Covalent Organic Framework (COF): A Drug and Carrier to Attenuate Retinal Ganglion Cells Death in an Acute Glaucoma Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
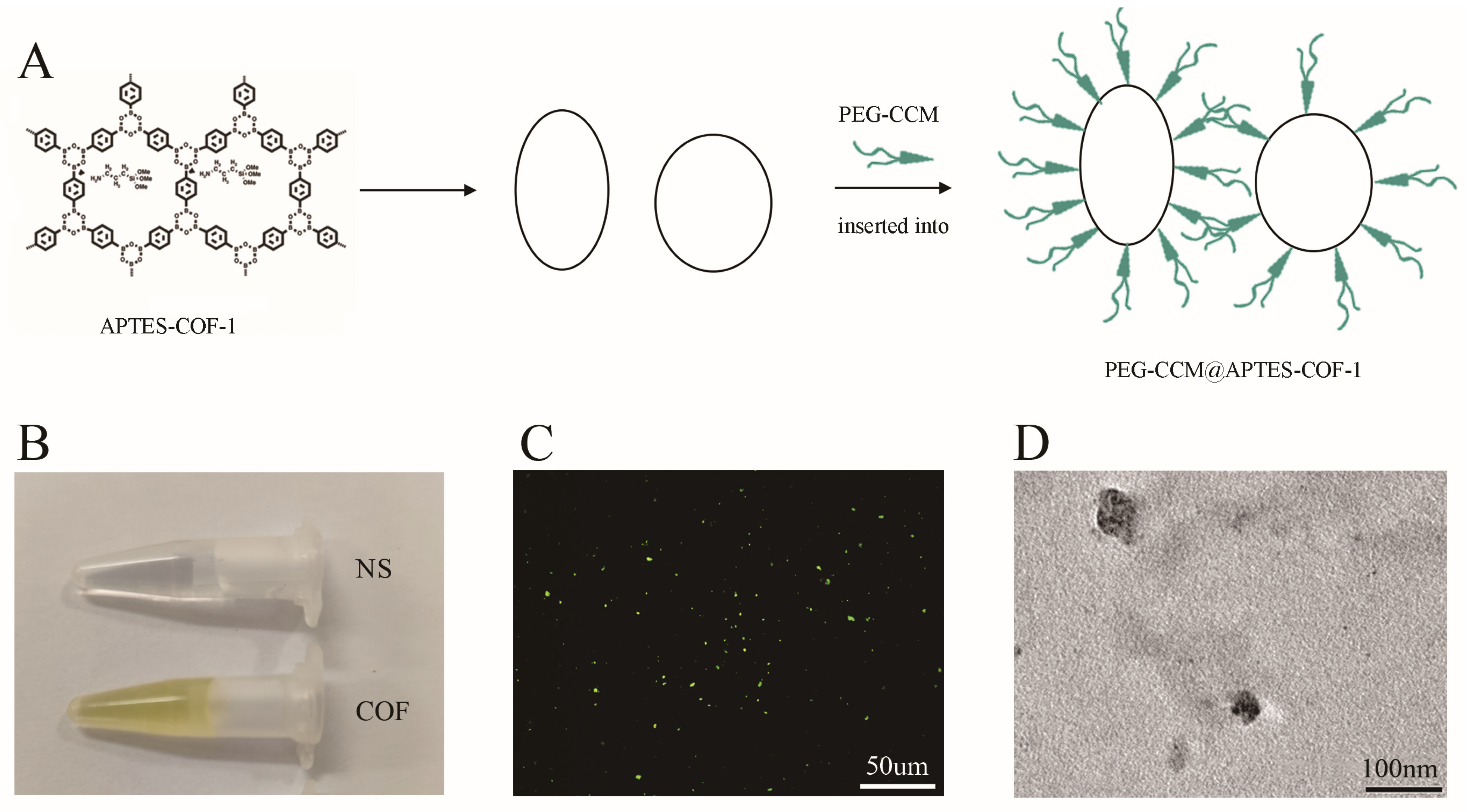
2.1. Synthesis and Self-Assembly of APTES-COF-1@Rapamycin/Null and PEG-CCM
2.2. Transmission Electron Microscopy (TEM)
2.3. Cell Culture and Cell Viability
2.4. Animals
2.5. Acute Ocular Hypertension (AOH) Glaucomatous Model
2.6. Intravitreal Administration
2.7. RGC Labelling and Quantification
2.8. Electroretinogram (ERG)
2.9. Immunofluorescence and Immunohistochemistry
2.10. Real-Time Quantitative PCR (qPCR)
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of COF Nanoparticles
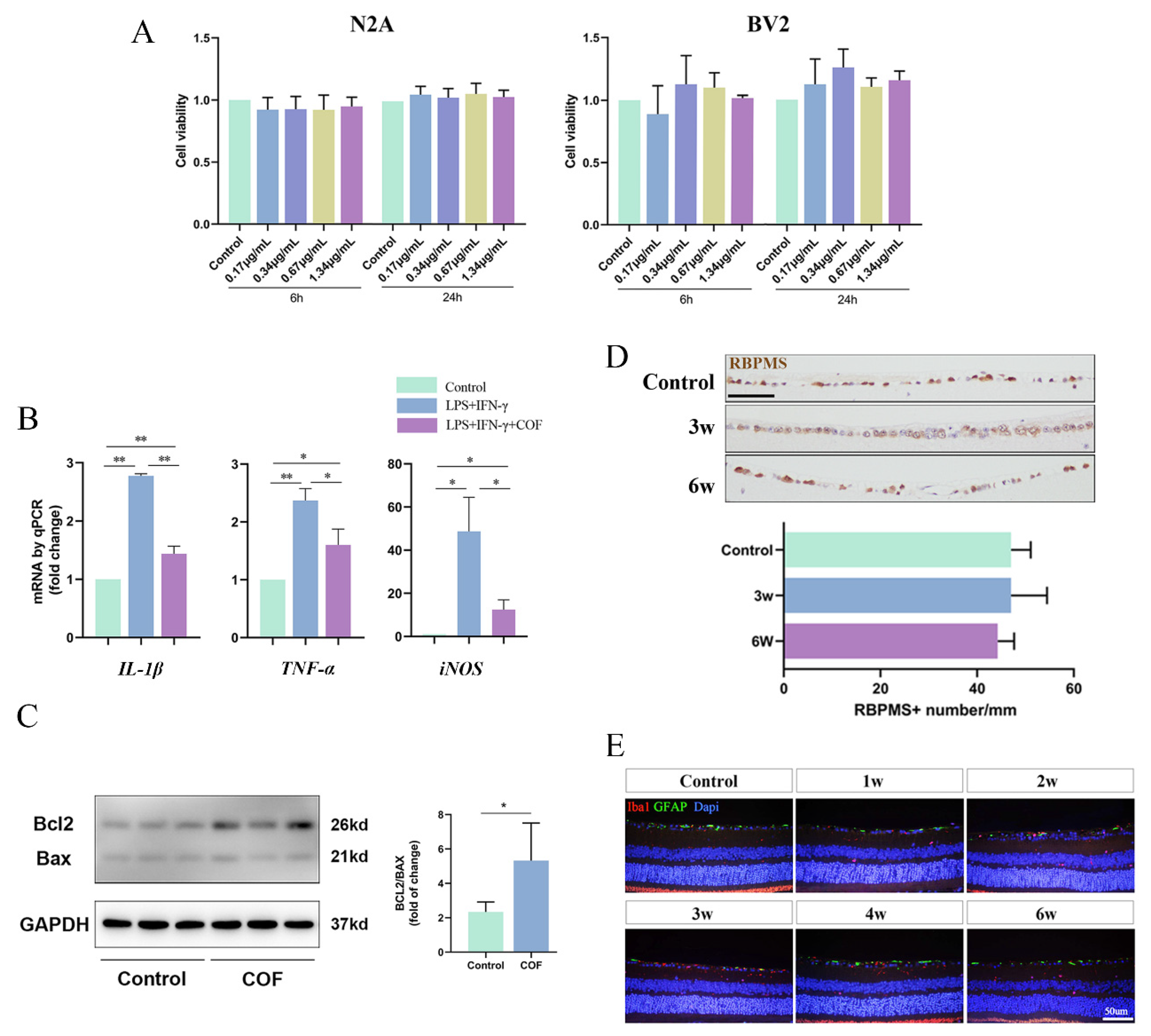
3.2. The Safety and Effect of COF Delivered In Vitro and In Vivo
3.3. COF Could Efficiently Attenuate RGC Death, Increase Phototransduction Function and Alleviate the Overactivation of Microglia after Retinal I/R Injury
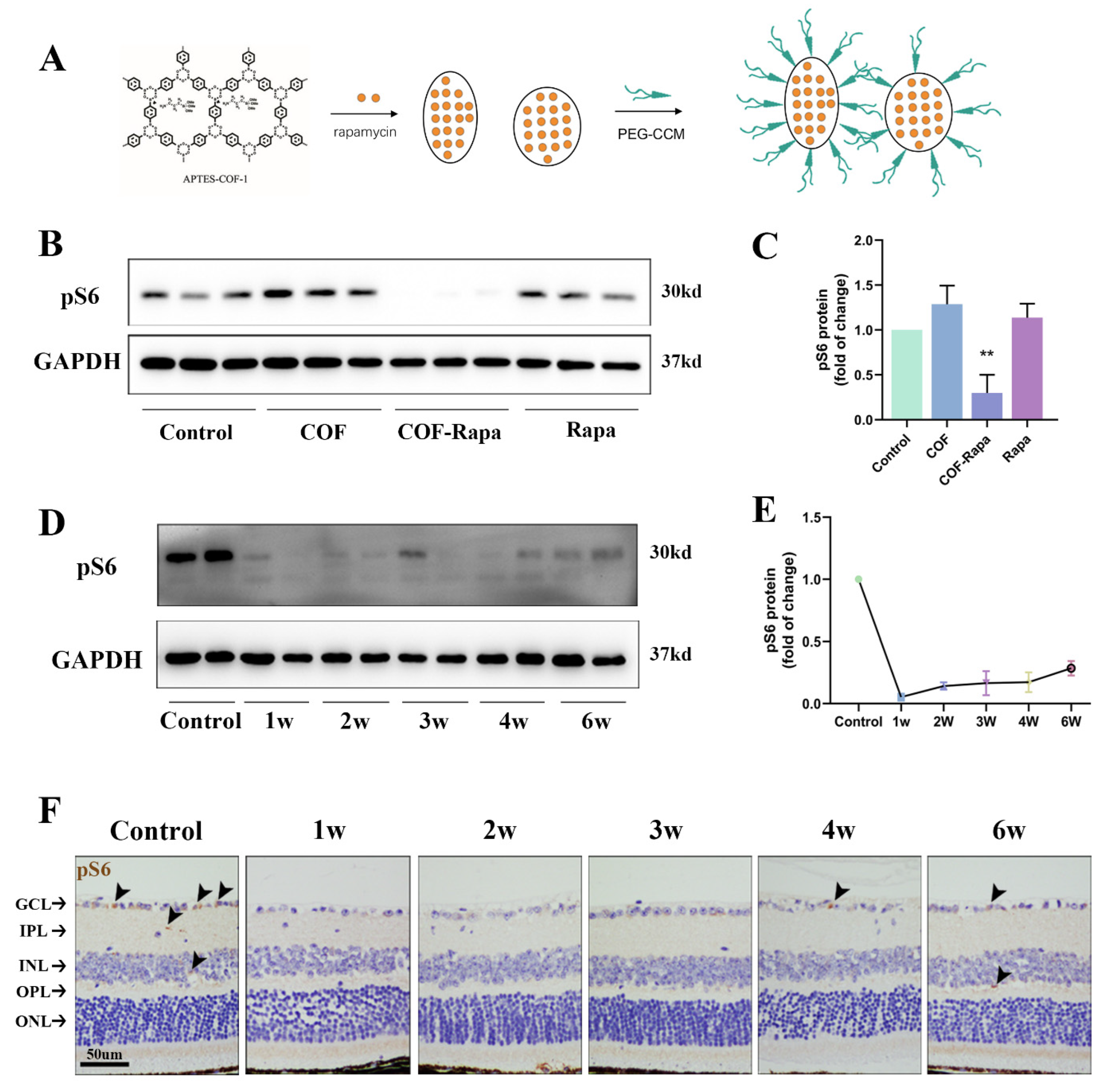
3.4. Construction of COF-Rapamycin Nanoparticles: Single Intravitreal Injection, Long-Lasting Effect
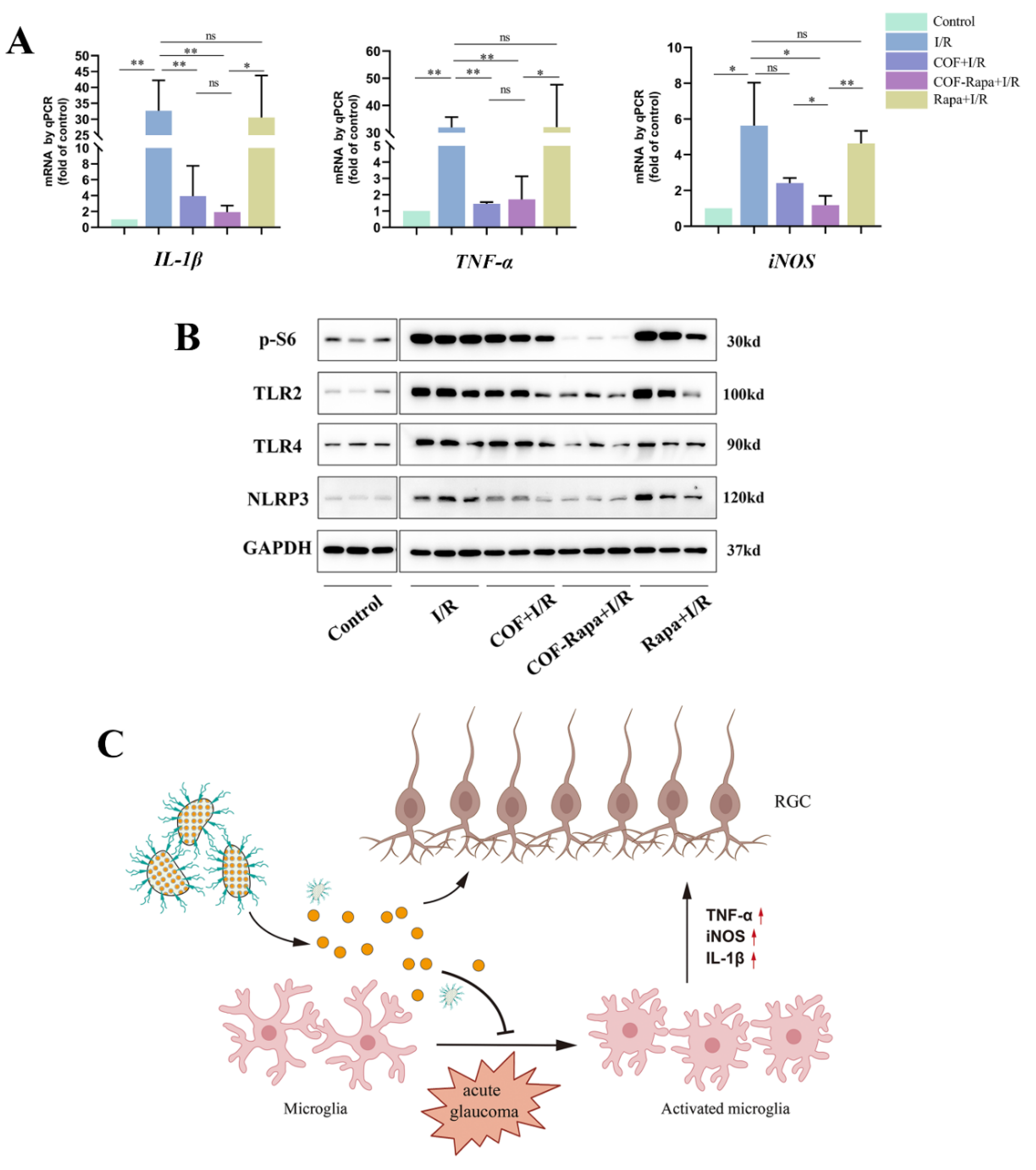
3.5. COF-Rapa Could Efficiently Alleviate the Inflammatory Reaction in Retinal I/R Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGC | Retinal ganglion cell |
| IOP | Intraocular pressure |
| I/R | Ischemia–reperfusion |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| COF | Covalent organic frameworks |
| AOH | Acute ocular hypertension |
