Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications
Abstract
1. Introduction
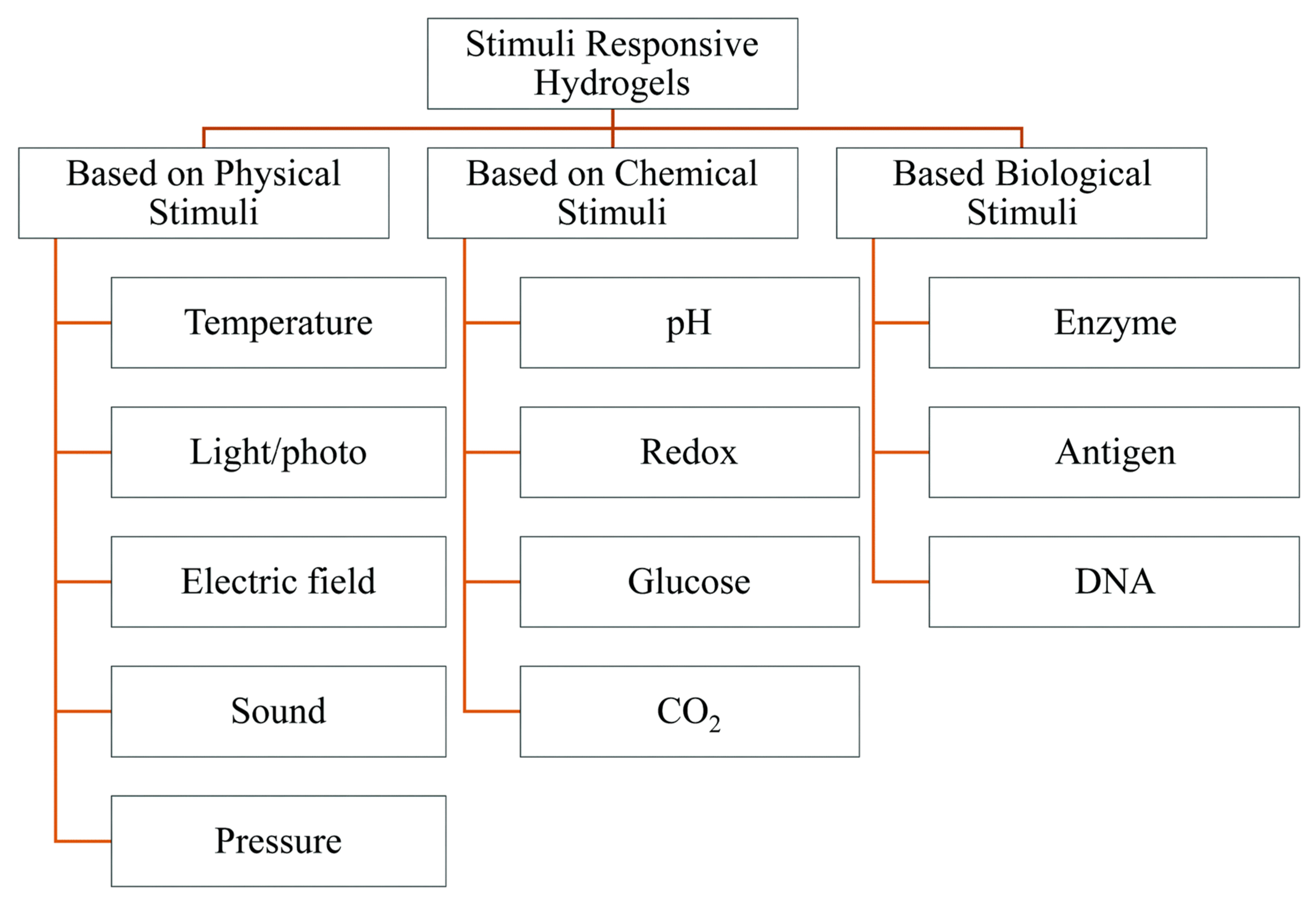
2. Stimuli-Responsive Polymers
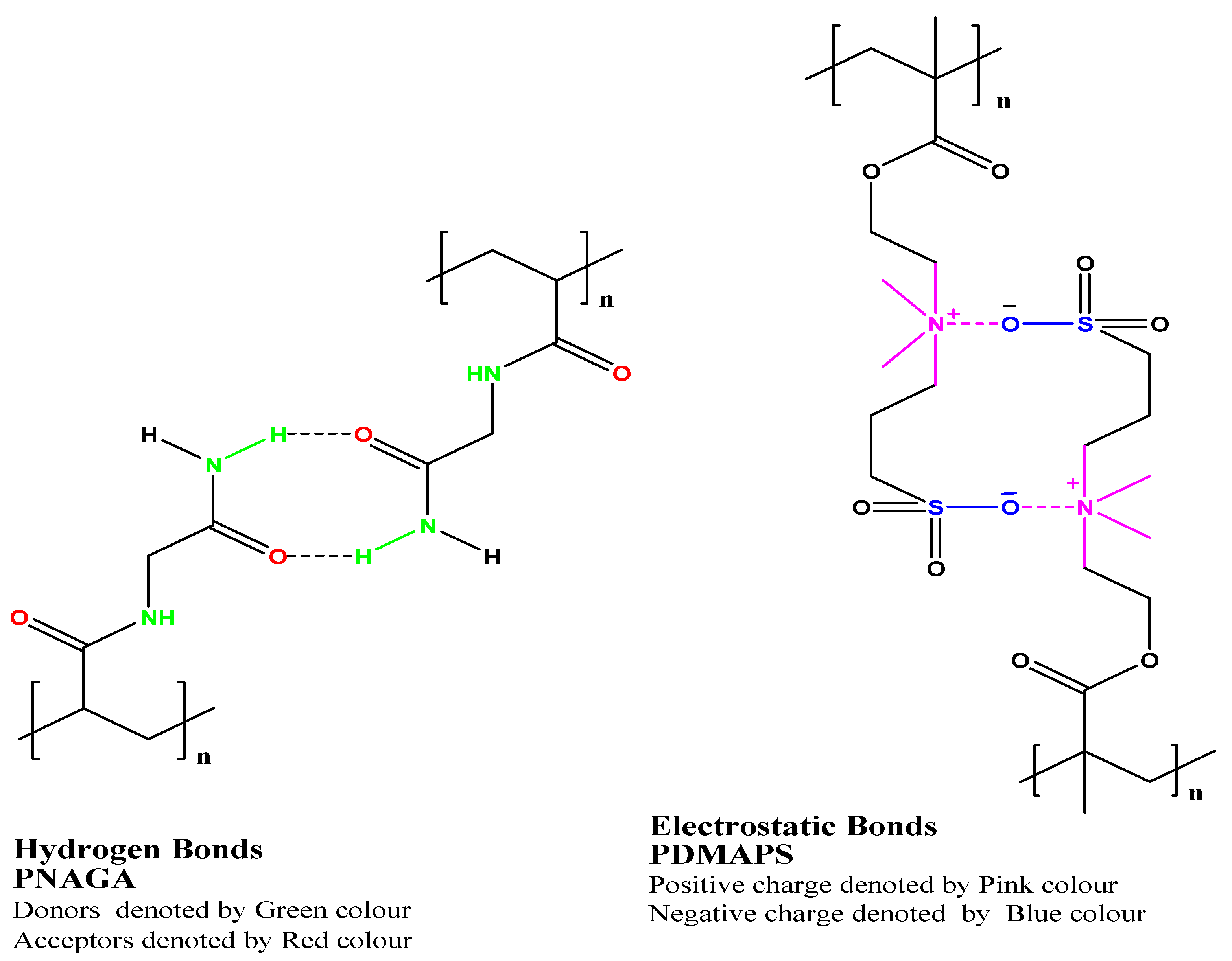
2.1. Physicochemical Properties of Thermoresponsive Hydrogel
2.1.1. Mechanical Strength
2.1.2. Adhesion
2.1.3. Optical Property
Lasting Time
2.2. Natural Polymers and Their Derivatives
2.2.1. Polysaccharides
Cellulose Derivatives
Chitosan
Dextran
Xyloglucan
2.2.2. Proteins
Gelatin
2.2.3. Synthetic polymers and their derivatives
N-Isopropylacrylamide(pNiPAAm)-Based Systems
2.2.4. PEO/PPO-Based Systems
2.2.5. PEG/Biodegradable Polyester Copolymers
2.2.6. Poly(organophosphazenes)
3. Method of Functional Thermoresponsive Hydrogel Synthesis
3.1. Bulk Polymerization
3.2. Solution Polymerization
3.3. Suspension Polymerization (Including Inverse-Suspension Polymerization)
3.4. Emulsion Polymerization
3.5. Hydrogel Synthesis by Chemical Mechanism
3.5.1. Chain Growth Polymerization
