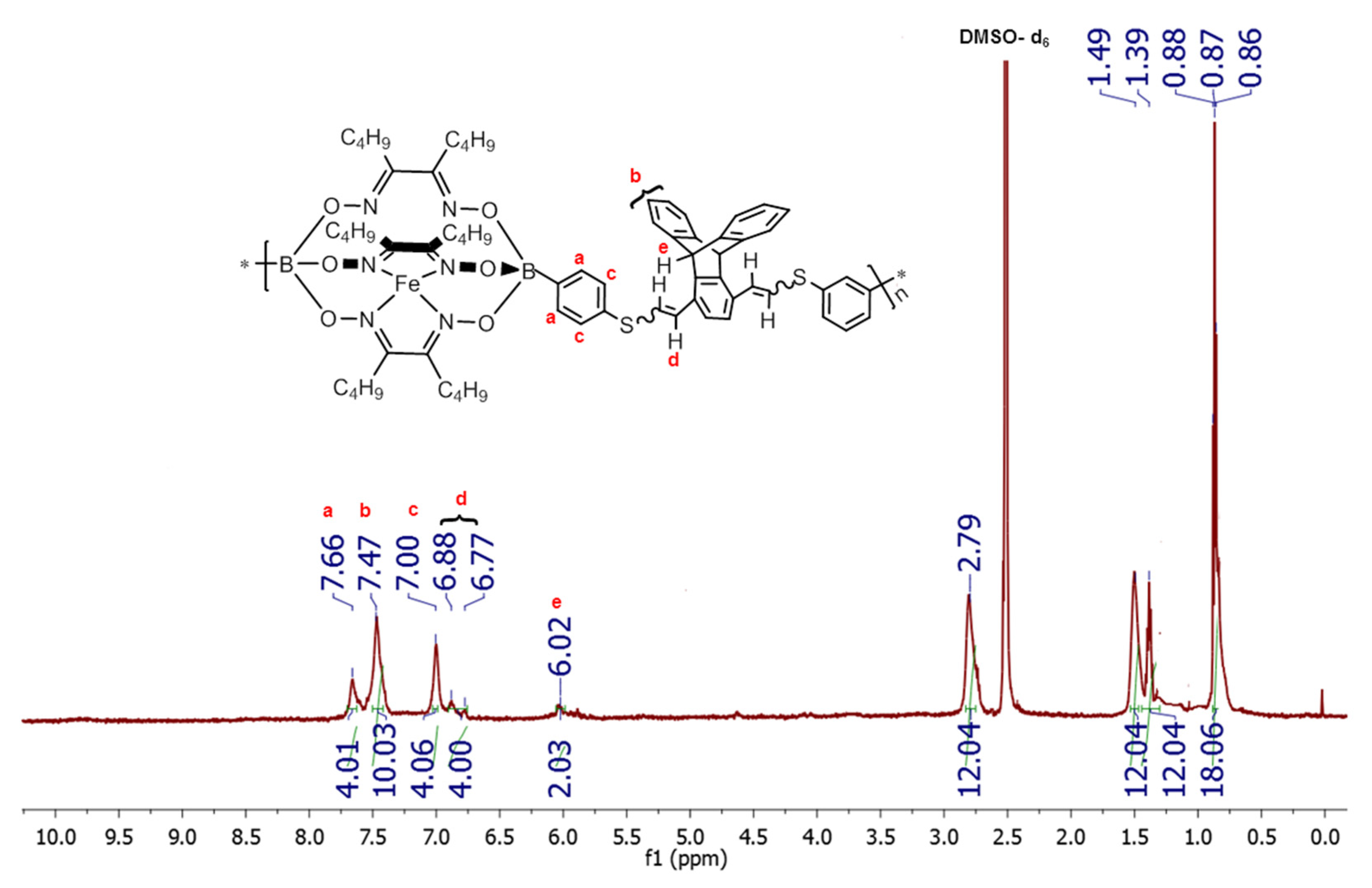
3.2. Synthesis of Copolymers TCP1–3
3.3. Synthesis of Copolymers OTCP1–3
3.4. Copolymers’ Adsorption of Lithium Ions
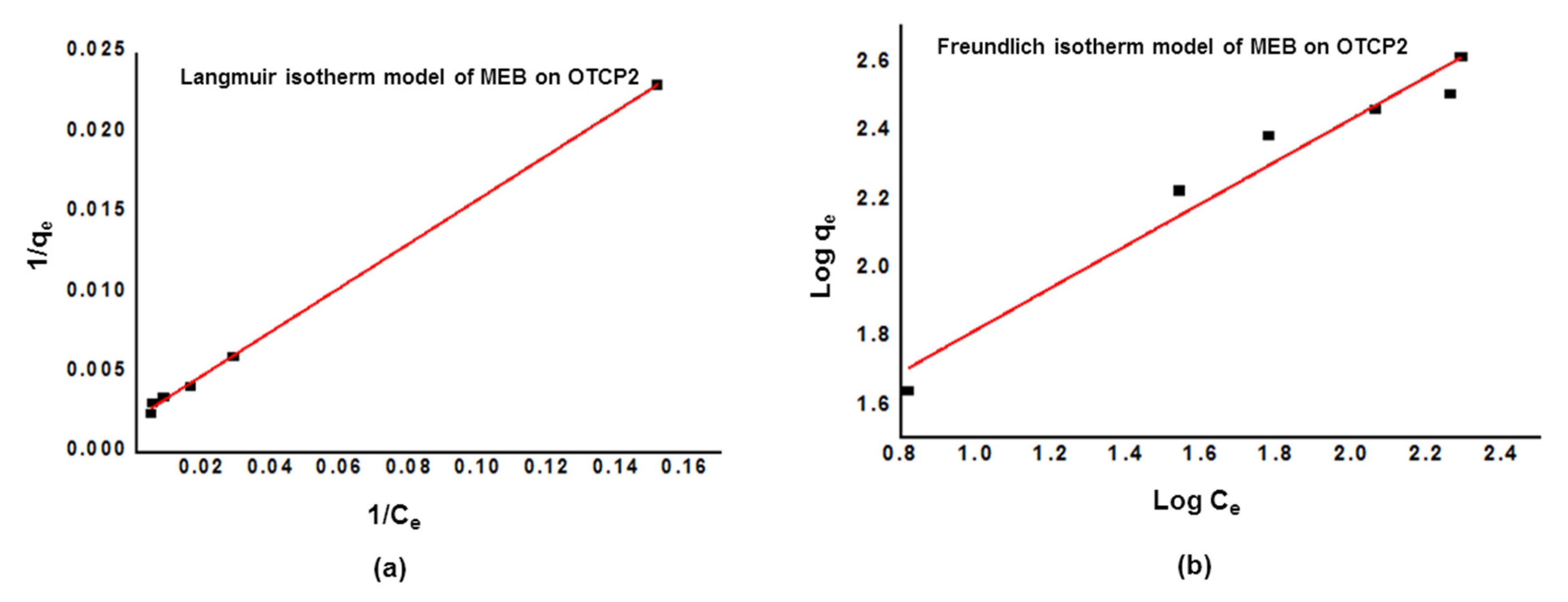
3.5. Methylene Blue Adsorption Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, A.-D.; Wei, D.; Nie, H.-J.; Hu, H.; Peng, C.; Li, S.-T.; Yan, K.-N.; Zhou, B.-S.; Feng, L.; Fang, C.; et al. Light-induced primary amines and o-nitrobenzyl alcohols cyclization as a versatile photoclick reaction for modular conjugation. Nat. Commun. 2020, 11, 5472. [Google Scholar] [CrossRef] [PubMed]
- Konuray, O.; Fernández-Francos, X.; De la Flor, S.; Ramis, X.; Serra, À. The Use of Click-Type Reactions in the Preparation of Thermosets. Polymers 2020, 12, 1084. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, D.; Lim, D.-K.; Koo, H.; Kim, K. Copper-Free Click Chemistry: Applications in Drug Delivery, Cell Tracking, and Tissue Engineering. Adv. Mater. 2022, 34, 2107192. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, K.; Zhang, Z.; Feng, Y.; Rao, L.; Chen, X.; Yu, G. Metal-free bioorthogonal click chemistry in cancer theranostics. Chem. Soc. Rev. 2022, 51, 1336–1376. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, J.; Huang, Y.; Luo, J. Synthesis of Xylan-Click-Quaternized Chitosan via Click Chemistry and Its Application in the Preparation of Nanometal Materials. Molecules 2022, 27, 3455. [Google Scholar] [CrossRef]
- Cicetti, S.; Maestre, E.; Spanevello, R.A.; Sarotti, A.M. Towards the Synthesis of Highly Hindered Pyrrolidines by Intramolecular AAC Click Reactions: What Can Be Learned from DFT Calculations? Eur. J. Org. Chem. 2022, 2022, e202200478. [Google Scholar] [CrossRef]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]
- Du, J.; Huang, D.; Li, H.; Qin, A.; Tang, B.Z.; Li, Y. Catalyst-Free Click Polymerization of Thiol and Activated Internal Alkynes: A Facile Strategy toward Functional Poly(β-thioacrylate)s. Macromolecules 2020, 53, 4932–4941. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Liu, X.; Li, Z. Photobase-catalysed anionic thiol-epoxy click photopolymerization under NIR irradiation: From deep curing to shape memory. Polym. Chem. 2022, 13, 3048–3052. [Google Scholar] [CrossRef]
- Capperucci, A.; Petrucci, A.; Faggi, C.; Tanini, D. Click Reaction of Selenols with Isocyanates: Rapid Access to Selenocarbamates as Peroxide-Switchable Reservoir of Thiol-Peroxidase-Like Catalysts. Adv. Synth. Catal. 2021, 363, 4256–4263. [Google Scholar] [CrossRef]
- Sinha, J.; Podgórski, M.; Tomaschke, A.; Ferguson, V.L.; Bowman, C.N. Phototriggered Base Amplification for Thiol-Michael Addition Reactions in Cross-linked Photopolymerizations with Efficient Dark Cure. Macromolecules 2020, 53, 6331–6340. [Google Scholar] [CrossRef]
- Tang, J.; Yin, S.; Liu, X.; Kan, Z.; Zhang, X.; Jiang, M.; Tao, F.; Wang, F.; Li, C. Thiol-yne click reaction mediated photoelectrochemical detection of multi-sulfhydryl compounds based on diacetylene functionalized conjugated polymer. Sens. Actuators B Chem. 2021, 344, 130207. [Google Scholar] [CrossRef]
- Daglar, O.; Luleburgaz, S.; Baysak, E.; Gunay, U.S.; Hizal, G.; Tunca, U.; Durmaz, H. Nucleophilic Thiol-yne reaction in Macromolecular Engineering: From synthesis to applications. Eur. Polym. J. 2020, 137, 109926. [Google Scholar] [CrossRef]
- Yao, B.; Mei, J.; Li, J.; Wang, J.; Wu, H.; Sun, J.Z.; Qin, A.; Tang, B.Z. Catalyst-Free Thiol–Yne Click Polymerization: A Powerful and Facile Tool for Preparation of Functional Poly(vinylene sulfide)s. Macromolecules 2014, 47, 1325–1333. [Google Scholar] [CrossRef]