| FG | Fluoro-Gold |
| pS6 | Phospho-S6 ribosomal protein |
| IL-1β | Interleukin-1β |
| iNOS | Inducible nitric oxide synthase |
| TNF-α | Tumor necrosis factor-α |
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, N.; Chen, X.; Krishna, R.; Jiang, D. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. Int. Ed. Engl. 2015, 54, 2986–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulzer, C.R.; Shen, L.; Bisbey, R.P.; McKone, J.R.; Zhang, N.; Abruña, H.D.; Dichtel, W.R. Superior Charge Storage and Power Density of a Conducting Polymer-Modified Covalent Organic Framework. ACS Cent. Sci. 2016, 2, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 2014, 5, 4503. [Google Scholar] [CrossRef]
- Bai, L.; Phua, S.Z.F.; Lim, W.Q.; Jana, A.; Luo, Z.; Tham, H.P.; Zhao, L.; Gao, Q.; Zhao, Y. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 2016, 52, 4128–4131. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Wang, J.; Gu, S.; Kaspar, R.B.; Zhuang, Z.; Zheng, J.; Guo, H.; Qiu, S.; Yan, Y. 3D Porous Crystalline Polyimide Covalent Organic Frameworks for Drug Delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Liao, Q.; Liu, Y.; Xi, K.; Huang, W.; Jia, X. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018, 9, 2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Li, Z.; Jia, Y.; Zhuo, Y. Rapamycin is neuroprotective in a rat chronic hypertensive glaucoma model. PLoS ONE 2019, 14, e0213489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nazio, F.; Tettamanti, G.; Girardello, R.; Cianfanelli, V.; Cavaliere, F.; Morrone, L.A.; Corasaniti, M.T.; et al. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 2018, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.M.; Guymer, C.; Wood, J.P.M.; Daskalaki, E.; Chidlow, G.; Zhang, C.; Balasubramanian, R.; Cardozo, B.H.; Foxworth, N.E.; Deering, K.E.; et al. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or rapamycin. Proc. Natl. Acad. Sci. USA 2020, 117, 33619–33627. [Google Scholar] [CrossRef]
- Doshi, R.R.; Bakri, S.J.; Fung, A.E. Intravitreal injection technique. Semin. Ophthalmol. 2011, 26, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sampat, K.M.; Garg, S.J. Complications of intravitreal injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.-S.; Feng, Q.; Lo, A.C.Y.; Chang, R.C.-C.; Lin, B.; Chung, S.K.; So, K.-F. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS ONE 2012, 7, e45469. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, Y.; Zhang, H. P16INK4a upregulation mediated by TBK1 induces retinal ganglion cell senescence in ischemic injury. Cell Death Dis. 2017, 8, e2752. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Zhao, Y.; Jin, P.; Lou, X.; Luo, Z.; Zhang, H.; Li, F. Involvement of the NLRC4 inflammasome in promoting retinal ganglion cell death in an acute glaucoma mouse model. Exp. Eye Res. 2021, 203, 108388. [Google Scholar] [CrossRef]