3.5.2. Graft Polymerization Mechanism
3.5.3. Step-Growth Polymerization
3.5.4. Crosslinking Method
3.5.5. Synthesis of Thermo-Responsive Hydrogels (TRHs)
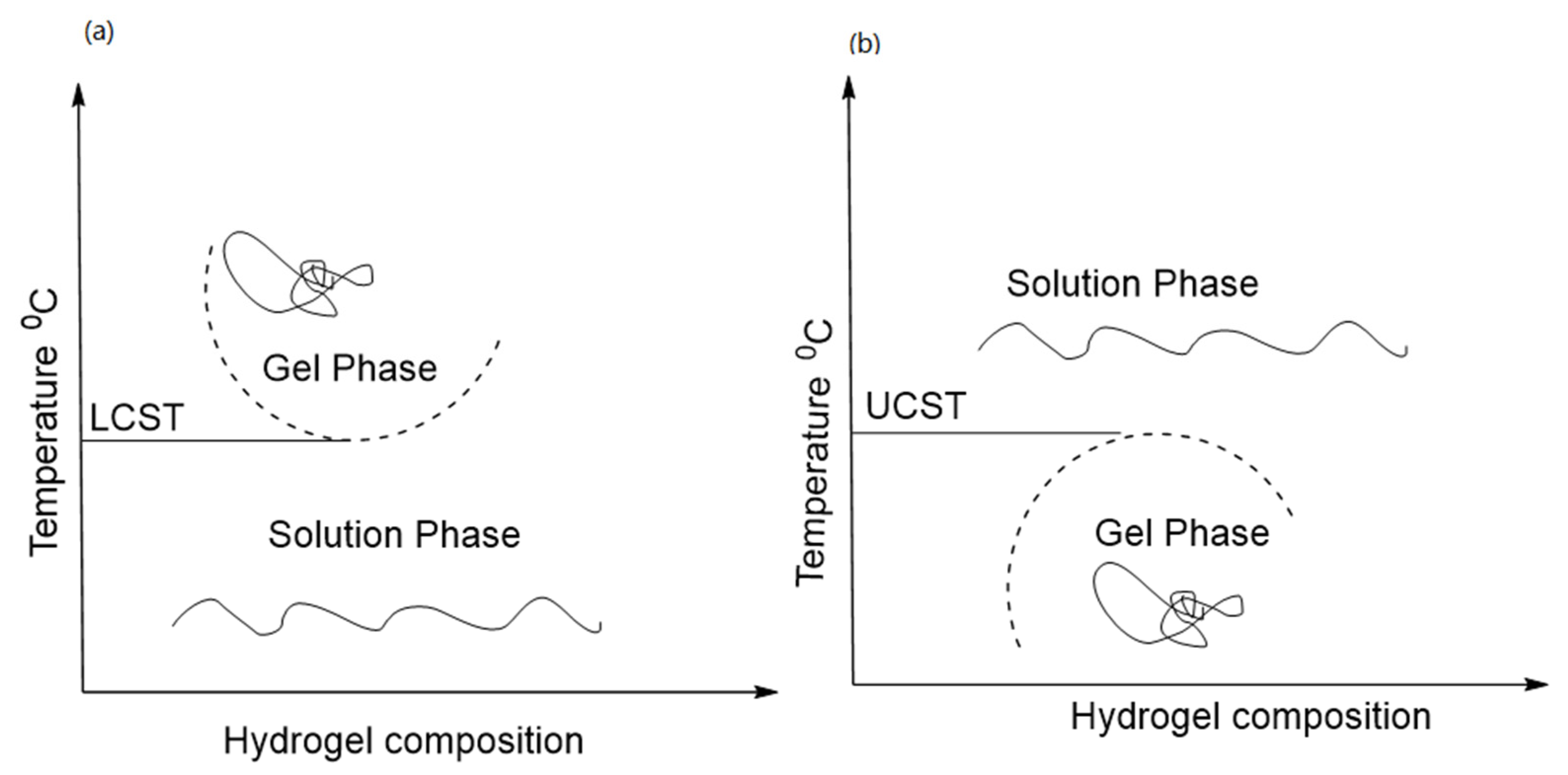
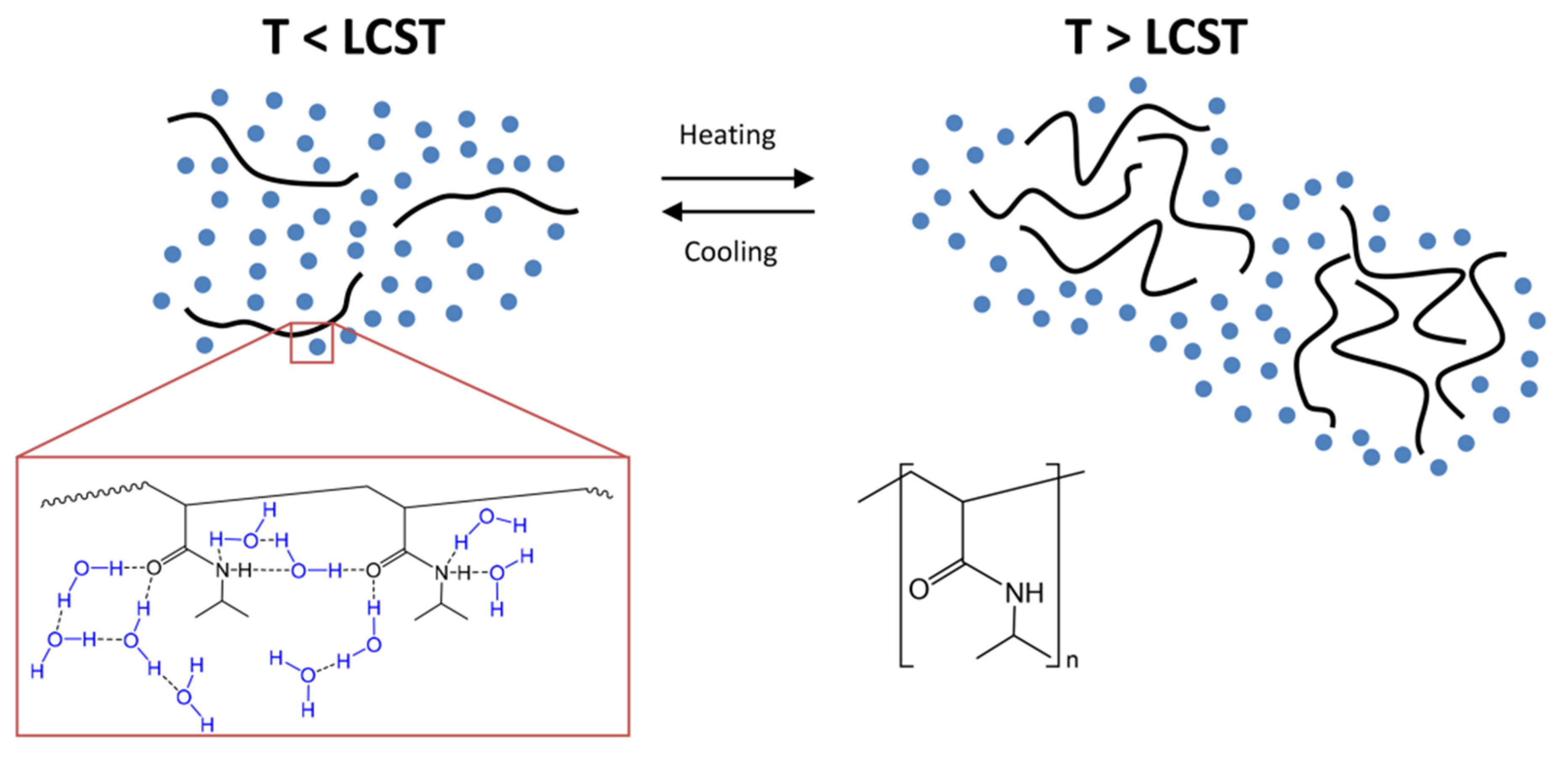
4. Mechanism of Thermoresponsive Hydrogel
4.1. LCST Polymers
4.2. UCST Polymers
4.3. Mechanism of Phase Transition in Thermoresponsive Hydrogels
5. Mathematical Models for Drug Release from the Hydrogel-Based Formulations
- Post-loading (drug)
- In-situ drug loading
- Diffusion-controlled hydrogel drug delivery systems
- Swelling-controlled hydrogel drug delivery systems
- Chemically controlled hydrogel drug delivery systems
5.1. Diffusion-Controlled Hydrogel Drug Delivery Systems
- Dg is drug diffusion coefficients in the swollen hydrogel network or matrix
- Do, the drug diffusion coefficients in pure solvent
- rs, the size of the drug to be delivered
- Gel structure
- Polymer composition
- Size of the molecule/drug
- Water content in the systems
5.2. Swelling-Controlled Hydrogel Drug Delivery Systems
5.3. Chemically Controlled Hydrogel Drug Delivery Systems
- Pure kinetic controlled hydrogel drug release mechanism, where polymer degradation (bond cleavage) takes place. This is the rate-limiting step. However, diffusion is considered a negligible parameter in the modeling.
- Reaction diffusion-controlled hydrogel drug release mechanism. Both reaction (drug-polymer and protein-drug interactions and polymer degradation) and diffusion terms should be considered in the modeling to predicate the accurate drug release from the hydrogel systems. This mechanism is mainly considered in the interest of the synthetic hydrogel systems, which are developed and designed with drug binding capacity and are used in drug delivery, biomedical, and tissue engineering applications [121,122,123,124]. The kinetically and reaction controlled hydrogel drug release has been classified into different types, as follows in Table 2.