- Caron, E.; Wolf, M.O. Soluble Oligo- and Polythienyl Sulfides and Sulfones: Synthesis and Photophysics. Macromolecules 2017, 50, 7543–7549. [Google Scholar] [CrossRef]
- Marrocchi, A.; Facchetti, A.; Lanari, D.; Santoro, S.; Vaccaro, L. Click-chemistry approaches to π-conjugated polymers for organic electronics applications. Chem. Sci. 2016, 7, 6298–6308. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Takasu, A. Synthesis of non-ionic poly(ester-sulfone) via low-temperature polycondensation for anode-selective electrophoretic deposition and subsequent photo cross-linking. Polym. J. 2018, 50, 187–196. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Moustafa, M.S.; Al-Mousawi, S.; Alameddine, B. Selective removal of toxic organic dyes using Tröger base-containing sulfone copolymers made from a metal-free thiol-yne click reaction followed by oxidation. RSC Adv. 2021, 11, 21170–21178. [Google Scholar] [CrossRef]
- Albada, B.; Keijzer, J.F.; Zuilhof, H.; van Delft, F. Oxidation-Induced “One-Pot” Click Chemistry. Chem. Rev. 2021, 121, 7032–7058. [Google Scholar] [CrossRef]
- Geven, M.; d’Arcy, R.; Turhan, Z.Y.; El-Mohtadi, F.; Alshamsan, A.; Tirelli, N. Sulfur-based oxidation-responsive polymers. Chemistry, (chemically selective) responsiveness and biomedical applications. Eur. Polym. J. 2021, 149, 110387. [Google Scholar] [CrossRef]
- Rajangam, V.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef]
- Oroujzadeh, M.; Mehdipour-Ataei, S. Evaluation of properties and performance of poly(ether sulfone ketone) membranes in proton exchange membrane fuel cells. Polym. Adv. Technol. 2022, 16, 133–138. [Google Scholar] [CrossRef]
- Wijaya, F.; Woo, S.; Lee, H.; Nugraha, A.F.; Shin, D.; Bae, B. Sulfonated poly(phenylene-co-arylene ether sulfone) multiblock membranes for application in high-performance fuel cells. J. Membr. Sci. 2022, 645, 120203. [Google Scholar] [CrossRef]
- Kim, M.; Ko, H.; Nam, S.; Kim, K. Study on Control of Polymeric Architecture of Sulfonated Hydrocarbon-Based Polymers for High-Performance Polymer Electrolyte Membranes in Fuel Cell Applications. Polymers 2021, 13, 3520. [Google Scholar] [CrossRef] [PubMed]
- Kosai, J.; Masuda, Y.; Chikayasu, Y.; Takahashi, Y.; Sasabe, H.; Chiba, T.; Kido, J.; Mori, H. S-Vinyl Sulfide-Derived Pendant-Type Sulfone/Phenoxazine-Based Polymers Exhibiting Thermally Activated Delayed Fluorescence: Synthesis and Photophysical Property Characterization. ACS Appl. Polym. Mater. 2020, 2, 3310–3318. [Google Scholar] [CrossRef]
- Jürgensen, N.; Kretzschmar, A.; Höfle, S.; Freudenberg, J.; Bunz, U.H.F.; Hernandez-Sosa, G. Sulfone-Based Deep Blue Thermally Activated Delayed Fluorescence Emitters: Solution-Processed Organic Light-Emitting Diodes with High Efficiency and Brightness. Chem. Mater. 2017, 29, 9154–9161. [Google Scholar] [CrossRef]
- Hirose, A.; Tanaka, K.; Yoshii, R.; Chujo, Y. Film-type chemosensors based on boron diiminate polymers having oxidation-induced emission properties. Polym. Chem. 2015, 6, 5590–5595. [Google Scholar] [CrossRef]
- Gomaa, M.; Sánchez-Ramos, A.; Ureña, N.; Pérez-Prior, M.; Levenfeld, B.; García-Salaberri, P.A. Characterization and Modeling of Free Volume and Ionic Conduction in Multiblock Copolymer Proton Exchange Membranes. Polymers 2022, 14, 1688. [Google Scholar] [CrossRef]
- Singh, S.; Varghese, A.M.; Reddy, K.S.K.; Romanos, G.E.; Karanikolos, G.N. Polysulfone Mixed-Matrix Membranes Comprising Poly(ethylene glycol)-Grafted Carbon Nanotubes: Mechanical Properties and CO2 Separation Performance. Ind. Eng. Chem. Res. 2021, 60, 11289–11308. [Google Scholar] [CrossRef]
