Fabrication, Property and Application of Calcium Alginate Fiber: A Review
Abstract
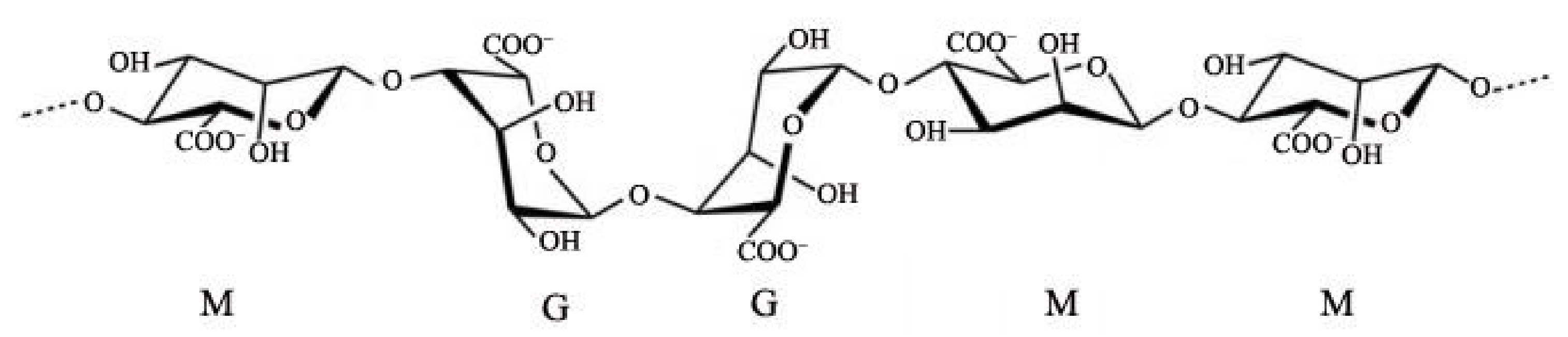
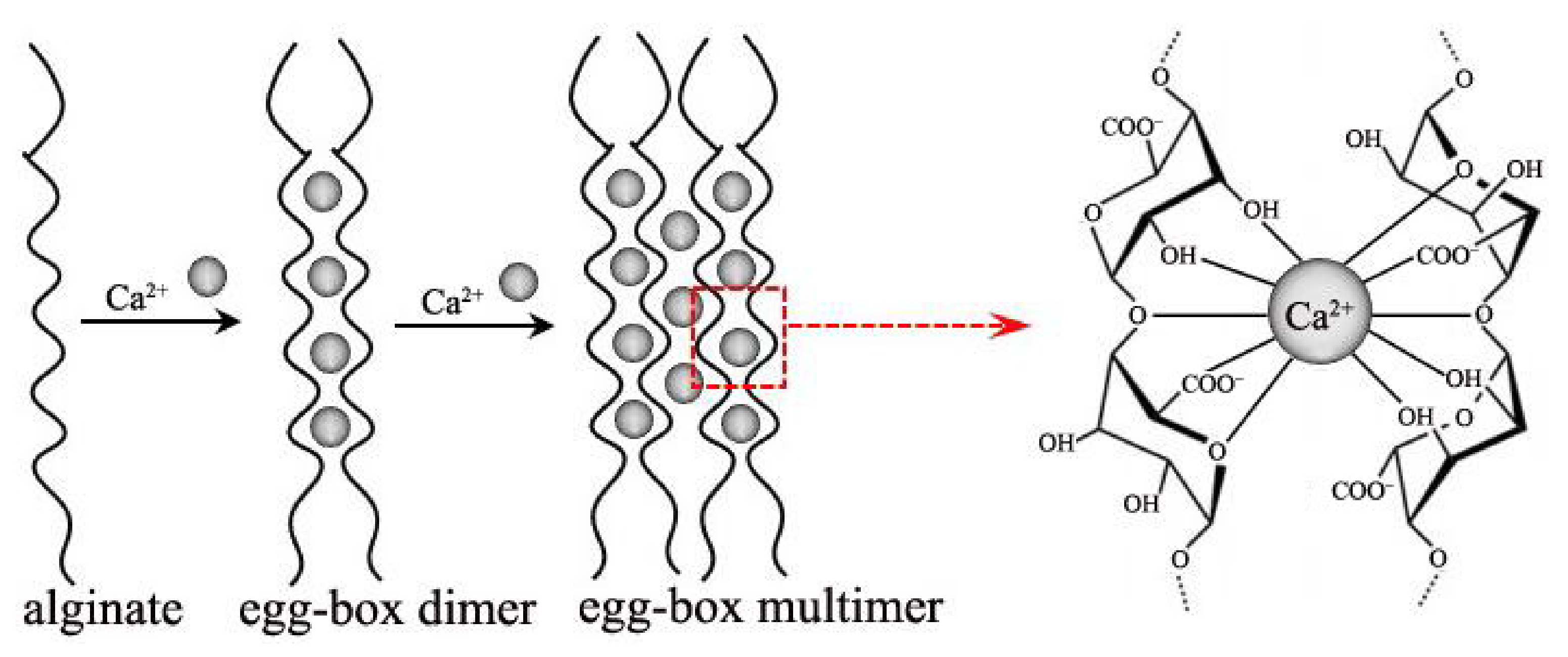
:1. Introduction
2. Preparation Method of Calcium Alginate Fiber
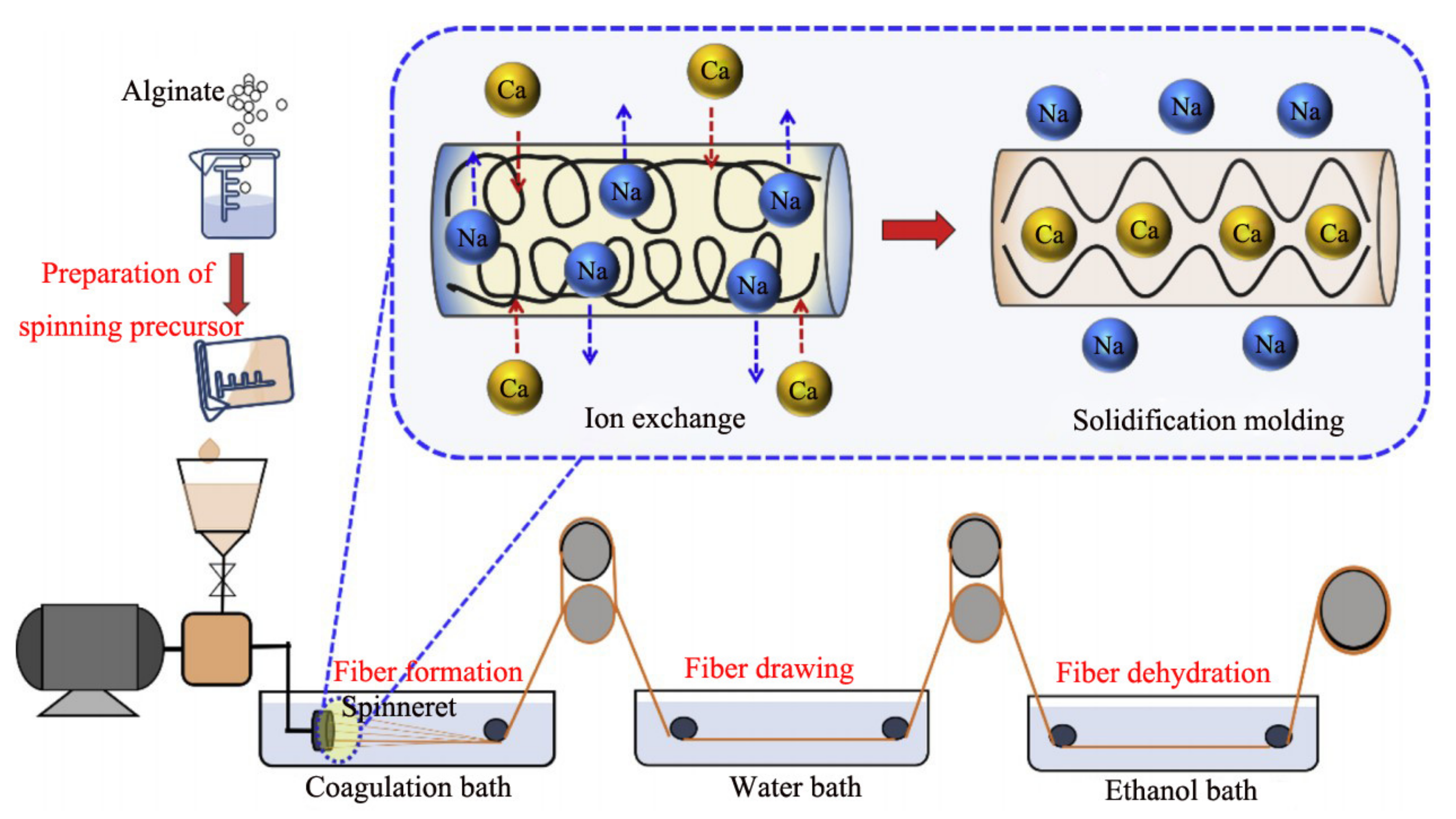
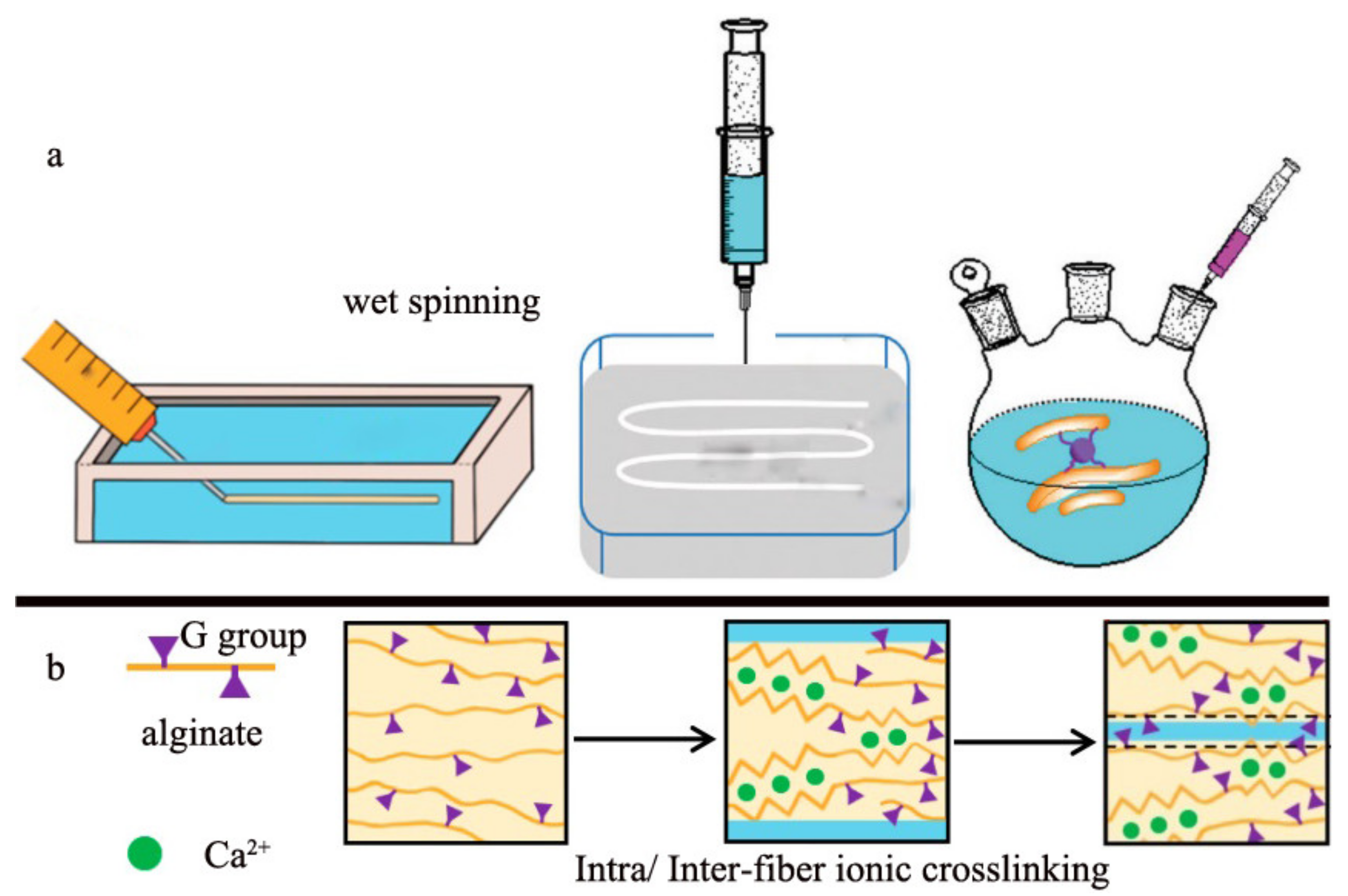
2.1. Wet Spinning
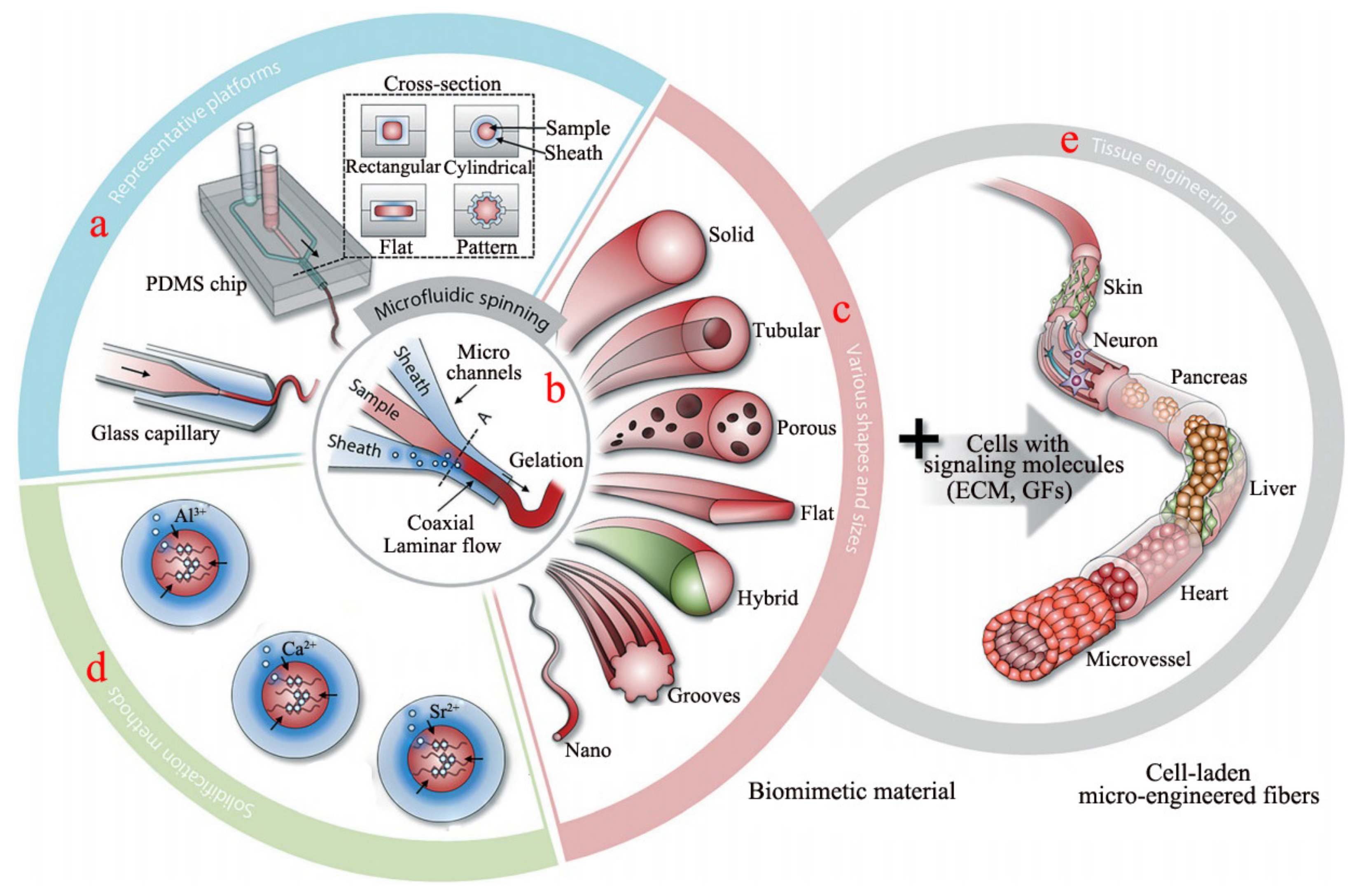
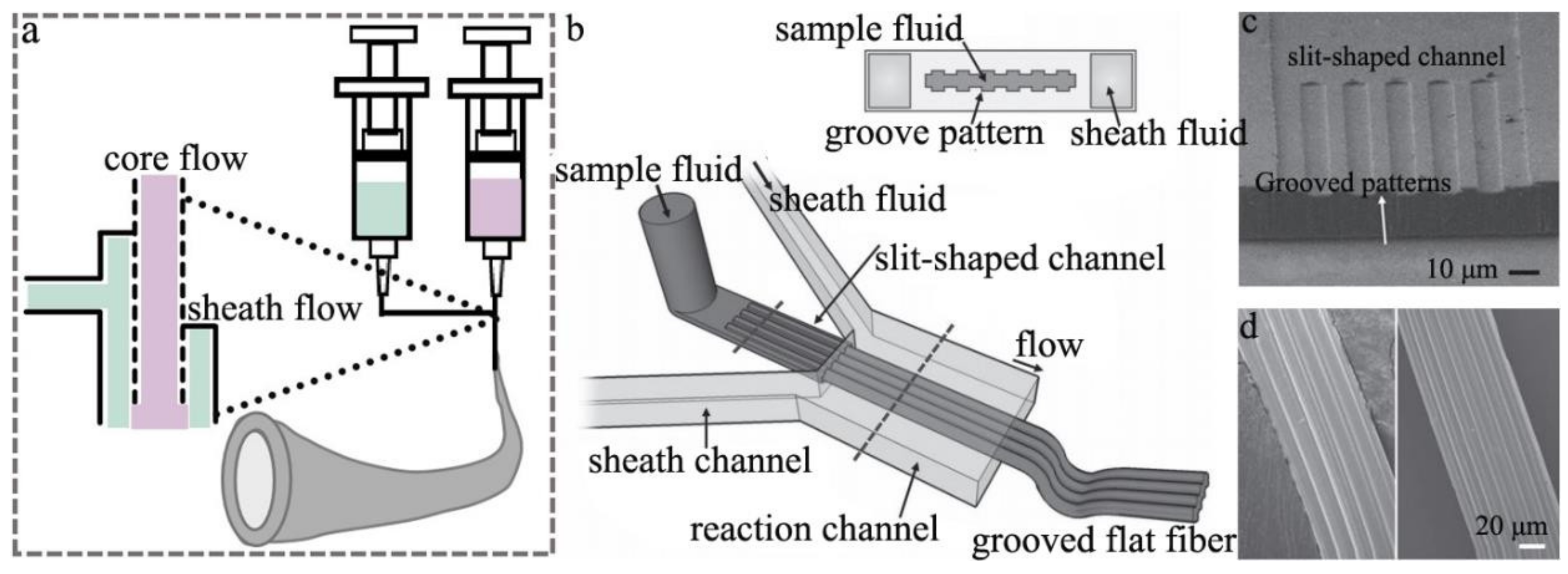
2.2. Microfluidic Spinning
3. Physicochemical Properties of Calcium Alginate Fiber
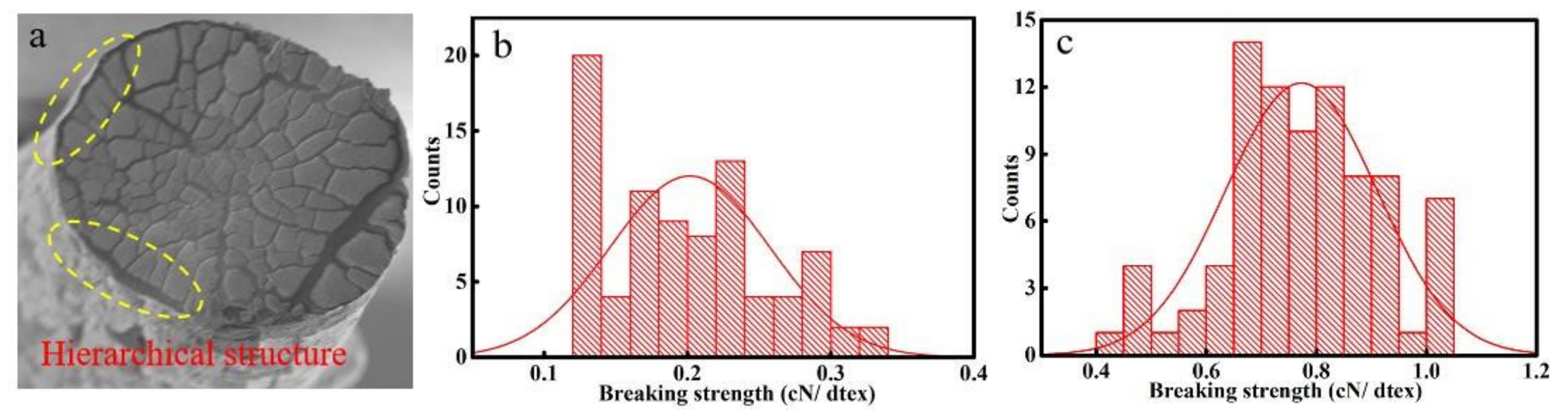
3.1. Mechanical Performance
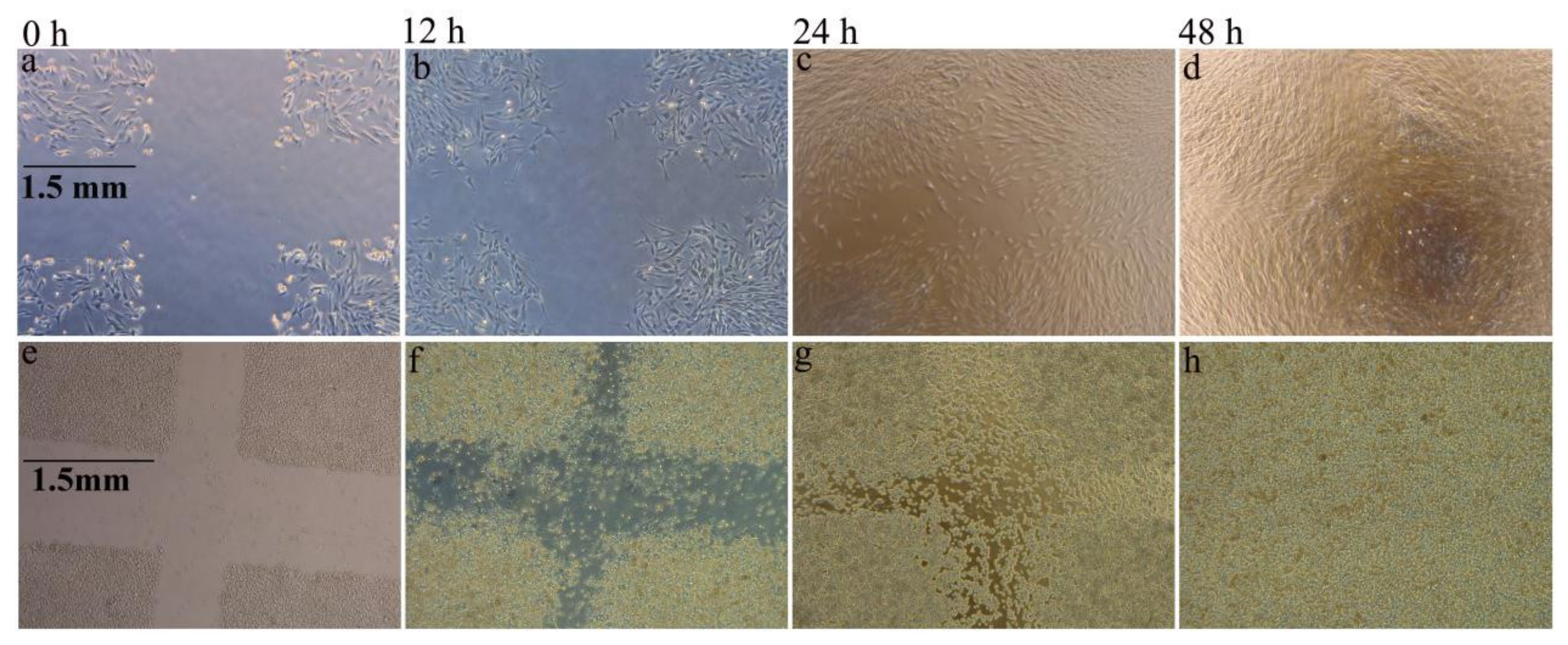
3.2. Moisture Absorption and Biological Compatibility Performance
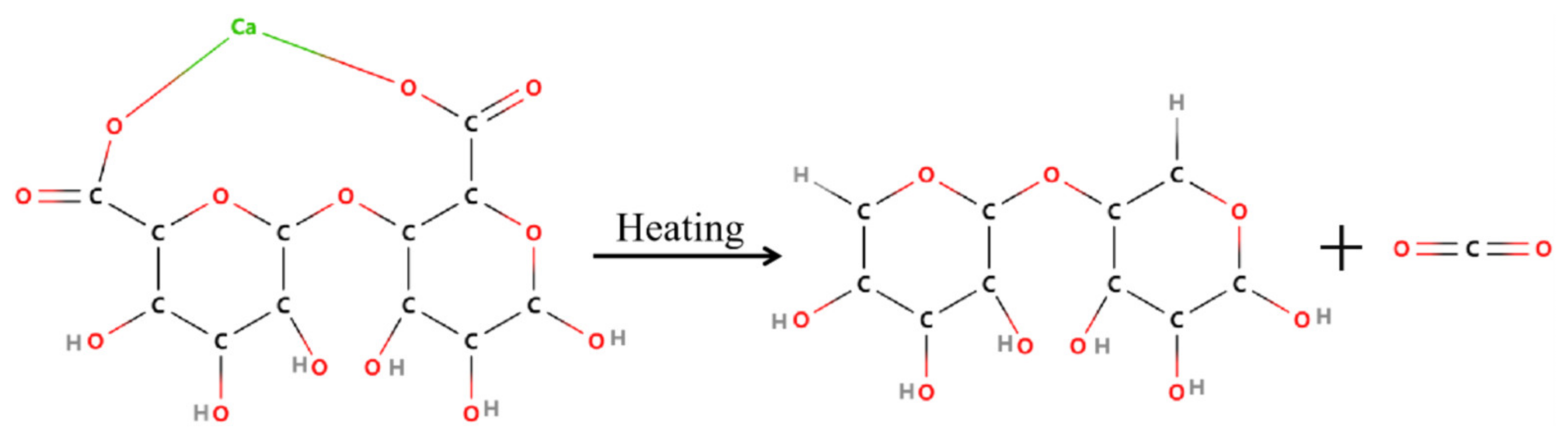
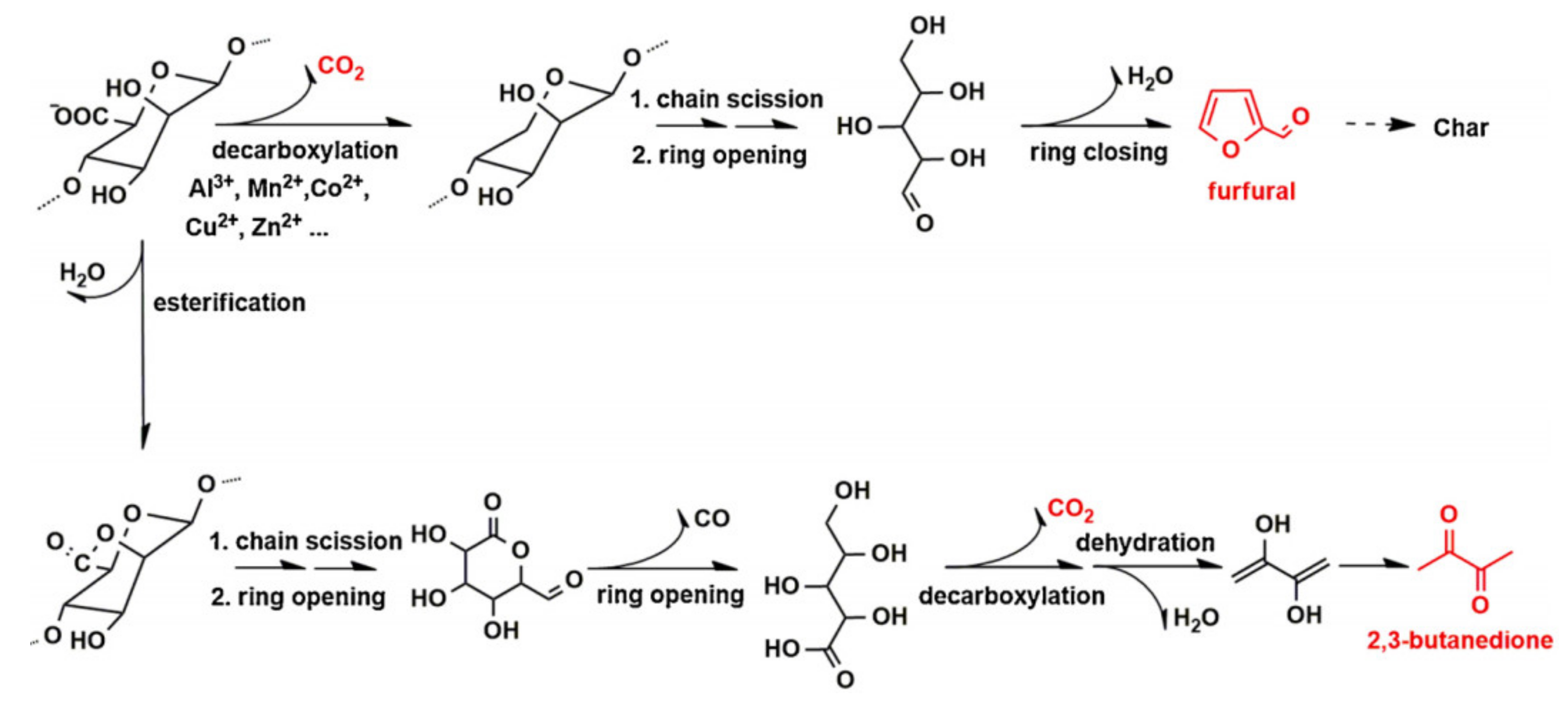
3.3. Flame Retardant Property
4. The Application of Calcium Alginate Fiber
4.1. Wound Dressing
4.2. Tissue Engineering Scaffold
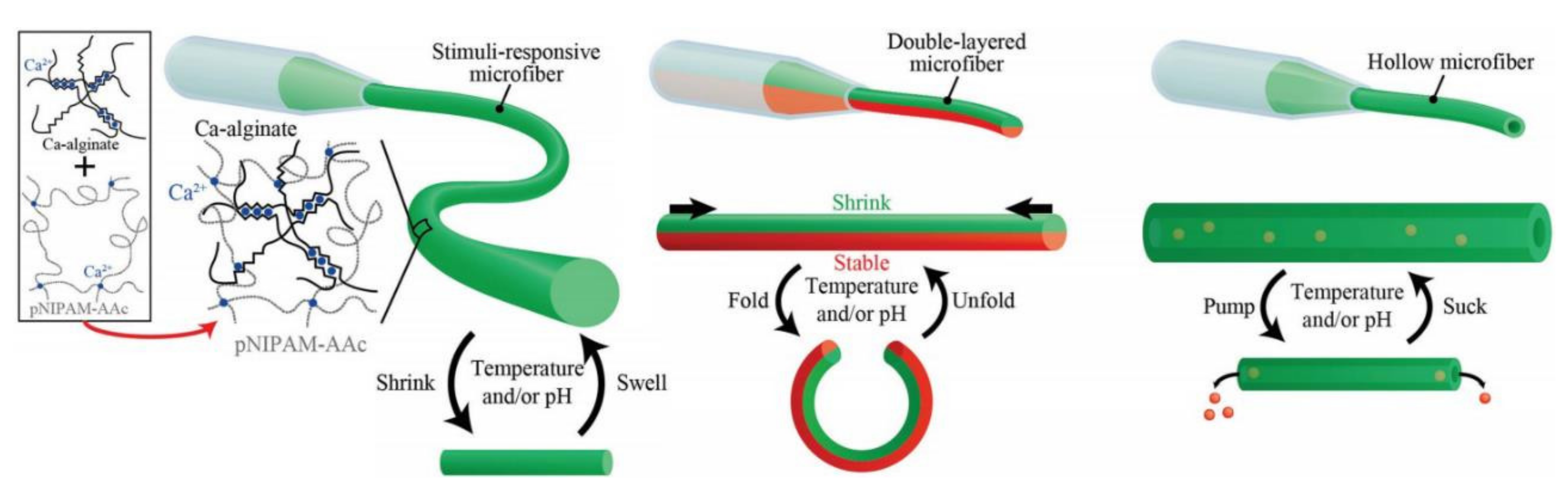
4.3. Smart Material
4.4. Fire-Resistant Material
5. Conclusions and Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.C.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium−Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Xu, Y.J.; Qu, L.Y.; Liu, Y.; Zhu, P. An overview of alginates as flame-retardant materials: Pyrolysis behaviors, flame retardancy, and applications. Carbohydr. Polym. 2021, 260, 117827. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Weng, L.; Deng, B. Strontium ion substituted alginate-based hydrogel fibers and its coordination binding model. J. Appl. Polym. Sci. 2020, 137, 48571. [Google Scholar] [CrossRef]
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Li, S.; Nasri, M.; Zouari, N. The brown seaweed Cystoseira schiffneri as a source of sodium alginate: Chemical and structural characterization, and antioxidant activities. Food Biosci. 2020, 40, 100873. [Google Scholar] [CrossRef]
- Bierhalz, A.C.; da Silva, M.A.; Braga, M.E.; Sousa, H.J.; Kieckbusch, T.G. Effect of calcium and/or barium crosslinking on the physical and antimicrobial properties of natamycin-loaded alginate films. LWT Food Sci. Technol. 2014, 57, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, L. Multiple steps and critical behaviors of the binding of calcium to alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef]
- Vakilian, S.; Jamshidi-Adegani, F.; Yahmadi, A.A.; Al-Broumi, M.; Al-Hashmi, S. A Competitive Nature-derived Multilayered Scaffold based on Chitosan and Alginate, for Full-thickness Wound Healing. Carbohydr. Polym. 2021, 262, 117921. [Google Scholar] [CrossRef]
- García-Gareta, E. Poly-ε-Caprolactone/Fibrin-Alginate Scaffold: A New Pro-Angiogenic Composite Biomaterial for the Treatment of Bone Defects. Polymers 2021, 13, 3399. [Google Scholar]
- Zheng, Y.; Wang, L.; Bai, X.; Xiao, Y.; Che, J. Bio-inspired composite by hydroxyapatite mineralization on (bis)phosphonate-modified cellulose-alginate scaffold for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2021, 635, 127958. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatt, A.N.; Singh, L.; Karim, Z.; Ansari, M.S. Alginate-based hydrogels for tissue engineering. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Woodhead Publishing: Sawston, UK, 2021. [Google Scholar]
