Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor
Abstract
1. Introduction
2. Materials and Methods
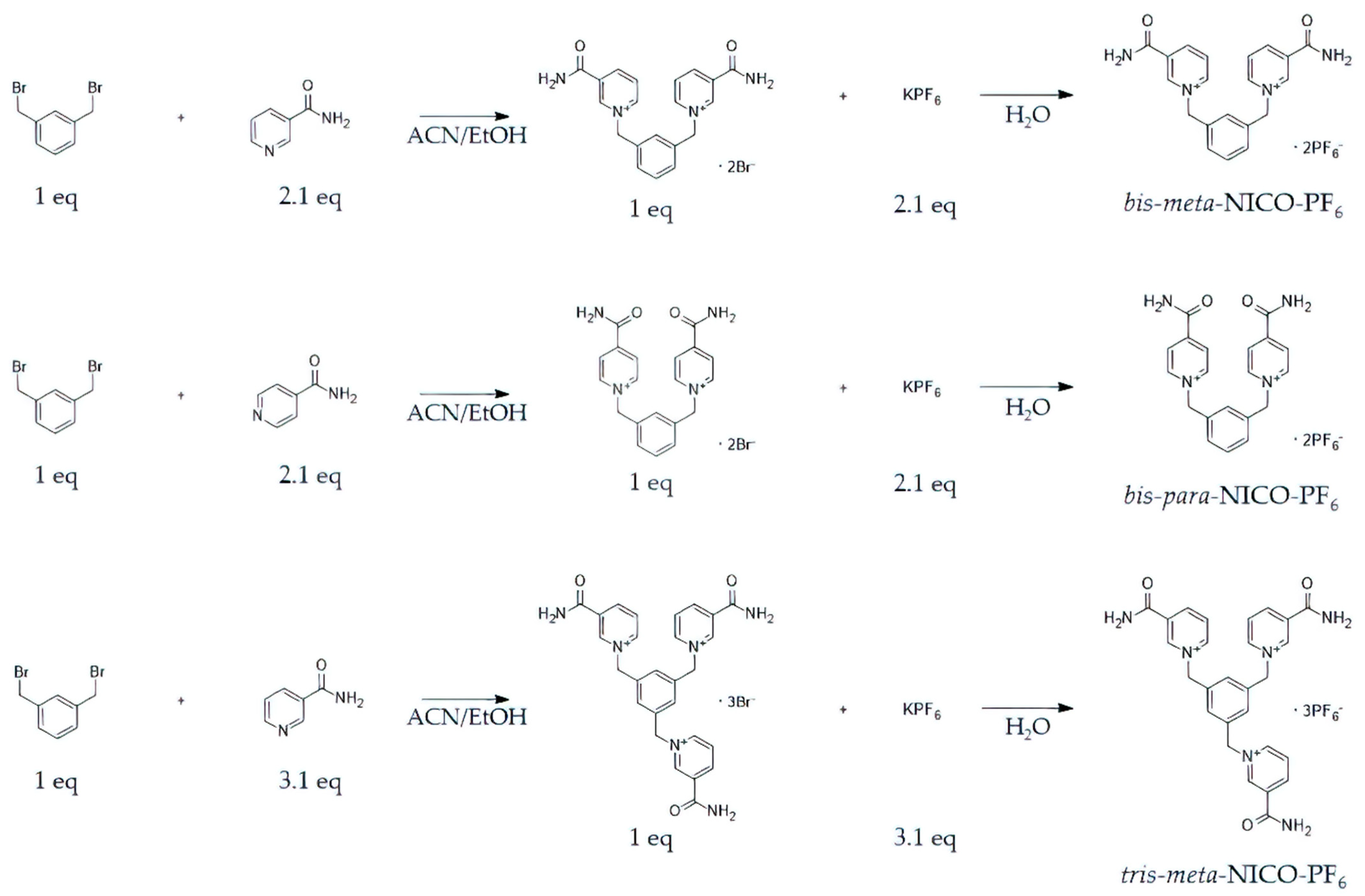
2.1. Synthesis of Ionophores
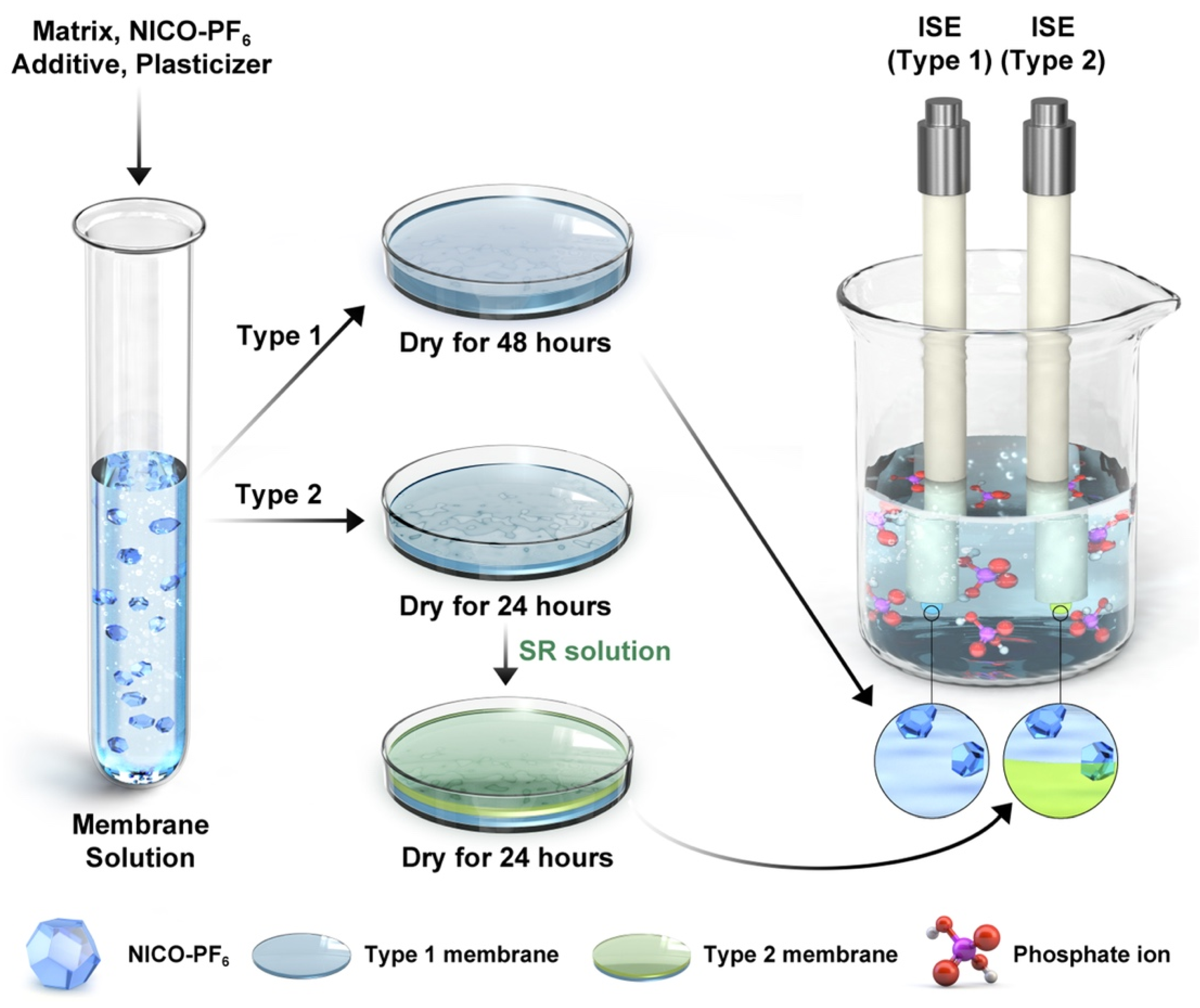
2.2. Membrane
2.3. Characterization
2.4. Electromotive Force (EMF) Measurement
3. Results
3.1. Synthesis of Ionophore and Its Interaction with Phosphate