- Xu, C.; Yu, T.; Peng, J.; Zhao, L.; Li, J.; Zhai, M. Efficient Adsorption Performance of Lithium Ion onto Cellulose Microspheres with Sulfonic Acid Groups. Quantum Beam Sci. 2020, 4, 6. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, E.-h.; Yan, G.; Yang, Y.-z.; Liu, W.-f.; Liu, X.-g. A lithium ion-imprinted adsorbent using magnetic carbon nanospheres as a support for the selective recovery of lithium ions. New Carbon Mater. 2020, 35, 696–706. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium Market Research–Global Supply, Future Demand and Price Development. Energy Storage Mater. 2016, 6, 171–179. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Sun, S. Performance of a synthetic resin for lithium adsorption in waste liquid of extracting aluminum from fly-ash. Chin. J. Chem. Eng. 2022, 44, 115–123. [Google Scholar] [CrossRef]
- Wahib, S.A.; Da’na, D.A.; Zaouri, N.; Hijji, Y.M.; Al-Ghouti, M.A. Adsorption and recovery of lithium ions from groundwater using date pits impregnated with cellulose nanocrystals and ionic liquid. J. Hazard. Mater. 2022, 421, 126657. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, J.; Svecova, L.; Lagallarde, F.; Laucournet, R.; Thivel, P.-X. Lithium recovery from aqueous solution by sorption/desorption. Hydrometallurgy 2014, 143, 1–11. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, R.; Verma, S.; Singh, S. Antimicrobials and Antibiotic Resistance Genes in Water Bodies: Pollution, Risk, and Control. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar] [CrossRef]
- Delgado-González, C.R.; Madariaga-Navarrete, A.; Fernández-Cortés, J.M.; Islas-Pelcastre, M.; Oza, G.; Iqbal, H.M.N.; Sharma, A. Advances and Applications of Water Phytoremediation: A Potential Biotechnological Approach for the Treatment of Heavy Metals from Contaminated Water. Int. J. Environ. Res. Public Health 2021, 18, 5215. [Google Scholar] [CrossRef]
- Elgarahy, A.; Al-wakeel, K.; Mohammad, S.; Shoubaky, G. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Lashen, Z.M.; Shams, M.S.; El-Sheshtawy, H.S.; Slaný, M.; Antoniadis, V.; Yang, X.; Sharma, G.; Rinklebe, J.; Shaheen, S.M.; Elmahdy, S.M. Remediation of Cd and Cu contaminated water and soil using novel nanomaterials derived from sugar beet processing- and clay brick factory-solid wastes. J. Hazard. Mater. 2022, 428, 128205. [Google Scholar] [CrossRef]
- Zhou, Y.; Elchalakani, D.M.; Liu, H.; Briseghella, B.; Sun, C. Photocatalytic concrete for degrading organic dyes in water. Environ. Sci. Pollut. Res. 2022, 29, 39027–39040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, N.; Soldatov, M.; Liu, H. A novel phosphazene-based amine-functionalized porous polymer with high adsorption ability for I2, dyes and heavy metal ions. React. Funct. Polym. 2022, 173, 105235. [Google Scholar] [CrossRef]
- Qiao, A.; Cui, M.; Huang, R.; Ding, G.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Kumar, Y.; Rani, S.; Shabir, J.; Kumar, L.S. Nitrogen-Rich and Porous Graphitic Carbon Nitride Nanosheet-Immobilized Palladium Nanoparticles as Highly Active and Recyclable Catalysts for the Reduction of Nitro Compounds and Degradation of Organic Dyes. ACS Omega 2020, 5, 13250–13258. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Alameddine, B. Conjugated microporous polymers using a copper-catalyzed [4 + 2] cyclobenzannulation reaction: Promising materials for iodine and dye adsorption. Polym. Chem. 2021, 12, 2282–2292. [Google Scholar] [CrossRef]