- Gunatilake, U.B.; Garcia-Rey, S.; Ojeda, E.; Basabe-Desmonts, L.; Benito-Lopez, F. TiO2 Nanotubes Alginate Hydrogel Scaffold for Rapid Sensing of Sweat Biomarkers: Lactate and Glucose. ACS Appl. Mater. Interfaces 2021, 13, 37734–37745. [Google Scholar] [CrossRef]
- Dangi, Y.R.; Lin, X.; Choi, J.W.; Lim, C.R.; Song, M.H.; Han, M.; Bediako, J.K.; Cho, C.-W.; Yun, Y.-S. Polyethyleneimine functionalized alginate composite fiber for fast recovery of gold from acidic aqueous solutions. Environ. Technol. Innov. 2022, 28, 102605. [Google Scholar] [CrossRef]
- Weng, L.; Zhang, X.; Fan, W.; Lu, Y. Development of the inorganic nanoparticles reinforced alginate-based hybrid fiber for wound care and healing. J. Appl. Polym. Sci. 2021, 138, 51228. [Google Scholar] [CrossRef]
- Chen, Z.; Song, J.; Xia, Y.; Jiang, Y.; Li, Y. High strength and strain alginate fibers by a novel wheel spinning technique for knitting stretchable and biocompatible wound-care materials. Mater. Sci. Eng. C 2021, 127, 112204. [Google Scholar] [CrossRef]
- Li, S.-Q.; Tang, R.-C.; Yu, C.-B. Flame retardant treatment of jute fabric with chitosan and sodium alginate. Polym. Degrad. Stab. 2022, 196, 109826. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, J.; Sun, F.; Sun, L.; Li, M. Flexible wearable graphene/alginate composite non-woven fabric temperature sensor with high sensitivity and anti-interference. Cellulose 2020, 27, 2369–2380. [Google Scholar] [CrossRef]
- Huang, C.C. Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin. Polymers 2021, 13, 3464. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of composite alginate-based electrospun membranes loaded with ZnO nanoparticles. Carbohydr. Polym. 2019, 227, 115371. [Google Scholar] [CrossRef]
- Anokhina, T.; Dmitrieva, E.; Volkov, A. Recovery of Model Pharmaceutical Compounds from Water and Organic Solutions with Alginate-Based Composite Membranes. Membranes 2022, 12, 235. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Ye, D.; Wu, Y.; Fan, L.; Yu, H. Injectable alginate fibrous hydrogel with a three-dimensional network structure fabricated by microfluidic spinning. Compos. Commun. 2019, 15, 1–5. [Google Scholar] [CrossRef]
- Jamnezhad, S.; Motififard, M.; Saber-Samandari, S.; Asefnejad, A.; Khandan, A. Development and investigation of novel alginate-hyaluronic acid bone fillers using freeze drying technique for orthopedic field. Nanomed. Res. J. 2020, 5, 306–315. [Google Scholar]
- Zhang, X.; Chen, H.; Jin, X. Influence of K+ and Na+ ions on the degradation of wet-spun alginate fibers for tissue engineering. J. Appl. Polym. Sci. 2017, 134, 44396. [Google Scholar] [CrossRef]
- Gonzalez, G.M.; Macqueen, L.A.; Lind, J.U.; Fitzgibbons, S.A.; Chantre, C.O.; Huggler, I.; Golecki, H.M.; Goss, J.A.; Parker, K.K. Production of Synthetic, Para-Aramid and Biopolymer Nanofibers by Immersion Rotary Jet-Spinning. Macromol. Mater. Eng. 2017, 302, 1600365. [Google Scholar] [CrossRef]
- Bielanin, J. The Development and Evaluation of Alginate Nanofibers as a Neuroprotective Nano-scaffold for Amyotrophic Lateral Sclerosis (ALS). Chemistry & Biochemistry Undergraduate Honors Theses. Bachelor’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2020. [Google Scholar]
- Parker, A.C.; Hannah, L.; Prashanth, R.; Tara, S.; Jeffrey, L.; Kartik, B.; Ryan, T.Z. Anti-microbial alginate for wound healing applications. Am. Chem. Soc. 2018, 256, 1155. [Google Scholar]