5.4. Miscellaneous Types of Hydrogel Systems and Release Mechanism
5.5. Advanced Hydrogel Systems and Their Drug Release Challenges
6. Applications of Functional Thermo-Responsive Hydrogels
6.1. Applications in Biosensing
6.2. Applications in Drug Delivery
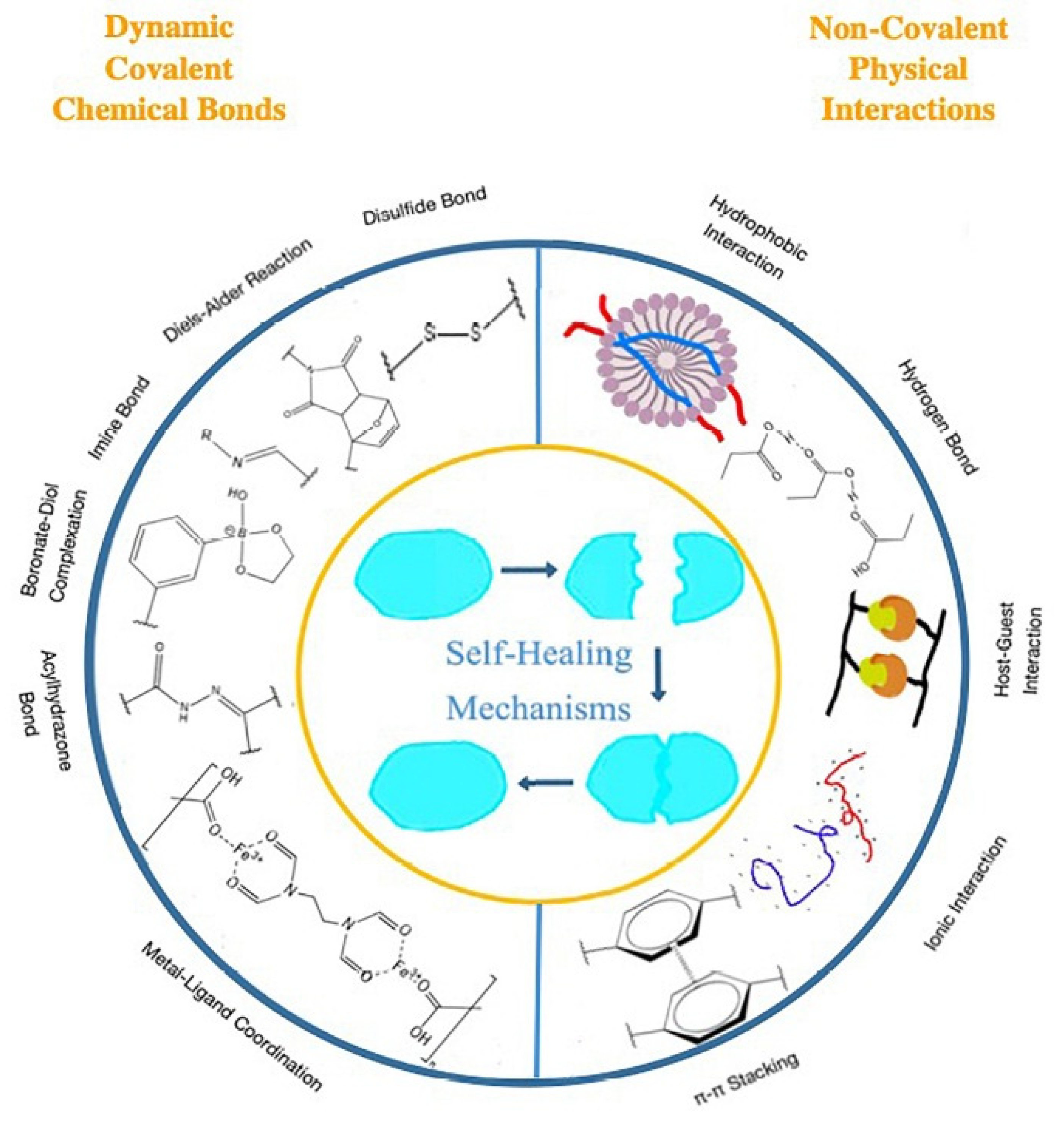
6.3. Applications in Self-Healing
7. Patent and Current Clinical Trial Status of the Hydrogel Drug Delivery System
8. Future Scope
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-Sensitive Hydrogels for Delivering Biotherapeutic Molecules: A Review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Naik, J.B.; Pardeshi, S.R.; Patil, R.P.; Patil, P.B.; Mujumdar, A. Mucoadhesive Micro-/Nano Carriers in Ophthalmic Drug Delivery: An Overview. BioNanoScience 2020, 10, 564–582. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent Advances in the Synthesis of Smart Hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Komatsu, S.; Asoh, T.-A.; Ishihara, R.; Kikuchi, A. Fabrication of Thermoresponsive Degradable Hydrogel Made by Radical Polymerization of 2-Methylene-1,3-Dioxepane: Unique Thermal Coacervation in Hydrogel. Polymer 2019, 179, 121633. [Google Scholar] [CrossRef]
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive Polymers: Insights into Decisive Hydrogel Characteristics, Mechanisms of Gelation, and Promising Biomedical Applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Ghaeini-Hesaroeiye, S.; Razmi Bagtash, H.; Boddohi, S.; Vasheghani-Farahani, E.; Jabbari, E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels 2020, 6, 20. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Kozuch, O.; Mayer, V. Pig Kidney Epithelial (PS) Cells: A Perfect Tool for the Study of Flaviviruses and Some Other Arboviruses. Acta Virol 1975, 19, 498. [Google Scholar]
- Ranganathan, N.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Synthesis and Properties of Hydrogels Prepared by Various Polymerization Reaction Systems. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar]
- Tang, S.; Floy, M.; Bhandari, R.; Sunkara, M.; Morris, A.J.; Dziubla, T.D.; Hilt, J.Z. Synthesis and Characterization of Thermoresponsive Hydrogels Based on N -Isopropylacrylamide Crosslinked with 4,4′-Dihydroxybiphenyl Diacrylate. ACS Omega 2017, 2, 8723–8729. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-Responsive Polydopamine-Based Smart Materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, L.; Tian, R.; Wu, H.; Gu, Z.; Li, Y. Versatile Polyphenolic Platforms in Regulating Cell Biology. Chem. Soc. Rev. 2022, 51, 4175–4198. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired Integration of Naturally Occurring Molecules towards Universal and Smart Antibacterial Coatings. Adv Funct Mater. 2022, 32, 2108749. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering-A Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F. (Kuo); Zhao, B.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F.; Chen, X.; Wang, X.-W.; Zhang, W.-B.; Peng, J.; Li, J.; Zhai, M. Stretchable, Conductive, and Self-Healing Hydrogel with Super Metal Adhesion. Chem. Mater. 2018, 30, 4289–4297. [Google Scholar] [CrossRef]
- Hua, M.; Wu, D.; Wu, S.; Ma, Y.; Alsaid, Y.; He, X. 4D Printable Tough and Thermoresponsive Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 12689–12697. [Google Scholar] [CrossRef]
- Shou, Y. 4D-Printable Thermoresponsive Hydrogel Exhibits High Mechanical Properties. MRS Bull. 2021, 46, 301. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Zhong, W. Mussel-Inspired Hydrogel Tissue Adhesives for Wound Closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L. Polyphenolic Substance of Mytilus Edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Naveen, N.R.; Patel, B.; Manne, R.; Patel, D.B. Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Peng, L.; Yu, G. Nanostructured Conducting Polymer Hydrogels for Energy Storage Applications. Nanoscale 2015, 7, 12796–12806. [Google Scholar] [CrossRef]
- Ge, D.; Lee, E.; Yang, L.; Cho, Y.; Li, M.; Gianola, D.S.; Yang, S. A Robust Smart Window: Reversibly Switching from High Transparency to Angle-Independent Structural Color Display. Adv. Mater. 2015, 27, 2489–2495. [Google Scholar] [CrossRef]
- Strozyk, M.S.; Chanana, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Stimuli-Responsive Materials: Protein/Polymer-Based Dual-Responsive Gold Nanoparticles with PH-Dependent Thermal Sensitivity (Adv. Funct. Mater. 7/2012). Adv. Funct. Mater. 2012, 22, 1322. [Google Scholar] [CrossRef]
- Owusu-Nkwantabisah, S.; Gillmor, J.; Switalski, S.; Mis, M.R.; Bennett, G.; Moody, R.; Antalek, B.; Gutierrez, R.; Slater, G. Synergistic Thermoresponsive Optical Properties of a Composite Self-Healing Hydrogel. Macromolecules 2017, 50, 3671–3679. [Google Scholar] [CrossRef]
- Han, F.; Soeriyadi, A.H.; Vivekchand, S.R.C.; Gooding, J.J. Simple Method for Tuning the Optical Properties of Thermoresponsive Plasmonic Nanogels. ACS Macro Lett. 2016, 5, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Lee, B.H.; Pauken, C.; Vernon, B.L. Degradation, Cytotoxicity, and Biocompatibility of NIPAAm-Based Thermosensitive, Injectable, and Bioresorbable Polymer Hydrogels. J. Biomed. Mater. Res. 2011, 98A, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-Trimethyl Chitosan Embedded in Situ Pluronic F127 Hydrogel for the Treatment of Brain Tumor. Carbohydr. Polym. 2019, 203, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Lu, W.W.; Li, X.; Zhu, D.; De Yao, K.; Wang, Q.; Zhao, C.; Wang, C. A Rapid Temperature-Responsive Sol-Gel Reversible Poly(N-Isopropylacrylamide)-g-Methylcellulose Copolymer Hydrogel. Biomaterials 2004, 25, 3005–3012. [Google Scholar] [CrossRef]