- Khare, P.; Singh, A.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Sunlight-Induced Selective Photocatalytic Degradation of Methylene Blue in Bacterial Culture by Pollutant Soot Derived Nontoxic Graphene Nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 579–589. [Google Scholar] [CrossRef]
- Nedu, M.-E.; Tertis, M.; Cristea, C.; Georgescu, A.V. Comparative Study Regarding the Properties of Methylene Blue and Proflavine and Their Optimal Concentrations for In Vitro and In Vivo Applications. Diagnostics 2020, 10, 223. [Google Scholar] [CrossRef]
- Manippady, S.R.; Singh, A.; Basavaraja, B.M.; Samal, A.K.; Srivastava, S.; Saxena, M. Iron–Carbon Hybrid Magnetic Nanosheets for Adsorption-Removal of Organic Dyes and 4-Nitrophenol from Aqueous Solution. ACS Appl. Nano Mater. 2020, 3, 1571–1582. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Dabholkar, N.; Gorantla, S.; Dubey, S.K.; Alexander, A.; Taliyan, R.; Singhvi, G. Repurposing methylene blue in the management of COVID-19: Mechanistic aspects and clinical investigations. Biomed. Pharmacother. 2021, 142, 112023. [Google Scholar] [CrossRef]
- Porizka, M.; Kopecky, P.; Dvorakova, H.; Kunstyr, J.; Lips, M.; Michalek, P.; Balik, M. Methylene blue administration in patients with refractory distributive shock-A retrospective study. Sci. Rep. 2020, 10, 1828. [Google Scholar] [CrossRef] [PubMed]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Adel, M.; Ahmed, M.A.; Elabiad, M.A.; Mohamed, A.A. Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100719. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; AlZahrani, I.I.S.; Naglah, A.M.; El-Desouky, M.G.; Khalil, M.A.; El-Bindary, A.A.; El-Bindary, M.A. Effective Removal of Methylene Blue From Aqueous Solution Using Metal-Organic Framework; Modelling Analysis, Statistical Physics Treatment and DFT Calculations. ChemistrySelect 2021, 6, 11431–11447. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Tenhu, H.; Khattab, S.A.; Eldeeb, A.A.; Azaam, M.M. Highly efficient adsorbent material for removal of methylene blue dye based on functionalized polyacrylonitrile. Eur. Polym. J. 2022, 169, 111138. [Google Scholar] [CrossRef]
- Tarekegn, M.M.; Balakrishnan, R.M.; Hiruy, A.M.; Dekebo, A.H. Removal of methylene blue dye using nano zerovalent iron, nanoclay and iron impregnated nanoclay–A comparative study. RSC Adv. 2021, 11, 30109–30131. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Liu, W.; Xu, Y.; Cao, Y.; Chen, B.; Xu, M. Preparation of hyper-cross-linked hydroxylated polystyrene for adsorptive removal of methylene blue. RSC Adv. 2021, 11, 25551–25560. [Google Scholar] [CrossRef]
- Han, Q.; Wang, J.; Goodman, B.A.; Xie, J.; Liu, Z. High adsorption of methylene blue by activated carbon prepared from phosphoric acid treated eucalyptus residue. Powder Technol. 2020, 366, 239–248. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Dotto, G.L. Treatment of water containing methylene by biosorption using Brazilian berry seeds (Eugenia uniflora). Environ. Sci. Pollut. Res. 2020, 27, 20831–20843. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Netto, M.S.; Georgin, J.; Luiz Dotto, G.; Abu Shayeb, M.K.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Preparation and characterization of a novel mountain soursop seeds powder adsorbent and its application for the removal of crystal violet and methylene blue from aqueous solutions. Chem. Eng. J. 2020, 391, 123617. [Google Scholar] [CrossRef]