- Homem, N.C.; Tavares, T.D.; Miranda, C.; Antunes, J.C.; Felgueiras, H.P. Functionalization of Crosslinked Sodium Alginate/Gelatin Wet-Spun Porous Fibers with Nisin Z for the Inhibition of Staphylococcus aureus-Induced Infections. Int. J. Mol. Sci. 2021, 22, 1930. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F.; Dolatyar, B.; Zeynali, B. In vitro study of alginate–gelatin scaffolds incorporated with silica NPs as injectable, biodegradable hydrogels. RSC Adv. 2021, 11, 16688–16697. [Google Scholar] [CrossRef]
- Pska, B.; Np, A.; Skaa, B. Fabrication and characterization of Chrysin—A plant polyphenol loaded alginate -chitosan composite for wound healing application. Colloids Surf. B Biointerfaces 2021, 206, 111922. [Google Scholar]
- Zhang, M.; Wang, G.; Wang, D.; Zheng, Y.; Lee, S. Ag@MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications. Int. J. Biol. Macromol. 2021, 175, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Shi, J.; Tang, S.; Liu, L.; Zhu, H.; Zhou, G.; Zeng, J.; Zhang, H.; Yu, Y.; Guo, J. Strengthening and toughening sodium alginate fibers using a dynamically cross-linked network of inorganic nanoparticles and sodium alginate through the hydrogen bonding strategy. New J. Chem. 2021, 45, 10362–10372. [Google Scholar] [CrossRef]
- Kim, K.; Choi, J.H.; Shin, M. Mechanical Stabilization of Alginate Hydrogel Fiber and 3D Constructs by Mussel-Inspired Catechol Modification. Polymers 2021, 13, 892. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Li, Y.; Wang, Y.; Yao, M.; Zhang, K.; Chen, Z.; Yue, H.; Shi, J.; Guan, F. Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell Tissue Res. 2021, 383, 809–821. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021, 131, 185–197. [Google Scholar] [CrossRef]
- Lim, J.; Choi, G.; Joo, K.I.; Cha, H.J.; Kim, J. Embolization of Vascular Malformations via In Situ Photocrosslinking of Mechanically Reinforced Alginate Microfibers using an Optical-Fiber-Integrated Microfluidic Device. Adv. Mater. 2021, 33, 2006759. [Google Scholar] [CrossRef]
- Liu, R.; Kong, B.; Chen, Y.; Liu, X.; Mi, S. Formation of helical alginate microfibers using different G/M ratios of sodium alginate based on microfluidics. Sens. Actuators B Chem. 2020, 304, 127069. [Google Scholar] [CrossRef]
- Sa, V.; Kornev, K.G. A method for wet spinning of alginate fibers with a high concentration of single-walled carbon nanotubes. Carbon 2011, 49, 1859–1868. [Google Scholar] [CrossRef]
- He, Y.; Zhang, N.; Gong, Q.; Qiu, H.; Wei, W.; Yu, L.; Gao, J. Alginate/graphene oxide fibers with enhanced mechanical strength prepared by wet spinning. Carbohydr. Polym. 2012, 88, 1100–1108. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, L.; Liu, Q.; Li, D.; Deng, B. Facile fabrication and characterization on alginate microfibres with grooved structure via microfluidic spinning. R. Soc. Open Sci. 2019, 6, 181928. [Google Scholar] [CrossRef] [Green Version]
- Ci, M.; Liu, J.; Shang, S.; Jiang, Z.; Sui, S. The Effect of HPMC and CNC on the Structure and Properties of Alginate Fibers. Fibers Polym. 2020, 21, 2179–2185. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and functional assessment of alginate fibers prepared by metal-calcium ion complex coagulation bath. Carbohydr. Polym. 2020, 232, 115693. [Google Scholar] [CrossRef]