- Stabenfeldt, S.E.; García, A.J.; LaPlaca, M.C. Thermoreversible Laminin-Functionalized Hydrogel for Neural Tissue Engineering. J. Biomed. Mater. Res. A 2006, 77A, 718–725. [Google Scholar] [CrossRef]
- Bhattarai, N.; Matsen, F.A.; Zhang, M. PEG-Grafted Chitosan as an Injectable Thermoreversible Hydrogel. Macromol. Biosci. 2005, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Zeng, S.-Y. Fabrication of Quaternized Chitosan Nanoparticles Using Tripolyphosphate/Genipin Dual Cross-Linkers as a Protein Delivery System. Polymers 2018, 10, 1226. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Thermo-Responsive Chitosan-Graft-Poly(N-Isopropylacrylamide) Injectable Hydrogel for Cultivation of Chondrocytes and Meniscus Cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef]
- Crompton, K.E.; Goud, J.D.; Bellamkonda, R.V.; Gengenbach, T.R.; Finkelstein, D.I.; Horne, M.K.; Forsythe, J.S. Polylysine-Functionalised Thermoresponsive Chitosan Hydrogel for Neural Tissue Engineering. Biomaterials 2007, 28, 441–449. [Google Scholar] [CrossRef]
- Bölgen, N.; Aguilar, M.R.; Fernández, M. del M.; Gonzalo-Flores, S.; Villar-Rodil, S.; San Román, J.; Pişkin, E. Thermoresponsive Biodegradable HEMA-Lactate-Dextran-Co-NIPA Cryogels for Controlled Release of Simvastatin. Artif. Cells Nanomed. Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Joly-Duhamel, C.; Hellio, D.; Djabourov, M. All Gelatin Networks: 1. Biodiversity and Physical Chemistry. Langmuir 2002, 18, 7208–7217. [Google Scholar] [CrossRef]
- Yang, H.; Kao, W.J. Thermoresponsive Gelatin/Monomethoxy Poly(Ethylene GlycolI_Poly(d,l-Lactide) Hydrogels: Formulation, Characterization, and Antibacterial Drug Delivery. Pharm. Res. 2005, 23, 205–214. [Google Scholar] [CrossRef]
- Ohya, S.; Matsuda, T. Poly(N-Isopropylacrylamide) (PNIPAM)-Grafted Gelatin as Thermoresponsive Three-Dimensional Artificial Extracellular Matrix: Molecular and Formulation Parameters vs. Cell Proliferation Potential. J. Biomater. Sci. Polym. Ed. 2005, 16, 809–827. [Google Scholar] [CrossRef]
- Gil, E.S.; Frankowski, D.J.; Spontak, R.J.; Hudson, S.M. Swelling Behavior and Morphological Evolution of Mixed Gelatin/Silk Fibroin Hydrogels. Biomacromolecules 2005, 6, 3079–3087. [Google Scholar] [CrossRef]
- Coughlan, D.C.; Quilty, F.P.; Corrigan, O.I. Effect of Drug Physicochemical Properties on Swelling/Deswelling Kinetics and Pulsatile Drug Release from Thermoresponsive Poly(N-Isopropylacrylamide) Hydrogels. J. Control. Release 2004, 98, 97–114. [Google Scholar] [CrossRef]
- Na, K.; Park, J.H.; Kim, S.W.; Sun, B.K.; Woo, D.G.; Chung, H.-M.; Park, K.-H. Delivery of Dexamethasone, Ascorbate, and Growth Factor (TGF Beta-3) in Thermo-Reversible Hydrogel Constructs Embedded with Rabbit Chondrocytes. Biomaterials 2006, 27, 5951–5957. [Google Scholar] [CrossRef]
- Liu, X.-M.; Wang, L.-S.; Wang, L.; Huang, J.; He, C. The Effect of Salt and PH on the Phase-Transition Behaviors of Temperature-Sensitive Copolymers Based on N-Isopropylacrylamide. Biomaterials 2004, 25, 5659–5666. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-Isopropylacrylamide-Co-Propylacrylic Acid) Copolymers That Respond Sharply to Temperature and PH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef]
- Xu, F.-J.; Kang, E.-T.; Neoh, K.-G. ph- and Temperature-Responsive Hydrogels from Crosslinked Triblock Copolymers Prepared via Consecutive Atom Transfer Radical Polymerizations. Biomaterials 2006, 27, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-C.; Lee, S.B.; Jin, J.-I.; Sohn, Y.S. A New Class of Biodegradable Thermosensitive Polymers. I. Synthesis and Characterization of Poly(Organophosphazenes) with Methoxy-Poly(Ethylene Glycol) and Amino Acid Esters as Side Groups. Macromolecules 1999, 32, 2188–2193. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T.; Miyazaki, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular Design of Biodegradable Polymeric Micelles for Temperature-Responsive Drug Release. J. Control. Release 2006, 115, 46–56. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Kikuchi, A.; Yamato, M.; Okano, T. Bio-Functionalized Thermoresponsive Interfaces Facilitating Cell Adhesion and Proliferation. Biomaterials 2006, 27, 5069–5078. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic Block Copolymers as Novel Polymer Therapeutics for Drug and Gene Delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(Ethylene Oxide) poly(Propylene Oxide) poly(Ethylene Oxide) Block Copolymer Surfactants in Aqueous Solutions and at Interfaces: Thermodynamics, Structure, Dynamics, and Modeling. Colloids Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Cortiella, J.; Nichols, J.E.; Kojima, K.; Bonassar, L.J.; Dargon, P.; Roy, A.K.; Vacant, M.P.; Niles, J.A.; Vacanti, C.A. Tissue-Engineered Lung: An in Vivo and in Vitro Comparison of Polyglycolic Acid and Pluronic F-127 Hydrogel/Somatic Lung Progenitor Cell Constructs to Support Tissue Growth. Tissue Eng. 2006, 12, 1213–1225. [Google Scholar] [CrossRef]
- Weinand, C.; Pomerantseva, I.; Neville, C.M.; Gupta, R.; Weinberg, E.; Madisch, I.; Shapiro, F.; Abukawa, H.; Troulis, M.J.; Vacanti, J.P. Hydrogel-Beta-TCP Scaffolds and Stem Cells for Tissue Engineering Bone. Bone 2006, 38, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Sosnik, A.; Levy, A. Improved Reverse Thermo-Responsive Polymeric Systems. Biomaterials 2003, 24, 3707–3714. [Google Scholar] [CrossRef]
- Cellesi, F.; Tirelli, N.; Hubbell, J.A. Towards a Fully-Synthetic Substitute of Alginate: Development of a New Process Using Thermal Gelation and Chemical Cross-Linking. Biomaterials 2004, 25, 5115–5124. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. In Situ Gelation of PEG-PLGA-PEG Triblock Copolymer Aqueous Solutions and Degradation Thereof. J. Biomed. Mater. Res. 2000, 50, 171–177. [Google Scholar] [CrossRef]