3.2. Membrane Characteristics
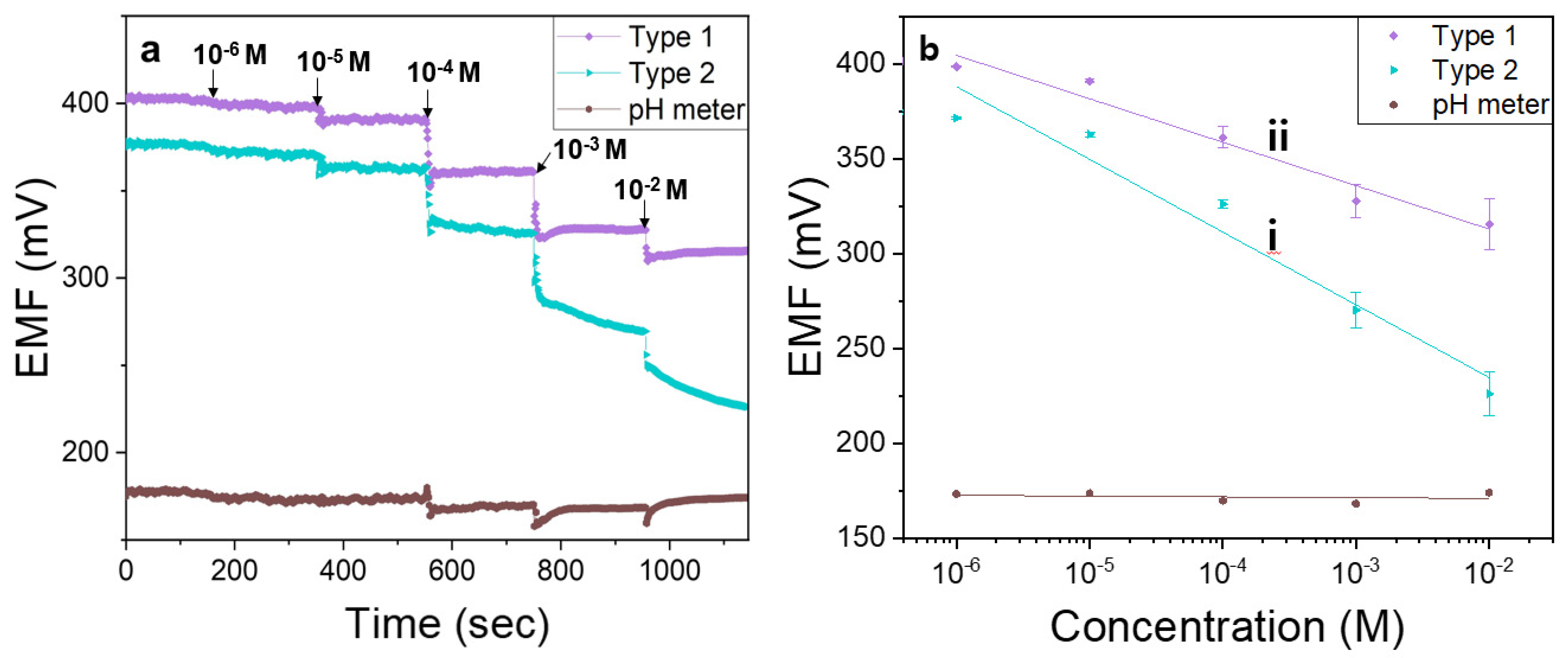
3.3. Evaluation of ISE Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Zhu, B.; Struewing, I.; Xu, N.; Duan, S. Nitrogen–Phosphorus-Associated Metabolic Activities during the Development of a Cyanobacterial Bloom Revealed by Metatranscriptomics. Sci. Rep. 2019, 9, 2480. [Google Scholar] [CrossRef] [PubMed]
- McAdams, H.H. Bacterial Stalks Are Nutrient-Scavenging Antennas. Proc. Natl. Acad. Sci. USA 2006, 103, 11435. [Google Scholar] [CrossRef] [PubMed]
- Litke, D.W. Review of Phosphorus Control Measures in the United States and Their Effects on Water Quality; US Department of the Interior, US Geological Survey: Denver, CO, USA, 1999.