- Fu, X.; Liang, Y.; Wu, R.; Shen, J.; Chen, Z.; Chen, Y.; Wang, Y.; Xia, Y. Conductive core-sheath calcium alginate/graphene composite fibers with polymeric ionic liquids as an intermediate. Carbohydr. Polym. 2019, 206, 328–335. [Google Scholar] [CrossRef]
- Ci, M.; Liu, J.; Liu, L.; Shang, S.; Sui, S. Preparation and Characterization of Hydroxypropyl Methylcellulose Modified Alginate Fiber. J. Phys. Conf. Ser. 2021, 1790, 012073. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Gong, Y.; Guo, Y.; Shi, Q. Study on polysaccharide polyelectrolyte complex and fabrication of alginate/chitosan derivative composite fibers. Int. J. Biol. Macromol. 2021, 184, 181–187. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab A Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef]
- Xie, R.; Xu, P.; Liu, Y.; Li, L.; Luo, G.; Ding, M.; Liang, Q. Necklace-Like Microfibers with Variable Knots and Perfusable Channels Fabricated by an Oil-Free Microfluidic Spinning Process. Adv. Mater. 2018, 30, e1705082. [Google Scholar] [CrossRef]
- Hancock, M.J.; Piraino, F.; Camci-Unal, G.; Rasponi, M.; Khademhosseini, A. Anisotropic material synthesis by capillary flow in a fluid stripe. Biomaterials 2011, 32, 6493–6504. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Selimović, Š.; Cox JP, A.; Ribas, J.; Bakooshli, M.A.; Heintze, D.; Weiss, A.S.; Cropek, D.; Khademhosseini, A. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab A Chip 2013, 13, 3569–3577. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Ostrovidov, S.; Zhao, Y.; Liang, X.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Wu, H.; Khademhosseini, A. Microfluidic Spinning of Cell-Responsive Grooved Microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Mun, C.H.; Lee, S.H. Microfluidic spinning of fibrous alginate carrier having highly enhanced drug loading capability and delayed release profile. RSC Adv. 2015, 5, 15172–15181. [Google Scholar] [CrossRef]
- Chung, B.G.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab A Chip 2011, 12, 45–59. [Google Scholar] [CrossRef]
- Kang, E.; Choi, Y.Y.; Choi, Y.J.; Sang, H.L. Microfludics spinning of flat fiber with micro grooves for cell-aligning scaffolds. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS, Okinawa, Japan, 28 October–1 November 2012. [Google Scholar]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaolds for tissue engineering: Part 1. structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Sharabi, M.; Benayahu, D.; Benayahu, Y.; Isaacs, J.; Haj-Ali, R. Laminated collagen-fiber bio-composites for soft-tissue bio-mimetics. Compos. Sci. Technol. 2015, 117, 268–276. [Google Scholar] [CrossRef]
- Elkady, M.; Salama, E.; Amer, W.A.; Ebeid, E.; Ayad, M.M.; Shokry, H. Novel eco-friendly electrospun nanomagnetic zinc oxide hybridized PVA/alginate/chitosan nanofibers for enhanced phenol decontamination. Environ. Sci. Pollut. Res. 2020, 27, 43077–43092. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Zhao, Y.; Jin, X. Ampicillin-incorporated alginate-chitosan fibers from microfluidic spinning and for vitro release. J. Biomater. Sci. 2017, 28, 1408–1425. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, J.H.; Choi, Y.H.; Eom, Y. Review on cellulose nanocrystal-reinforced polymer nanocomposites: Processing, properties, and rheology. Korea Aust. Rheol. J. 2021, 33, 165–185. [Google Scholar] [CrossRef]