- Losytskyy, M.; Chornenka, N.; Vakarov, S.; Meier-Menches, S.M.; Gerner, C.; Potocki, S.; Arion, V.B.; Gumienna-Kontecka, E.; Voloshin, Y.; Kovalska, V. Sensing of Proteins by ICD Response of Iron(II) Clathrochelates Functionalized by Carboxyalkylsulfide Groups. Biomolecules 2020, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Selin, R.O.; Klemt, I.; Chernii, V.Y.; Losytskyy, M.Y.; Chernii, S.; Mular, A.; Gumienna-Kontecka, E.; Kovalska, V.B.; Voloshin, Y.Z.; Vologzhanina, A.V.; et al. Synthesis and spectral characterization of the first fluorescein-tagged iron(ii) clathrochelates, their supramolecular interactions with globular proteins, and cellular uptake. RSC Adv. 2021, 11, 8163–8177. [Google Scholar] [CrossRef]
- Varzatskii, O.A.; Oranskiy, D.A.; Vakarov, S.V.; Chornenka, N.V.; Belov, A.S.; Vologzhanina, A.V.; Pavlov, A.A.; Grigoriev, S.A.; Pushkarev, A.S.; Millet, P.; et al. Hydrogen production with a designed clathrochelate-based electrocatalytic materials: Synthesis, X-ray structure and redox-properties of the iron cage complexes with pendant (poly)aryl-terminated ribbed substituents. Int. J. Hydrog. Energy 2017, 42, 27894–27909. [Google Scholar] [CrossRef]
- Voloshin, Y.Z.; Buznik, V.M.; Dedov, A.G. New types of the hybrid functional materials based on cage metal complexes for (electro) catalytic hydrogen production. Pure Appl. Chem. 2020, 92, 1159–1174. [Google Scholar] [CrossRef]
- Alameddine, B.; Shetty, S.; Baig, N.; Al-Mousawi, S.; Al-Sagheer, F. Synthesis and characterization of metalorganic polymers of intrinsic microporosity based on iron(II) clathrochelate. Polymer 2017, 122, 200–207. [Google Scholar] [CrossRef]
- Zlobin, I.S.; Aisin, R.R.; Novikov, V.V. Iron(II) Clathrochelates in Molecular Spintronic Devices: A Vertical Spin Valve. Russ. J. Coord. Chem. 2022, 48, 33–40. [Google Scholar] [CrossRef]
- Alameddine, B.; Shetty, S.; Anju, R.S.; Al-Sagheer, F.; Al-Mousawi, S. Highly soluble metal-organic polymers based on iron(II) clathrochelates and their gelation induced by sonication. Eur. Polym. J. 2017, 95, 566–574. [Google Scholar] [CrossRef]
- Jansze, S.M.; Severin, K. Clathrochelate Metalloligands in Supramolecular Chemistry and Materials Science. Acc. Chem. Res. 2018, 51, 2139–2147. [Google Scholar] [CrossRef]
- Antipin, I.; Alfimov, M.; Arslanov, V.; Burilov, V.; Vatsadze, S.; Voloshin, Y.; Volcho, K.; Gorbatchuk, V.; Gorbunova, Y.; Gromov, S.; et al. Functional supramolecular systems: Design and applications. Russ. Chem. Rev. 2021, 90, 895. [Google Scholar] [CrossRef]
- Cecot, G.; Alameddine, B.; Prior, S.; Zorzi, R.D.; Geremia, S.; Scopelliti, R.; Fadaei, F.T.; Solari, E.; Severin, K. Large heterometallic coordination cages with gyrobifastigium-like geometry. Chem. Commun. 2016, 52, 11243–11246. [Google Scholar] [CrossRef] [PubMed]
- Cecot, G.; Marmier, M.; Geremia, S.; Zorzi, R.D.; Vologzhanina, A.V.; Pattison, P.; Solari, E.; Tirani, F.F.; Scopelliti, R.; Severin, K. The Intricate Structural Chemistry of MII2nLn-Type Assemblies. J. Am. Chem. Soc. 2017, 39, 8371–8381. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Xie, Y.; Pham, T.D.; Shetty, S.; Son, F.A.; Idrees, K.B.; Chen, Z.; Xie, H.; Liu, Y.; Snurr, R.Q.; et al. Creating Optimal Pockets in a Clathrochelate-Based Metal–Organic Framework for Gas Adsorption and Separation: Experimental and Computational Studies. J. Am. Chem. Soc. 2022, 144, 3737–3745. [Google Scholar] [CrossRef]