- Osborne, A.; Khatib, T.Z.; Songra, L.; Barber, A.C.; Hall, K.; Kong, G.Y.; Widdowson, P.S.; Martin, K.R. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018, 9, 1007. [Google Scholar] [CrossRef] [Green Version]
- Tsujinaka, H.; Fu, J.; Shen, J.; Yu, Y.; Hafiz, Z.; Kays, J.; McKenzie, D.; Cardona, D.; Culp, D.; Peterson, W.; et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat. Commun. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-L.; Huang, S.-P.; Chang, C.-H.; Lin, K.-H.; Sheu, M.-M.; Tsai, R.-K. Factors Influencing the Retrograde Labeling of Retinal Ganglion Cells with Fluorogold in an Animal Optic Nerve Crush Model. Ophthalmic Res. 2014, 51, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.E.; Schlamp, C.L.; Nickells, R.W. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin. Eye Res. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, R.M.; Moskowitz, A.; Akula, J.; Fulton, A.B. The neural retina in retinopathy of prematurity. Prog. Retin. Eye Res. 2017, 56, 32–57. [Google Scholar] [CrossRef] [Green Version]
- Silverman, S.M.; Wong, W.T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, M.; Bai, Y.; Huang, L.; Yu, W.; Bian, Z.; Zhao, M.; Li, X. Retinal ischemia/reperfusion injury is mediated by Toll-like receptor 4 activation of NLRP3 inflammasomes. Investig. Opthalmol. Vis. Sci. 2014, 55, 5466–5475. [Google Scholar] [CrossRef]
- Dumas, A.A.; Pomella, N.; Rosser, G.; Guglielmi, L.; Vinel, C.; Millner, T.O.; Rees, J.; Aley, N.; Sheer, D.; Wei, J.; et al. Microglia promote glioblastoma via mTOR-mediated immunosuppression of the tumour microenvironment. EMBO J. 2020, 39, e103790. [Google Scholar] [CrossRef] [PubMed]