- Sims, J.T.; Goggin, N.; McDermott, J. Nutrient Management for Water Quality Protection: Integrating Research into Environmental Policy. Water Sci. Technol. 1999, 39, 291–298. [Google Scholar] [CrossRef]
- Manuel, J. Nutrient Pollution: A Persistent Threat to Waterways. Environ. Health Perspect. 2014, 122, A304–A309. [Google Scholar] [CrossRef]
- Kivlehan, F.; Mace, W.J.; Moynihan, H.A.; Arrigan, D.W.M. Potentiometric Evaluation of Calix[4]Arene Anion Receptors in Membrane Electrodes: Phosphate Detection. Anal. Chim. Acta 2007, 585, 154–160. [Google Scholar] [CrossRef]
- Chambers, P. Standard Methods for the Examination of Water and Wastewater; Scientific e-Resources: Delhi, India, 2019; ISBN 1-83947-166-2. [Google Scholar]
- Crespo, G.A. Recent Advances in Ion-Selective Membrane Electrodes for in Situ Environmental Water Analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Fiedler, U.; Růžička, J. Selectrode—The Universal Ion-Selective Electrode. Anal. Chim. Acta 1973, 67, 179–193. [Google Scholar] [CrossRef]
- Saleh, M.A.; Ewane, E.; Wilson, B.L. Monitoring the Houston Ship Channel for Inorganic Pollutants by Ion Selective Electrodes, Ion Chromatography, and Inductively Coupled Plasma Spectroscopy. Chemosphere 1999, 39, 2357–2364. [Google Scholar] [CrossRef]
- De Marco, R.; Clarke, G.; Pejcic, B. Ion-Selective Electrode Potentiometry in Environmental Analysis. Electroanalysis 2007, 19, 1987–2001. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Lamb, D.; Megharaj, M.; Naidu, R. Application of Ion Selective Electrode Array to Simultaneously Determinate Multi-Free Ions in Solution. Environ. Technol. Innov. 2019, 15, 100424. [Google Scholar] [CrossRef]
- Warwick, C.; Guerreiro, A.; Soares, A. Sensing and Analysis of Soluble Phosphates in Environmental Samples: A Review. Biosens. Bioelectron. 2013, 41, 1–11. [Google Scholar] [CrossRef]
- Strehlitz, B.; Grundig, B.; Vorlop, K.-D.; Bartholmes, P.; Kotte, H.; Stottmeister, U. Artificial Electron Donors for Nitrate and Nitrite Reductases Usable as Mediators in Amperometric Biosensors. Fresenius J. Anal. Chem. 1994, 349, 676–678. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P. Interactions between Macromolecules and Ions: The Hofmeister Series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Vlachy, N.; Jagoda-Cwiklik, B.; Vácha, R.; Touraud, D.; Jungwirth, P.; Kunz, W. Hofmeister Series and Specific Interactions of Charged Headgroups with Aqueous Ions. Adv. Colloid Interface Sci. 2009, 146, 42–47. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Liu, P.; Xu, X.; Du, D.; Lin, Y. High-Performance Dual-Channel Ratiometric Colorimetric Sensing of Phosphate Ion Based on Target-Induced Differential Oxidase-like Activity Changes of Ce-Zr Bimetal-Organic Frameworks. Sens. Actuators B Chem. 2020, 321, 128546. [Google Scholar] [CrossRef]
- Pinyorospathum, C.; Rattanarat, P.; Chaiyo, S.; Siangproh, W.; Chailapakul, O. Colorimetric Sensor for Determination of Phosphate Ions Using Anti-Aggregation of 2-Mercaptoethanesulfonate-Modified Silver Nanoplates and Europium Ions. Sens. Actuators B Chem. 2019, 290, 226–232. [Google Scholar] [CrossRef]
- Patel, V.; Selvaganapathy, P.R. Enhancing the Sensitivity of Cobalt Based Solid-State Phosphate Sensor Using Electrical Pretreatment. Sens. Actuators B Chem. 2021, 349, 130789. [Google Scholar] [CrossRef]