- Chen, S.; Singh, J. Controlled Delivery of Testosterone from Smart Polymer Solution Based Systems: In Vitro Evaluation. Int. J. Pharm. 2005, 295, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Debeli, D.K.; Shan, G. A Novel Drug Loading and Release from a Thermoresponsive Hydrogel Formed in Situ Emulsion Polymerization. J. Appl. Polym. Sci. 2020, 137, 48669. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent Hydrogels via Graft Polymerization of Acrylic Acid from Chitosan-Cellulose Hybrid and Their Potential in Controlled Release of Soil Nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive Sol–Gel Reversible Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels: A Review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Ohar, H.; Budkowski, A. Impact of the Various Buffer Solutions on the Temperature-Responsive Properties of POEGMA-Grafted Brush Coatings. Colloid Polym. Sci. 2022, 300, 487–495. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bajpai, M.; Sharma, L. Inverse Suspension Polymerization of Poly(Methacrylic Acid-Co-Partially Neutralized Acrylic Acid) Superabsorbent Hydrogels: Synthesis and Water Uptake Behavior. Des. Monomers Polym. 2007, 10, 181–192. [Google Scholar] [CrossRef]
- Park, J.; Doyle, P.S. Multifunctional Hierarchically-Assembled Hydrogel Particles with Pollen Grains via Pickering Suspension Polymerization. Langmuir 2018, 34, 14643–14651. [Google Scholar] [CrossRef] [PubMed]
- Yiamsawas, D.; Kangwansupamonkon, W.; Kiatkamjornwong, S. Lignin-Based Microgels by Inverse Suspension Polymerization: Syntheses and Dye Removal. Macromol. Chem. Phys. 2021, 222, 2100285. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. Effects of the Poly(Ethylene Glycol) Hydrogel Crosslinking Mechanism on Protein Release. Biomater. Sci. 2016, 4, 405–411. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Kloxin, A.M.; Sawicki, L.A.; Anseth, K.S. Mechanical Properties and Degradation of Chain and Step-Polymerized Photodegradable Hydrogels. Macromolecules 2013, 46, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, X.; Tao, Y.; Deng, C.; Yu, K.; Zhang, W.; Deng, L.; Xiong, W. Two-Step Freezing Polymerization Method for Efficient Synthesis of High-Performance Stimuli-Responsive Hydrogels. ACS Omega 2020, 5, 5921–5930. [Google Scholar] [CrossRef]
- Boere, K.W.M.; Soliman, B.G.; Rijkers, D.T.S.; Hennink, W.E.; Vermonden, T. Thermoresponsive Injectable Hydrogels Cross-Linked by Native Chemical Ligation. Macromolecules 2014, 47, 2430–2438. [Google Scholar] [CrossRef]
- Klouda, L.; Perkins, K.R.; Watson, B.M.; Hacker, M.C.; Bryant, S.J.; Raphael, R.M.; Kurtis Kasper, F.; Mikos, A.G. Thermoresponsive, in Situ Cross-Linkable Hydrogels Based on N-Isopropylacrylamide: Fabrication, Characterization and Mesenchymal Stem Cell Encapsulation. Acta Biomater. 2011, 7, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Ormanci, O.; Akin, I.; Sahin, F.; Yucel, O.; Simon, V.; Cavalu, S.; Goller, G. Spark plasma sintered Al2O3–YSZ–TiO2 composites: Processing, characterization and in vivo evaluation. Mater. Sci. Eng. C 2014, 40, 16–23. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive Hydrogel Induced by Dual Supramolecular Assemblies and Its Controlled Release Property for Enhanced Anticancer Drug Delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of Phase Transition in Thermoresponsive Hydrogel PNIPAM: Role in Drug Delivery and Tissue Engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-Responsive and Multi-Responsive Grafted Polymer Brushes with Transitions Based on Critical Solution Temperature: Synthesis, Properties, and Applications. Colloid Polym. Sci. 2021, 299, 363–383. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, K.; Ding, Y.; Zhang, G.; Wu, C. Origin of Hysteresis Observed in Association and Dissociation of Polymer Chains in Water. Phys. Chem. Chem. Phys. 2010, 12, 3188. [Google Scholar] [CrossRef]
- Najafi, M.; Habibi, M.; Fokkink, R.; Hennink, W.E.; Vermonden, T. LCST Polymers with UCST Behavior. Soft Matter 2021, 17, 2132–2141. [Google Scholar] [CrossRef]
- Ougizawa, T.; Inoue, T. UCST and LCST Behavior in Polymer Blends and Its Thermodynamic Interpretation. Polym. J. 1986, 18, 521–527. [Google Scholar] [CrossRef]
- Bischofberger, I.; Trappe, V. New Aspects in the Phase Behaviour of Poly-N-Isopropyl Acrylamide: Systematic Temperature Dependent Shrinking of PNiPAM Assemblies Well beyond the LCST. Sci. Rep. 2015, 5, 15520. [Google Scholar] [CrossRef] [PubMed]
- Kohori, F.; Sakai, K.; Aoyagi, T.; Yokoyama, M.; Yamato, M.; Sakurai, Y.; Okano, T. Control of Adriamycin Cytotoxic Activity Using Thermally Responsive Polymeric Micelles Composed of Poly(N-Isopropylacrylamide-Co-N,N-Dimethylacrylamide)-b-Poly(d,l-Lactide). Colloids Surf. B Biointerfaces 1999, 16, 195–205. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2021, 31, 2008123. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Non-Ionic Homo- and Copolymers with H-Donor and H-Acceptor Units with an UCST in Water. Macromol. Chem. Phys. 2010, 211, 2109–2117. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive Polymer Nanocarriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zheng, S. Poly(Acrylic Acid)-Grafted Poly(N-Isopropyl Acrylamide) Networks: Preparation, Characterization and Hydrogel Behavior. J. Biomater. Sci., Polym. Ed. 2011, 22, 2305–2324. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Huglin, M.R. Hydrogels in Medicine and Pharmacy; Peppas, N.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1986; Volume 21, p. 184. [Google Scholar] [CrossRef]