- Shu, H.J.; Chun-Xuan, W.U.; Yang, K.; Liu, T.W.; Chen, L.I.; Cao, C.L. Preparation of rapid expansion alginate/silica fiber composite scaffold and application of rapid hemostatic function. J. Mater. Eng. 2019, 47, 124–129. [Google Scholar]
- Zhang, X.; Huang, C.; Zhao, Y.; Jin, X. Preparation and characterization of nanoparticle reinforced alginate fibers with high porosity for potential wound dressing application. RSC Adv. 2017, 7, 39349–39358. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, J.; Perwez, M.S.; Das, A.; Saha, P. Development of hydroxyapatite reinforced alginate–chitosan based printable biomaterial-ink. Nano Struct. Nano Objects 2021, 25, 100630. [Google Scholar] [CrossRef]
- D’Elía, N.; Silva, R.R.; Sartuqui, J.; Ercoli, D.; Mestres, G. Development and characterisation of bilayered periosteum-inspired composite membranes based on sodium alginate-hydroxyapatite nanoparticles. J. Colloid Interface Sci. 2020, 572, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, H.; Li, D.; Wang, B.; Wang, G. A graphene assembled porous fiber-based Janus membrane for highly effective solar steam generation. J. Colloid Interface Sci. 2021, 592, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Ko, D.; Rd, F.C.; Liu, W.; Smink, A.M.; De, H.B.; De, V.P.; Lakey, J.R. Immunological Challenges Facing Translation of Alginate Encapsulated Porcine Islet Xenotransplantation to Human Clinical Trials. Methods Mol. Biol. 2017, 1479, 305–333. [Google Scholar]
- Zhe, S.; Xia, Y.; Quan, F.; Li, H.; Zhu, L. Adsorption properties of alginate fiber for basic fuchsin. China Synth. Fiber Ind. 2012, 4, 4–7. [Google Scholar]
- Kong, Q.S.; Jiang, L.P.; Gao, J.X.; Quan, J.I.; Xia, Y.Z.; Jian, Y.U. Study on the Adsorption Property of Alginate Fiber to Zn~(2+). Synth. Fiber China 2008, 40, 1130–1136. [Google Scholar]
- Zhang, X.; Yang, J.; Weng, L.; Fan, W.; Xu, Y. Enhanced mechanical performance of cellulose nanocrystal doped eco-friendly calcium-alginate based bio-composite fiber with superior flame retardancy. Text. Res. J. 2022, 92, 1820–1829. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, J.; Wang, F.; Tan, L.; Xia, Y. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibres. Polym. Degrad. Stab. 2012, 97, 1034–1040. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, Y.; Wang, B.; Li, P.; Xu, Y.; Jiang, Z.; Dong, C.; Zhu, P. Fully bio-based fire-safety viscose/alginate blended nonwoven fabrics: Thermal degradation behavior, flammability, and smoke suppression. Cellulose 2020, 27, 6037–6053. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tanihara, M.; Nishimura, Y.; Suzuki, K.; Yamawaki, Y.; Kudo, H.; Kakimaru, Y.; Shimizu, Y. In vivo evaluation of a novel alginate dressing. J. Biomed. Mater. Res. 1999, 48, 522–527. [Google Scholar] [CrossRef]
- Mirean, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Rasheed, A.; Ahmad, S. Preparation and characterization of wool fiber reinforced nonwoven alginate hydrogel for wound dressing. Cellulose 2021, 28, 7941–7951. [Google Scholar] [CrossRef]