- Xu, K.; Kitazumi, Y.; Kano, K.; Shirai, O. Phosphate Ion Sensor Using a Cobalt Phosphate Coated Cobalt Electrode. Electrochim. Acta 2018, 282, 242–246. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, Y.; Zhao, G.; Wu, X.; Kan, Y.; Mur, L.; Han, J.; Qin, H. An All-Solid-State Phosphate Electrode with H3PO4 Doped Polyaniline as the Sensitive Layer. Int. J. Electrochem. Sci. 2017, 12, 4677–4691. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, S.; Mao, S.; Wu, X.; Li, Z.; Tao, W.; Jenkinson, I.R. Highly Sensitive and Selective Fluorescent Detection of Phosphate in Water Environment by a Functionalized Coordination Polymer. Water Res. 2019, 163, 114883. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, D.G.; Jeong, B.; Kim, Y.I.; Heo, J.; Lee, H.-K. Reusable and PH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate. Polymers 2022, 14, 190. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Mao, S.; Chen, J. Rapid Detection of Nutrients with Electronic Sensors: A Review. Environ. Sci. Nano 2018, 5, 837–862. [Google Scholar] [CrossRef]
- Modi, N.R.; Patel, B.; Patel, M.B.; Menon, S.K. Novel Monohydrogenphosphate Ion-Selective Polymeric Membrane Sensor Based on Phenyl Urea Substituted Calix[4]Arene. Talanta 2011, 86, 121–127. [Google Scholar] [CrossRef]
- Nishizawa, S.; Yokobori, T.; Kato, R.; Yoshimoto, K.; Kamaishi, T.; Teramae, N. Hydrogen-Bond Forming Ionophore for Highly Efficient Transport of Phosphate Anions across the Nitrobenzene–Water Interface. Analyst 2003, 128, 663–669. [Google Scholar] [CrossRef]
- Borba, A.; Lairion, F.; Disalvo, A.; Fausto, R. Interaction of Nicotinamide and Picolinamide with Phosphatidylcholine and Phosphatidylethanolamine Membranes: A Combined Approach Using Dipole Potential Measurements and Quantum Chemical Calculations. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 2553–2562. [Google Scholar] [CrossRef][Green Version]
- Nikas, I.P.; Paschou, S.A.; Ryu, H.S. The Role of Nicotinamide in Cancer Chemoprevention and Therapy. Biomolecules 2020, 10, 477. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.-H.; Warner, J.C. Synthesis of phenylene 1,3- and 1,44 bis (methylene)-3-carbamoyl pyridinium bromides. Org. Prep. Proced. Int. 2004, 36, 173–177. [Google Scholar] [CrossRef]
- Craggs, A.; Moody, G.J.; Thomas, J.D.R. PVC Matrix Membrane Ion-Selective Electrodes. Construction and Laboratory Experiments. J. Chem. Educ. 1974, 51, 541. [Google Scholar] [CrossRef]
- Joon, N.K.; He, N.; Ruzgas, T.; Bobacka, J.; Lisak, G. PVC-Based Ion-Selective Electrodes with a Silicone Rubber Outer Coating with Improved Analytical Performance. Anal. Chem. 2019, 91, 10524–10531. [Google Scholar] [CrossRef]
- Amemiya, S. 7—Ion-Selective Electrodes. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 261–294. ISBN 978-0-444-51958-0. [Google Scholar]
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Part I. Inorganic Cations (Technical Report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Uspenskaya, E.; Pleteneva, T.; Syroeshkin, A.; Kasymova, I.; Zakharova, N. Development of an Effective Way to Increase the Biological Activity of Nicotinamide—A New Strategy to Protect against Photoageing and Skin Neoplasia. BIO Web Conf. 2020, 22, 01005. [Google Scholar] [CrossRef]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A.K. FTIR Studies of PVC/PMMA Blend Based Polymer Electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Ding, X.; Ren, C.; Li, W.; Wu, H.; Yang, H. Preparation of Magnetic Porous NiFe2O4/SiO2 Composite Xerogels for Potential Application in Adsorption of Ce(Iv) Ions from Aqueous Solution. RSC Adv. 2017, 7, 16513–16523. [Google Scholar] [CrossRef]