- Chen, Z.; Idrees, K.B.; Shetty, S.; Xie, H.; Wasson, M.C.; Gong, W.; Zhang, X.; Alameddine, B.; Farha, O.K. Regulation of Catenation in Metal–Organic Frameworks with Tunable Clathrochelate-Based Building Blocks. Cryst. Growth Des. 2021, 21, 6665–6670. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Al-Mousawi, S.; Alameddine, B. Removal of anionic and cationic dyes using porous copolymer networks made from a Sonogashira cross-coupling reaction of diethynyl iron (II) clathrochelate with various arylamines. J. Appl. Polym. Sci. 2022, published online, e52966. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Hassan, A.; Al-Mousawi, S.; Das, N.; Alameddine, B. Fluorinated Iron(ii) clathrochelate units in metalorganic based copolymers: Improved porosity, iodine uptake, and dye adsorption properties. RSC Adv. 2021, 11, 14986–14995. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Moustafa, M.S.; Al-Mousawi, S.; Alameddine, B. Sizable iodine uptake of porous copolymer networks bearing Tröger’s base units. Polymer 2021, 229, 123996. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F.; Alameddine, B. Synthesis of triptycene-derived covalent organic polymer networks and their subsequent in-situ functionalization with 1,2-dicarbonyl substituents. React. Funct. Polym. 2019, 139, 153–161. [Google Scholar] [CrossRef]
- Alameddine, B.; Baig, N.; Shetty, S.; Al-Sagheer, F.; Al-Mousawi, S. Microwave-Assisted [4+2] Diels–Alder Cycloaddition of 1,4-Diethynyl Triptycene with Various Cyclopentadienone Derivatives: Promising Building Blocks for Polymer Networks. Asian J. Org. Chem. 2018, 7, 378–382. [Google Scholar] [CrossRef]
- Alameddine, B.; Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F. Triptycene-containing Poly(vinylene sulfone) derivatives from a metal-free thiol-yne click polymerization followed by a mild oxidation reaction. Polymer 2018, 154, 233–240. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, L.; Madejová, J. Near-IR study of the impact of alkyl-ammonium and -phosphonium cations on the hydration of montmorillonite. J. Mol. Struct. 2022, 1256, 132568. [Google Scholar] [CrossRef]
- Liu, M.; Yao, C.; Liu, C.; Xu, Y. Thiophene-based porous organic networks for volatile iodine capture and effectively detection of mercury ion. Sci. Rep. 2018, 8, 14071. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, G.; Wang, J.; Xu, Y.; Chang, L. Template-free synthesis of porous carbon from triazine based polymers and their use in iodine adsorption and CO2 capture. Sci. Rep. 2018, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Fuente, E.; Menéndez, J.A.; Díez, M.A.; Suárez, D.; Montes-Morán, M.A. Infrared Spectroscopy of Carbon Materials: A Quantum Chemical Study of Model Compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Al-Mousawi, S.; Al-Sagheer, F.; Alameddine, B. Synthesis of secondary arylamine copolymers with Iron(II) clathrochelate units and their functionalization into tertiary Polyarylamines via Buchwald-Hartwig cross-coupling reaction. Polymer 2019, 178, 121606. [Google Scholar] [CrossRef]
- Bai, H.; Xue, C.; Lyu, J.L.; Li, J.; Chen, G.X.; Yu, J.H.; Lin, C.T.; Lv, D.J.; Xiong, L.M. Thermal conductivity and mechanical properties of flake graphite/copper composite with a boron carbide-boron nano-layer on graphite surface. Compos. Part A Appl. Sci. Manuf. 2018, 106, 42–51. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F.; Alameddine, B. Influence of size and nature of the aryl diborate spacer on the intrinsic microporosity of Iron(II) clathrochelate polymers. Polymer 2018, 151, 164–170. [Google Scholar] [CrossRef]