- Amsden, B. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed; John Wiley & Sons, Inc.: New York, NY, USA, 2002; ISBN 978-0-471-41077-5. [Google Scholar]
- Cohen, M.H.; Turnbull, D. Molecular Transport in Liquids and Glasses. J. Chem. Physics 1959, 31, 1164–1169. [Google Scholar] [CrossRef]
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical Foundations and Structural Design of Hydrogels in Medicine and Biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef]
- Siepmann, J. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute Diffusion in Swollen Membranes. IX. Scaling Laws for Solute Diffusion in Gels. J. Appl. Polym. Sci. 1988, 36, 735–747. [Google Scholar] [CrossRef]
- Mason, M.N.; Metters, A.T.; Bowman, C.N.; Anseth, K.S. Predicting Controlled-Release Behavior of Degradable PLA- b -PEG- b -PLA Hydrogels. Macromolecules 2001, 34, 4630–4635. [Google Scholar] [CrossRef]
- Anseth, K.S.; Metters, A.T.; Bryant, S.J.; Martens, P.J.; Elisseeff, J.H.; Bowman, C.N. In Situ Forming Degradable Networks and Their Application in Tissue Engineering and Drug Delivery. J. Control. Release 2002, 78, 199–209. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef]
- Yoshida, R.; Sakai, K.; Okano, T.; Sakurai, Y. Pulsatile Drug Delivery Systems Using Hydrogels. Adv. Drug Deliv. Rev. 1993, 11, 85–108. [Google Scholar] [CrossRef]
- Brazel, C. Dimensionless Analysis of Swelling of Hydrophilic Glassy Polymers with Subsequent Drug Release from Relaxing Structures. Biomaterials 1999, 20, 721–732. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Modeling of Drug Release from Swellable Polymers. Eur. J. Pharm. Biopharm. 2000, 49, 47–58. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Mechanisms of Solute and Drug Transport in Relaxing, Swellable, Hydrophilic Glassy Polymers. Polymer 1999, 40, 3383–3398. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the Morphology of Hydrophilic Polymeric Matrices on the Diffusion and Release of Water Soluble Drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between Mesh Size and Equilibrium Degree of Swelling of Polymeric Networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, D.; Peppas, N.A. Modelling of Water Transport and Solute Release in Physiologically Sensitive Gels. J. Control. Release 1993, 23, 123–135. [Google Scholar] [CrossRef]
- Hedenqvist, M.; Angelstok, A.; Edsberg, L.; Larsson, P.T.; Gedde, U.W. Diffusion of Small-Molecule Penetrants in Polyethylene: Free Volume and Morphology. Polymer 1996, 37, 2887–2902. [Google Scholar] [CrossRef]
- Siepmann, J.; Podual, K.; Sriwongjanya, M.; Peppas, N.A.; Bodmeier, R. A New Model Describing the Swelling and Drug Release Kinetics from Hydroxypropyl Methylcellulose Tablets. J. Pharm. Sci. 1999, 88, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Kranz, H.; Bodmeier, R.; Peppas, N.A. HPMC-Matrices for Controlled Drug Delivery: A New Model Combining Diffusion, Swelling, and Dissolution Mechanisms and Predicting the Release Kinetics. Pharm. Res. 1999, 16, 1748–1756. [Google Scholar] [CrossRef]
- Wu, N.; Wang, L.-S.; Tan, D.C.-W.; Moochhala, S.M.; Yang, Y.-Y. Mathematical Modeling and in Vitro Study of Controlled Drug Release via a Highly Swellable and Dissoluble Polymer Matrix: Polyethylene Oxide with High Molecular Weights. J. Control. Release 2005, 102, 569–581. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Development of Fibrin Derivatives for Controlled Release of Heparin-Binding Growth Factors. J. Control. Release 2000, 65, 389–402. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Panitch, A.; Hubbell, J.A. Development of Growth Factor Fusion Proteins for Cell-triggered Drug Delivery. FASEB J. 2001, 15, 1300–1302. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Controlled Release of Nerve Growth Factor from a Heparin-Containing Fibrin-Based Cell Ingrowth Matrix. J. Control. Release 2000, 69, 149–158. [Google Scholar] [CrossRef]
- Taylor, S.J.; McDonald, J.W.; Sakiyama-Elbert, S.E. Controlled Release of Neurotrophin-3 from Fibrin Gels for Spinal Cord Injury. J. Control. Release 2004, 98, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Metters, A.; Zammaretti, P.; Hubbell, J.A.; Zisch, A.H. Endothelial Cell Proliferation and Progenitor Maturation by Fibrin-Bound VEGF Variants with Differential Susceptibilities to Local Cellular Activity. J. Control. Release 2005, 101, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.A.; Sanchez-Adams, J.; Anseth, K.S. Exogenously Triggered, Enzymatic Degradation of Photopolymerized Hydrogels with Polycaprolactone Subunits: Experimental Observation and Modeling of Mass Loss Behavior. Biomacromolecules 2006, 7, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible Hydrogels Based on Photopolymerized Poly(Ethylene Glycol)-co-Poly(.Alpha.-Hydroxy Acid) Diacrylate Macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Edelman, E.R.; Nugent, M.A.; Smith, L.T.; Karnovsky, M.J. Basic Fibroblast Growth Factor Enhances the Coupling of Intimal Hyperplasia and Proliferation of Vasa Vasorum in Injured Rat Arteries. J. Clin. Invest. 1992, 89, 465–473. [Google Scholar] [CrossRef][Green Version]
- Ruppert, R.; Hoffmann, E.; Sebald, W. Human Bone Morphogenetic Protein 2 Contains a Heparin-Binding Site Which Modifies Its Biological Activity. Eur. J. Biochem 1996, 237, 295–302. [Google Scholar] [CrossRef]
- Rojekar, S.; Vora, L.K.; Tekko, I.A.; Volpe-Zanutto, F.; McCarthy, H.O.; Vavia, P.R.; Donnelly, R.F. Etravirine-Loaded Dissolving Microneedle Arrays for Long-Acting Delivery. Eur. J. Pharm. Biopharm. 2021, 165, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Damiri, F.; Rojekar, S.; Zehravi, M.; Ramproshad, S.; Dhoke, D.; Musale, S.; Mulani, A.A.; Modak, P.; Paradhi, R.; et al. Recent Advancements in Microneedle Technology for Multifaceted Biomedical Applications. Pharmaceutics 2022, 14, 1097. [Google Scholar] [CrossRef] [PubMed]