- Mulfaul, K.; Ozaki, E.; Fernando, N.; Brennan, K.; Chirco, K.R.; Connolly, E.; Greene, C.; Maminishkis, A.; Salomon, R.G.; Linetsky, M.; et al. Toll-like Receptor 2 Facilitates Oxidative Damage-Induced Retinal Degeneration. Cell Rep. 2020, 30, 2209–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmitriev, A.V.; Henderson, D.; Linsenmeier, R.A. Diabetes Alters pH Control in Rat Retina. Investig. Ophthalmol. Vis. Sci. 2019, 60, 723–730. [Google Scholar] [CrossRef]
- Lin, L.-T.; Chen, J.-T.; Tai, M.-C.; Chen, Y.-H.; Chen, C.-L.; Pao, S.-I.; Hsu, C.-R.; Liang, C.-M. Protective effects of hypercapnic acidosis on Ischemia-reperfusion-induced retinal injury. PLoS ONE 2019, 14, e0211185. [Google Scholar] [CrossRef] [Green Version]
- Birol, G.; Budzynski, E.; Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal arterial occlusion leads to acidosis in the cat. Exp. Eye Res. 2005, 80, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; He, T.; Yang, J.; Yang, N.; Li, Z.; Xing, Y. SIRT1 is required for the neuroprotection of resveratrol on retinal ganglion cells after retinal ischemia-reperfusion injury in mice. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Khan, R.S.; Lee, V.; Dine, K.; Wu, W.; Shindler, K.S. SIRT1 promotes RGC survival and delays loss of function following optic nerve crush. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5097–5102. [Google Scholar] [CrossRef]