- Louette, P.; Bodino, F.; Pireaux, J.J. Poly(ethylene terephthalate) (PET) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2005, 12, 1. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray Photoelectron Spectroscopy Sulfur 2p Study of Organic Thiol and Disulfide Binding Interactions with Gold Surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Yang, D.; Zheng, X.; Wang, Y.; Pan, J.; Zhang, T.; Xu, J.; Qiu, F.; Yan, Y.; et al. A facile strategy toward ion-imprinted hierarchical mesoporous material via dual-template method for simultaneous selective extraction of lithium and rubidium. J. Clean. Prod. 2018, 171, 264–274. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Park, M.J.; Shon, H.K.; Seo, J.G.; Koo, S.; Chung, W.-J. Synthesis and characterization of multi-walled carbon nanotubes-supported dibenzo-14-crown-4 ether with proton ionizable carboxyl sidearm as Li+ adsorbents. Chem. Eng. J. 2015, 264, 89–98. [Google Scholar] [CrossRef]
- Ding, T.; Wu, Q.; Nie, Z.; Zheng, M.; Wang, Y.; Yang, D. Selective recovery of lithium resources in salt lakes by polyacrylonitrile/ion-imprinted polymer: Synthesis, testing, and computation. Polym. Test. 2022, 113, 107647. [Google Scholar] [CrossRef]
- Ding, T.; Zheng, M.; Lin, Y. Adsorption of Li(I) Ions through New High-Performance Electrospun PAN/Kaolin Nanofibers: A Combined Experimental and Theoretical Calculation. ACS Omega 2022, 7, 11430–11439. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, S.; Liu, J.; Zhang, W.; Pan, J. 2-Methylol-12-crown-4 ether immobilized PolyHIPEs toward recovery of lithium(i). New J. Chem. 2018, 42, 16814–16822. [Google Scholar] [CrossRef]
- Hong, H.-J.; Ryu, T.; Park, I.-S.; Kim, M.; Shin, J.; Kim, B.-G.; Chung, K.-S. Highly porous and surface-expanded spinel hydrogen manganese oxide (HMO)/Al2O3 composite for effective lithium (Li) recovery from seawater. Chem. Eng. J. 2018, 337, 455–461. [Google Scholar] [CrossRef]
- Rosly, N.Z.; Abdullah, A.H.; Ahmad Kamarudin, M.; Ashari, S.E.; Alang Ahmad, S.A. Adsorption of Methylene Blue Dye by Calix[6]Arene-Modified Lead Sulphide (Pbs): Optimisation Using Response Surface Methodology. Int. J. Environ. Res. Public Health 2021, 18, 397. [Google Scholar] [CrossRef]
- Hongo, T.; Moriura, M.; Hatada, Y.; Abiko, H. Simultaneous Methylene Blue Adsorption and pH Neutralization of Contaminated Water by Rice Husk Ash. ACS Omega 2021, 6, 21604–21612. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, W.; He, S.; Yu, S.; Chen, Y.; Lu, L.; Shu, Z.; Cui, H.; Zhang, Y.; Jin, H. Rapid adsorption of cationic dye-methylene blue on the modified montmorillonite/graphene oxide composites. Appl. Clay Sci. 2019, 168, 304–311. [Google Scholar] [CrossRef]
- Dhar, L.; Hossain, S.; Rahman, M.S.; Quraishi, S.B.; Saha, K.; Rahman, F.; Rahman, M.T. Adsorption Mechanism of Methylene Blue by Graphene Oxide-Shielded Mg–Al-Layered Double Hydroxide from Synthetic Wastewater. J. Phys. Chem. A 2021, 125, 954–965. [Google Scholar] [CrossRef]
- Wei, S.; Wu, J.; Chen, P.; Fu, B.; Zhu, X.; Chen, M. Integration of Phosphotungstic Acid into Zeolitic Imidazole Framework-67 for Efficient Methylene Blue Adsorption. ACS Omega 2022, 7, 9900–9908. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Pasha, S.S.; Pramanik, S.K.; Alameddine, B. Copolymer networks with contorted units and highly polar groups for ultra-fast selective cationic dye adsorption and iodine uptake. Polymer 2022, 239, 124467. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Wang, H.; Du, Q.; Li, M.; Sun, Y.; Cui, M.; Li, L. Study on the Adsorption Performance of Casein/Graphene Oxide Aerogel for Methylene Blue. ACS Omega 2021, 6, 29243–29253. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.; Phuong, T.; Xuan, N.; Nguyet, D.M.; Quan, T.H.; Anh, T.N.M.; Thinh, D.B.; Tai, L.T.; Lan, N.T.; Trinh, D.N.; et al. Preparing three-dimensional graphene aerogels by chemical reducing method: Investigation of synthesis condition and optimization of adsorption capacity of organic dye. Surf. Interfaces 2021, 23, 101023. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Wang, Y.; Zhang, X.-F.; Yao, J. Construction of a hybrid graphene oxide/nanofibrillated cellulose aerogel used for the efficient removal of methylene blue and tetracycline. J. Phys. Chem. Solids 2021, 150, 109839. [Google Scholar] [CrossRef]