- Hopper, G.P.; Deakin, A.H.; Crane, E.O.; Clarke, J.V. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: A prospective, comparative audit. J. Wound Care 2013, 21, 200. [Google Scholar] [CrossRef]
- Erwin, E.; Etriwati, E.; Zamzami, R.S.; Hosea, C. Moist wound dressing and its application in distant skin flap in cats. Vet. World 2021, 14, 734. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, H.; Yang, K.; Wang, H.; Duan, C.; Ni, N.; An, L.; Luo, Y.; Zhao, P.; Gou, Y.; et al. Reversibly immortalized keratinocytes (iKera) facilitate re-epithelization and skin wound healing: Potential applications in cell-based skin tissue engineering. Bioact. Mater. 2022, 9, 523–540. [Google Scholar] [CrossRef]
- Asadi, L.; Mokhtari, J.; Abbasi, M. An alginate–PHMB–AgNPs based wound dressing polyamide nanocomposite with improved antibacterial and hemostatic properties. J. Mater. Sci. Mater. Med. 2021, 32, 7. [Google Scholar] [CrossRef]
- Das, D.; Zhang, S.; No, I. Synthesis and characterizations of alginate-α-tricalcium phosphate microparticle hybrid film with flexibility and high mechanical property as a biomaterial. Biomed. Mater. 2018, 13, 025008. [Google Scholar] [CrossRef]
- Liang, L.; Hou, T.; Ouyang, Q.; Xie, L.; Li, C. Antimicrobial sodium alginate dressing immobilized with polydopamine-silver composite nanospheres. Compos. Part B Eng. 2020, 188, 107877. [Google Scholar] [CrossRef]
- Filho, R.; Vasconcelos, N.F.; Andrade, F.K.; Rosa, M.; Vieira, R.S. Papain immobilized on alginate membrane for wound dressing application. Colloids Surf. B Biointerfaces 2020, 194, 111222. [Google Scholar] [CrossRef] [PubMed]
- Cesar, P.; Natarelli, C.; Oliveira, J.; Andrade, P.A.; Marcussi, S. Development and characterization of a poly (vinyl alcohol) and sodium alginate blend foam for wound dressing loaded with propolis and alltrans retinoic acid. J. Appl. Polym. Sci. 2021, 138, 50480. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Yuan, K.; Yang, S.; Tang, T. Dual-functional hybrid quaternized chitosan/Mg/alginate dressing with antibacterial and angiogenic potential for diabetic wound healing. J. Orthop. Transl. 2021, 30, 6–15. [Google Scholar] [CrossRef]
- Ashton, R.S.; Banerjee, A.; Punyani, S.; Schaffer, D.V.; Kane, R.S. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials 2007, 28, 5518–5525. [Google Scholar] [CrossRef]
- Roopavath, U.; Soni, R.; Mahanta, U.; Deshpande, A.S.; Rath, S.N. 3D printable SiO2 nanoparticle ink for patient specific bone regeneration. RSC Adv. 2019, 9, 23832–23842. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Liu, T.; Chew, S.Y. The application of nanofibrous scaffolds in neural tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1055–1064. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Nano/microscale topographically designed alginate/PCL scaffolds for inducing myoblast alignment and myogenic differentiation. Carbohydr. Polym. 2019, 223, 115041. [Google Scholar] [CrossRef]
- Tachizawa, S.; Takahashi, H.; Kim, Y.J.; Odawara, A.; Pauty, J.; Ikeuchi, Y.; Suzuki, I.; Kikuchi, A.; Matsunaga, Y.T. Bundle Gel Fibers with Tunable Microenvironment for In Vitro Neuron Cell Guiding. ACS Appl. Mater. Interfaces 2017, 9, 43250–43257. [Google Scholar] [CrossRef]
- Yeo, M.G.; Kim, G.H. Fabrication of cell-laden electrospun hybrid scaffolds of alginate-based bioink and PCL microstructures for tissue regeneration. Chem. Eng. J. 2015, 275, 27–35. [Google Scholar] [CrossRef]