- Cho, S.-H.; Chen, J.A.; Sayed, F.; Ward, M.E.; Gao, F.; Nguyen, T.A.; Krabbe, G.; Sohn, P.D.; Lo, I.; Minami, S.; et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. J. Neurosci. 2015, 35, 807–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Wang, X.; Zhu, Y.; Dong, X.; Liu, C.; Zhang, H.; Liu, L.; Huang, S.; Chen, L. Rapamycin ameliorates cadmium-induced activation of MAPK pathway and neuronal apoptosis by preventing mitochondrial ROS inactivation of PP2A. Neuropharmacology 2016, 105, 270–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.-G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuss, N.D.; Pierson, M.J.; Roehrich, H.; McPherson, S.W.; Gram, A.L.; Li, L.; Gregerson, D.S. Optic nerve as a source of activated retinal microglia post-injury. Acta Neuropathol. Commun. 2018, 6, 19–66. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Sun, S.-W.; Naismith, R.T.; Snyder, A.Z.; Cross, A.; Song, S.-K. Assessing optic nerve pathology with diffusion MRI: From mouse to human. NMR Biomed. 2008, 21, 928–940. [Google Scholar] [CrossRef] [Green Version]


| Antigen | Species | Supplier | Catalog Number | Dilution (WB) | Dilution (IF/IHC) |
|---|---|---|---|---|---|
| Bcl2 | Rabbit | Proteintech | 26593-1-AP | 1:1000 | - |
| BAX | Rabbit | Proteintech | 50599-2-Ig | 1:1000 | - |
| pS6 | Rabbit | CST | 5364 | 1:1000 | 1:500 |
| RBPMS | Rabbit | Abcam | ab194213 | - | 1:800 |
| caspase3 | Rabbit | CST | 9662 | 1:1000 | - |