- Cassol, G.O.; Gallon, R.; Schwaab, M.; Barbosa-Coutinho, E.; Júnior, J.B.S.; Pinto, J.C. Statistical Evaluation of Non-Linear Parameter Estimation Procedures for Adsorption Equilibrium Models. Adsorpt. Sci. Technol. 2014, 32, 257–273. [Google Scholar] [CrossRef]
- Xiao, Y.; Azaiez, J.; Hill, J.M. Erroneous Application of Pseudo-Second-Order Adsorption Kinetics Model: Ignored Assumptions and Spurious Correlations. Ind. Eng. Chem. Res. 2018, 57, 2705–2709. [Google Scholar] [CrossRef]

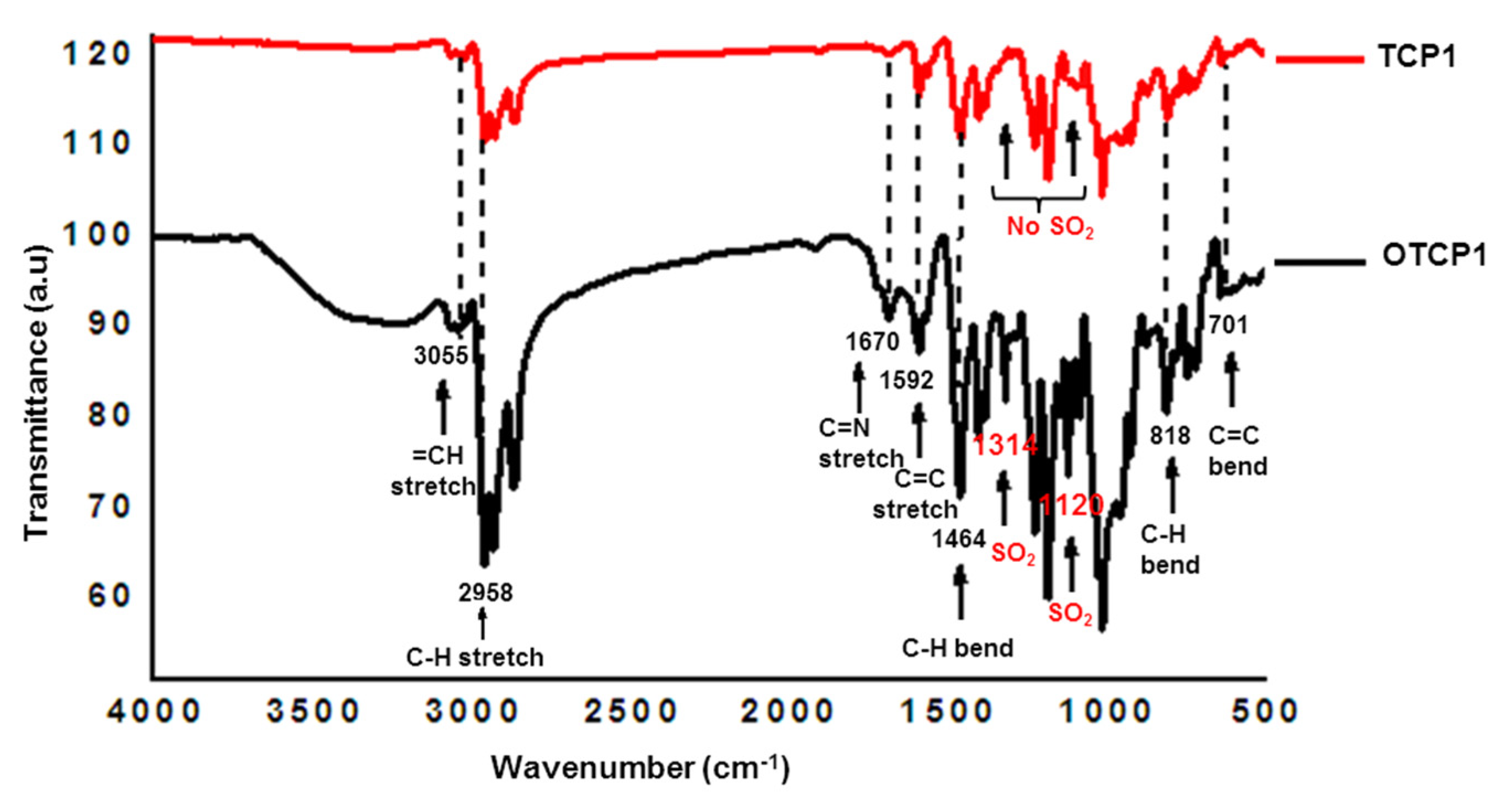
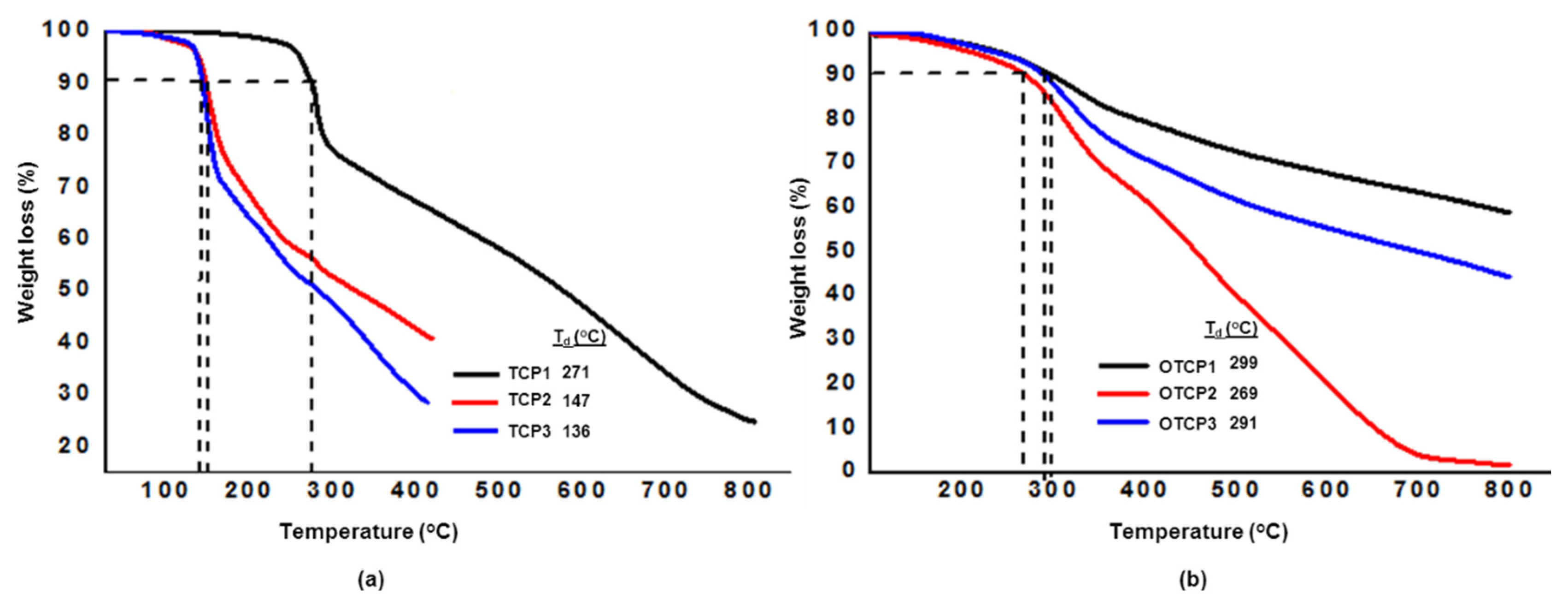
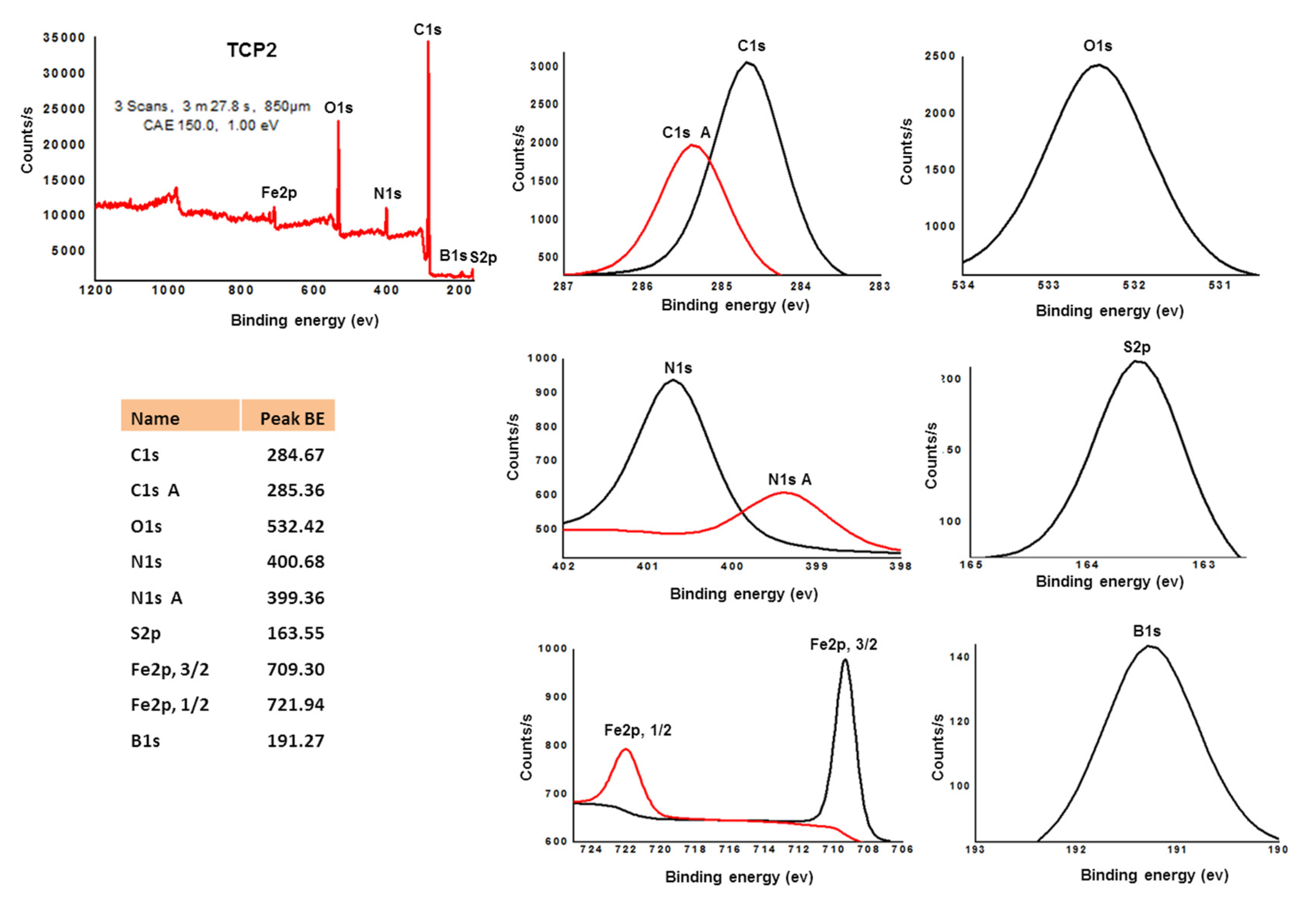
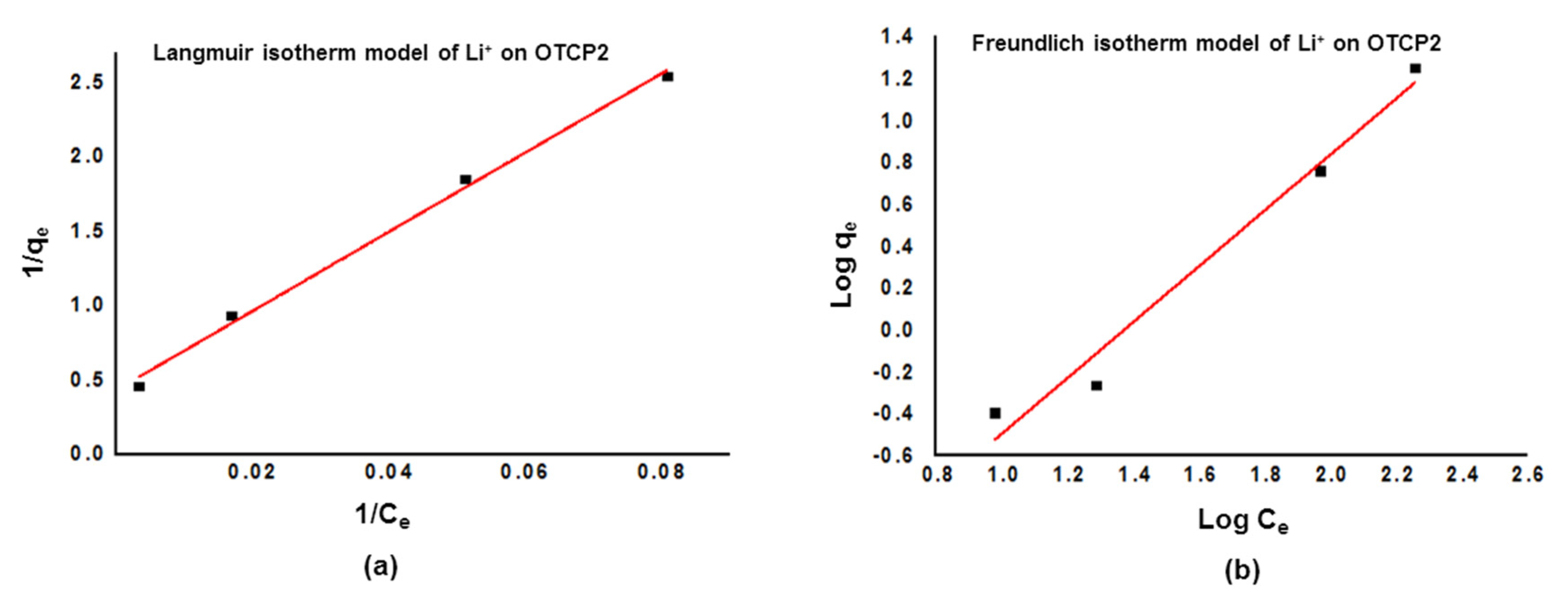
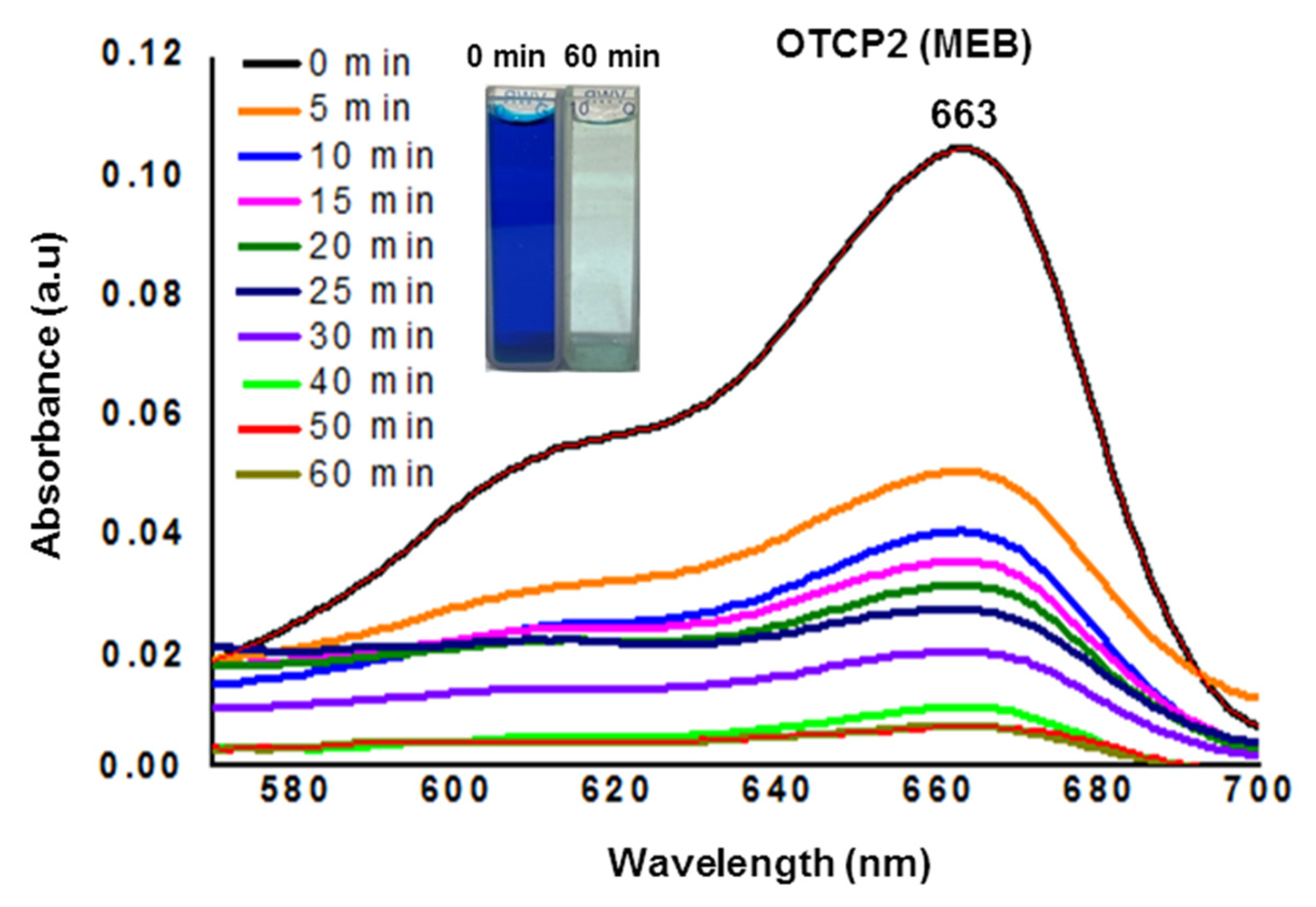

| Entry | Polymer | Mw (g·mol−1) | Mn (g·mol−1) | Đ |
|---|---|---|---|---|
| 1 | TCP1 | 30198 | 10066 | 3.0 |
| 2 | TCP2 | 21404 | 7493 | 2.8 |
| 3 | TCP3 | 21061 | 6315 | 3.3 |
| 4 | OTCP1 | 20017 | 7056 | 2.8 |
| Ion on OTCP2 | Langmuir Isotherm Parameters | Freundlich Isotherm Parameters | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL | R2 | 1/n | KF | R2 | |
| Li+ | 2.31 | 0.01691 | 0.9934 | 1.3302 | 0.1605 | 0.9645 |
| Dye on OTCP2 | Langmuir Isotherm Parameters | Freundlich Isotherm Parameters | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL | R2 | 1/n | KF | R2 | |
| MEB | 480.77 | 0.01508 | 0.9988 | 0.6136 | 3.3211 | 0.9480 |
| Dye on OTCP2 | Pseudo 1st Order Model | Pseudo 2nd Order Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Co (mg L−1) | qe,exp (mg g−1) | qe,cal (mg g−1) | k1 (min−1) | R2 | qe,cal (mg g−1) | k2 (min−1) | R2 | |
| MEB | 50 | 43.41 | 8.88 | −0.001 | 0.4983 | 45.40 | 0.0069 | 0.9919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shetty, S.; Baig, N.; Moustafa, M.S.; Al-Mousawi, S.; Alameddine, B. Synthesis of Metalorganic Copolymers Containing Various Contorted Units and Iron(II) Clathrochelates with Lateral Butyl Chains: Conspicuous Adsorbents of Lithium Ions and Methylene Blue. Polymers 2022, 14, 3394. https://doi.org/10.3390/polym14163394
Shetty S, Baig N, Moustafa MS, Al-Mousawi S, Alameddine B. Synthesis of Metalorganic Copolymers Containing Various Contorted Units and Iron(II) Clathrochelates with Lateral Butyl Chains: Conspicuous Adsorbents of Lithium Ions and Methylene Blue. Polymers. 2022; 14(16):3394. https://doi.org/10.3390/polym14163394
Chicago/Turabian StyleShetty, Suchetha, Noorullah Baig, Moustafa Sherief Moustafa, Saleh Al-Mousawi, and Bassam Alameddine. 2022. "Synthesis of Metalorganic Copolymers Containing Various Contorted Units and Iron(II) Clathrochelates with Lateral Butyl Chains: Conspicuous Adsorbents of Lithium Ions and Methylene Blue" Polymers 14, no. 16: 3394. https://doi.org/10.3390/polym14163394
APA StyleShetty, S., Baig, N., Moustafa, M. S., Al-Mousawi, S., & Alameddine, B. (2022). Synthesis of Metalorganic Copolymers Containing Various Contorted Units and Iron(II) Clathrochelates with Lateral Butyl Chains: Conspicuous Adsorbents of Lithium Ions and Methylene Blue. Polymers, 14(16), 3394. https://doi.org/10.3390/polym14163394