- Kleinsmann, A.J.; Weckenmann, N.M.; Nachtsheim, B.J. Phosphate-Triggered Self-Assembly of N-[(Uracil-5-Yl)Methyl]Urea: A Minimalistic Urea-Derived Hydrogelator. Chem. Eur. J. 2014, 20, 9753–9761. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Hu, K.; Li, Y.; Feng, J.; Shi, J.; Gu, J. Rapid and Specific Aqueous-Phase Detection of Nitroaromatic Explosives with Inherent Porphyrin Recognition Sites in Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2015, 7, 11956–11964. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, T.; Zhu, J.; Qin, Y.; Jiang, D. Polymer–Multiwall Carbon Nantubes Composites for Durable All Solid-Contact H2PO4−-Selective Electrodes. Sens. Actuators B Chem. 2015, 219, 100–104. [Google Scholar] [CrossRef]
- Jiang, H.; Ali, M.A.; Jiao, Y.; Yang, B.; Dong, L. In-Situ, Real-Time Monitoring of Nutrient Uptake on Plant Chip Integrated with Nutrient Sensor. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 289–292. [Google Scholar]
- Kim, H.J.; Hummel, J.W.; Sudduth, K.A.; Birrell, S.J. Evaluation of Phosphate Ion-Selective Membranes and Cobalt-Based Electrodes for Soil Nutrient Sensing. Trans. ASABE 2007, 50, 415–425. [Google Scholar] [CrossRef]
- Sasaki, S. Organic Tin Compounds Combined with Anionic Additives—An Ionophore System Leading to a Phosphate Ion-Selective Electrode? Talanta 2004, 63, 131–134. [Google Scholar] [CrossRef]



| Linear Range(M) | Slope (mV/decade) | LOD (μM) | Stability(Day) | Selectivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH3 COO− | Cl− | NO3− | Br− | SO42− | HCO3− | |||||
| ref [40] | 10−6–10−1 | −59.0 | 1.6 | 4.8 | - | −2.9 | −2.1 | −2.5 | −3.9 | - |
| ref [41] | 10−6–10−1 | −55.7 | 2.14 | - | - | −2.37 | - | - | −2.52 | −2.31 |
| ref [42] | 10−5–10−1 | −37.2 | 10 | 20 | - | 1.82 | −2.10 | 2.04 | - | −1.67 |
| ref [43] | 10−4–10−1 | −60.0 | 70 | - | - | −1.5 | −2.5 | −1.4 | −2.3 | −0.3 |
| Type 1 * | 10−6–10−2 | −23.5 | 1.54 | 30 | −6.09 | 0.02 | 1.71 | - | −0.68 | - |
| Type 2 * | 10−6–10−2 | −53.3 | 0.85 | 40 | −16.3 | −1.32 | −0.06 | - | −2.23 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, B.; Oh, J.S.; Kim, D.Y.; Kim, D.G.; Kim, Y.I.; Heo, J.; Lee, H.-K. Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor. Polymers 2022, 14, 3392. https://doi.org/10.3390/polym14163392
Jeong B, Oh JS, Kim DY, Kim DG, Kim YI, Heo J, Lee H-K. Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor. Polymers. 2022; 14(16):3392. https://doi.org/10.3390/polym14163392
Chicago/Turabian StyleJeong, Bongjin, Jin Seong Oh, Do Yeob Kim, Dong Gyu Kim, Young Il Kim, Jungseok Heo, and Hyung-Kun Lee. 2022. "Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor" Polymers 14, no. 16: 3392. https://doi.org/10.3390/polym14163392
APA StyleJeong, B., Oh, J. S., Kim, D. Y., Kim, D. G., Kim, Y. I., Heo, J., & Lee, H.-K. (2022). Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor. Polymers, 14(16), 3392. https://doi.org/10.3390/polym14163392