| Iba1 | Rabbit | Abcam | Ab178846 | - | 1:800 |
| TLR2 | Rabbit | Abcam | Ab209217 | 1:1000 | - |
| TLR4 | Rabbit | Sevicebio | GB11519 | 1:1000 | - |
| NLRP3 | Rabbit | Boster | BM4490 | 1:200 | - |
| GAPDH | Mouse | Proteintech | 600004-1-Ig | 1:10,000 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, K.; Liang, X.; Zhang, G.; Rong, Y.; Zhang, Q.; Liao, Q.; Zhang, H.; Xi, K.; Wang, J. Covalent Organic Framework (COF): A Drug and Carrier to Attenuate Retinal Ganglion Cells Death in an Acute Glaucoma Mouse Model. Polymers 2022, 14, 3265. https://doi.org/10.3390/polym14163265
Yao K, Liang X, Zhang G, Rong Y, Zhang Q, Liao Q, Zhang H, Xi K, Wang J. Covalent Organic Framework (COF): A Drug and Carrier to Attenuate Retinal Ganglion Cells Death in an Acute Glaucoma Mouse Model. Polymers. 2022; 14(16):3265. https://doi.org/10.3390/polym14163265
Chicago/Turabian StyleYao, Ke, Xin Liang, Guiyang Zhang, Yan Rong, Qiuxiang Zhang, Qiaobo Liao, Hong Zhang, Kai Xi, and Junming Wang. 2022. "Covalent Organic Framework (COF): A Drug and Carrier to Attenuate Retinal Ganglion Cells Death in an Acute Glaucoma Mouse Model" Polymers 14, no. 16: 3265. https://doi.org/10.3390/polym14163265
APA StyleYao, K., Liang, X., Zhang, G., Rong, Y., Zhang, Q., Liao, Q., Zhang, H., Xi, K., & Wang, J. (2022). Covalent Organic Framework (COF): A Drug and Carrier to Attenuate Retinal Ganglion Cells Death in an Acute Glaucoma Mouse Model. Polymers, 14(16), 3265. https://doi.org/10.3390/polym14163265