- Hu, W.W.; Yu, H.N. Coelectrospinning of chitosan/alginate fibers by dual-jet system for modulating material surfaces. Carbohydr. Polym. 2013, 95, 716–727. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Moon, H.; Troczynski, T.; Ko, F.K. Biomimetically Mineralized Alginate Nanocomposite Fibers for Bone Tissue Engineering: Mechanical Properties and in Vitro Cellular Interactions. ACS Appl. Bio Mater. 2020, 3, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Ting, J.C. Gene immobilization on alginate/polycaprolactone fibers through electrophoretic deposition to promote in situ transfection efficiency and biocompatibility. Int. J. Biol. Macromol. 2019, 121, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Ramakrishna, S.; Laurent, S.; Shokrgozar, M.A.; Semnani, D.; Sadeghi, D.; Bonakdar, S.; Akbari, M. Fabrication of Nanofibrous PVA/Alginate-Sulfate Substrates for Growth Factor Delivery. J. Biomed. Mater. Res. Part A 2019, 107, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Godeshala, S.; Nitiyanandan, R.; Islam, M.S.; Rege, K. Correction to Copper-Eluting Fibers for Enhanced Tissue Sealing and Repair. ACS Appl. Mater. Interfaces 2020, 12, 53568–53569. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Leung, V.; Ko, F.; Troczynski, T. Novel biomimetic hydroxyapatite/alginate nanocomposite fibrous scaffolds for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 1885–1894. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Ren, X.; Gao, G. Alginate Fiber Toughened Gels Similar to Skin Intelligence as Ionic Sensors. Carbohydr. Polym. 2020, 235, 116018. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, S.; Yoon, J. Highly Stretchable Strain Sensors Comprising Double Network Hydrogels Fabricated by Microfluidic Devices. Adv. Mater. Technol. 2019, 4, 1800739. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Huang, T.; Xu, H.G.; Jiao, K.X.; Zhu, L.P.; Wang, H.L. A Novel Hydrogel with High Mechanical Strength: A Macromolecular Microsphere Composite Hydrogel. Adv. Mater. 2010, 19, 1622–1626. [Google Scholar] [CrossRef]
- Nakajima, S.; Kawano, R.; Onoe, H. Stimuli-responsive hydrogel microfibers with controlled anisotropic shrinkage and cross-sectional geometries. Soft Matter. 2017, 13, 3710–3719. [Google Scholar] [CrossRef]
- Zou, X.; Shang, S.; Liu, J.; Ci, M.; Zhu, P. Facile Fabrication of Temperature Triggered Thermochromic Core-sheath Alginate Microfibers from Microfluidic Spinning. Fibers Polym. 2021, 22, 1535–1542. [Google Scholar] [CrossRef]
- Cui, L.; Hu, J.J.; Wang, W.; Yan, C.; Tu, C. Smart pH response flexible sensor based on calcium alginate fibers incorporated with natural dye for wound healing monitoring. Cellulose 2020, 27, 6367–6381. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.S.; Zhu, P.; Zhao, J.C.; Zhang, C.J.; Guo, Y.; Cui, L. Thermal degradation properties of biobased iron alginate film. J. Anal. Appl. Pyrolysis 2016, 119, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.J.; Zhao, J.C.; Guo, Y.; Zhu, P.; Wang, D.Y. Bio-based barium alginate film: Preparation, flame retardancy and thermal degradation behavior. Carbohydr. Polym. 2016, 139, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, C.; Guan, Y.; Zhang, Y.; Zhu, X.X. Enzymatically crosslinked alginate hydrogels with improved adhesion properties. Polym. Chem. 2015, 6, 2204–2213. [Google Scholar] [CrossRef]
- Ma, X.; Li, R.; Zhao, X.; Ji, Q.; Xing, Y.; Sunarso, J.; Xia, Y. Biopolymer composite fibres composed of calcium alginate reinforced with nanocrystalline cellulose. Compos. Part A Appl. Sci. Manuf. 2017, 96, 155–163. [Google Scholar] [CrossRef]
- Tian, G.; Quan, J.; Xu, D.; Tan, L.; Xia, Y. The effect of zinc ion content on flame retardance and thermal degradation of alginate fibers. Fibers Polym. 2013, 14, 767–771. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Yan, X.; Shi, M. Efficient suppression of flammability in flame retardant viscose fiber through incorporating with alginate fiber. Mater. Lett. 2018, 215, 106–109. [Google Scholar] [CrossRef]
- Zhang, C.J.; Liu, Y.; Cui, L.; Yan, C.; Zhu, P. Bio-based calcium alginate nonwoven fabrics: Flame retardant and thermal degradation properties. J. Anal. Appl. Pyrolysis 2016, 122, 13–23. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.; Zhao, H.B.; Schiraldi, D.A. Highly Efficient Flame Retardant Polyurethane Foam with Alginate/Clay Aerogel Coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Li, Y.; Wang, J.; Liu, X.; Song, T.; Yang, X.; Hao, J. Spray drying assisted Layer-by-Layer assembly of alginate, 3-aminopropyltriethoxysilane, and magnesium hydroxide flame retardant and its catalytic graphitization in ethylene-vinyl acetate resin. ACS Appl. Mater. Interfaces 2018, 10, 10490–10500. [Google Scholar] [CrossRef]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.T.; Wang, Y.Z.; Schiraldi, D.A. Nonflammable Alginate Nanocomposite Aerogels Prepared by a Simple Freeze-Drying and Post-Cross-Linking Method. ACS Appl. Mater. Interfaces 2016, 8, 643–650. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.-R.; Peng, Y.-L.; Wang, D.; Yang, L.; Peng, H.; Zhu, P.; Wang, D.-Y. Effect of reactive time on flame retardancy and thermal degradation behavior of bio-based zinc alginate film. Polym. Degrad. Stab. 2016, 127, 20–31. [Google Scholar] [CrossRef]
- Li, X.-L.; Chen, M.-J.; Chen, H.-B. Facile fabrication of mechanically-strong and flame retardant alginate/clay aerogels. Compos. Part B Eng. 2019, 164, 18–25. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.Z.; Wang, B.; Liu, Y.Y.; Zhu, P. Blending alginate fibers with polyester fibers for flame-retardant filling materials: Thermal decomposition behaviors and fire performance. Polym. Degrad. Stab. 2021, 183, 109470. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Fan, W.; Liu, Y.; Wang, Q.; Weng, L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers 2022, 14, 3227. https://doi.org/10.3390/polym14153227
Zhang X, Wang X, Fan W, Liu Y, Wang Q, Weng L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers. 2022; 14(15):3227. https://doi.org/10.3390/polym14153227
Chicago/Turabian StyleZhang, Xiaolin, Xinran Wang, Wei Fan, Yi Liu, Qi Wang, and Lin Weng. 2022. "Fabrication, Property and Application of Calcium Alginate Fiber: A Review" Polymers 14, no. 15: 3227. https://doi.org/10.3390/polym14153227
APA StyleZhang, X., Wang, X., Fan, W., Liu, Y., Wang, Q., & Weng, L. (2022). Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers, 14(15), 3227. https://doi.org/10.3390/polym14153227