- Trimukhe, A.; Rojekar, S.; Vavia, P.R.; Deshmukh, R.R. Pulsed Plasma Surface Modified Omeprazole Microparticles for Delayed Release Application. J. Drug Deliv. Sci. Technol. 2021, 66, 102905. [Google Scholar] [CrossRef]
- Rojekar, S.V.; Trimukhe, A.M.; Deshmukh, R.R.; Vavia, P.R. Novel Pulsed Oxygen Plasma Mediated Surface Hydrophılizatıon of Ritonavır for the Enhancement of Wettability and Solubility. J. Drug Deliv. Sci. Technol. 2021, 63, 102497. [Google Scholar] [CrossRef]
- Berkenblit, S.I.; Quinn, T.M.; Grodzinsky, A.J. Molecular Electromechanics of Cartilaginous Tissues and Polyelectrolyte Gels. J. Electrost. 1995, 34, 307–330. [Google Scholar] [CrossRef]
- Sohier, J.; Vlugt, T.J.H.; Cabrol, N.; Van Blitterswijk, C.; de Groot, K.; Bezemer, J.M. Dual Release of Proteins from Porous Polymeric Scaffolds. J. Control. Release 2006, 111, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. Dual Growth Factor Delivery from Degradable Oligo(Poly(Ethylene Glycol) Fumarate) Hydrogel Scaffolds for Cartilage Tissue Engineering. J. Control. Release 2005, 101, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Crotts, G.; Park, T.G. Protein Delivery from Poly(Lactic-Co-Glycolic Acid) Biodegradable Microspheres: Release Kinetics and Stability Issues. J. Microencapsul. 1998, 15, 699–713. [Google Scholar] [CrossRef]
- Gombotz, W. Protein Release from Alginate Matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.; Kan, C. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Health. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef]
- Jung, I.Y.; Lee, E.H.; Suh, A.Y.; Lee, S.J.; Lee, H. Oligonucleotide-Based Biosensors for in Vitro Diagnostics and Environmental Hazard Detection. Anal. Bioanal. Chem. 2016, 408, 2383–2406. [Google Scholar] [CrossRef]
- Gehrke, S.H.; Uhden, L.H.; McBride, J.F. Enhanced Loading and Activity Retention of Bioactive Proteins in Hydrogel Delivery Systems. J. Control. Release 1998, 55, 21–33. [Google Scholar] [CrossRef]
- Larsson, A.; Ekblad, T.; Andersson, O.; Liedberg, B. Photografted Poly(Ethylene Glycol) Matrix for Affinity Interaction Studies. Biomacromolecules 2007, 8, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Siegers, C.; Biesalski, M.; Haag, R. Self-Assembled Monolayers of Dendritic Polyglycerol Derivatives on Gold That Resist the Adsorption of Proteins. Chem. Eur. J. 2004, 10, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.W.; Kim, J.; Chapin, S.C.; Duong, T.; Donohue, E.; Pandey, P.; Broom, W.; Hill, W.A.; Doyle, P.S. Multiplexed Detection of MRNA Using Porosity-Tuned Hydrogel Microparticles. Anal. Chem. 2012, 84, 9370–9378. [Google Scholar] [CrossRef] [PubMed]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Scheller, F.; Schubert, F. Biosensors; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1992; ISBN 9780444987839. [Google Scholar]
- Öztop, H.N.; Akyildiz, F.; Saraydin, D. Poly(Acrylamide/Vinylsulfonic Acid) Hydrogel for Invertase Immobilization. Microsc. Res. Tech. 2020, 83, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Badalyan, A.; Neumann-Schaal, M.; Leimkühler, S.; Wollenberger, U. A Biosensor for Aromatic Aldehydes Comprising the Mediator Dependent PaoABC-Aldehyde Oxidoreductase. Electroanalysis 2013, 25, 101–108. [Google Scholar] [CrossRef]
- Khayyami, M. Development of an Amperometric Biosensor Based on Acetylcholine Esterase Covalently Bound to a New Support Material. Talanta 1998, 45, 557–563. [Google Scholar] [CrossRef]
- Sorber, J.; Steiner, G.; Schulz, V.; Guenther, M.; Gerlach, G.; Salzer, R.; Arndt, K.-F. Hydrogel-Based Piezoresistive PH Sensors: Investigations Using FT-IR Attenuated Total Reflection Spectroscopic Imaging. Anal. Chem. 2008, 80, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Bund, A.; Keller, M.; Arndt, K.-F. Characterization of a Microgravimetric Sensor Based on PH Sensitive Hydrogels. Sens. Actuators B Chem. 2004, 99, 579–585. [Google Scholar] [CrossRef]
- Zhan, Y.; Gonçalves, M.; Yi, P.; Capelo, D.; Zhang, Y.; Rodrigues, J.; Liu, C.; Tomás, H.; Li, Y.; He, P. Thermo/Redox/PH-Triple Sensitive Poly(N-Isopropylacrylamide-co-Acrylic Acid) Nanogels for Anticancer Drug Delivery. J. Mater. Chem. B 2015, 3, 4221–4230. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages. Sensors 2019, 19, 1199. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Li, Z.; Li, J.; Peng, H.; Su, S.; Li, Q.; Zhu, C.; Zuo, X.; Song, S.; Wang, L.; et al. Hybridization Chain Reaction Amplification for Highly Sensitive Fluorescence Detection of DNA with Dextran Coated Microarrays. Biosens. Bioelectron. 2016, 81, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Park, S.-Y. Poly(Acrylic Acid) Hydrogel Microspheres for a Metal-Ion Sensor. ACS Sens. 2021, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.K.; Lin, Y.; Wojciechowski, J.P.; Lee-Reeves, C.; Hachim, D.; Zhou, K.; Burdick, J.A.; Stevens, M.M. Expanding and Optimizing 3D Bioprinting Capabilities Using Complementary Network Bioinks. Sci. Adv. 2020, 6, eabc5529. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Wang, S.; Lee, J.M.; Yeong, W.Y. Smart Hydrogels for 3D Bioprinting. Int. J. Bioprinting 2015. [Google Scholar] [CrossRef]
- Furth, M.E.; Atala, A.; Van Dyke, M.E. Smart Biomaterials Design for Tissue Engineering and Regenerative Medicine. Biomaterials 2007, 28, 5068–5073. [Google Scholar] [CrossRef]
- Li, M.; Jilie, K. Smart Hydrogels. In Smart Polymers; Galaev, I., Mattiasson, B., Eds.; CRC Press: Cambridge, UK, 2007; pp. 247–268. ISBN 978-0-8493-9161-3. [Google Scholar]
- Ma, S.; Yu, B.; Pei, X.; Zhou, F. Structural Hydrogels. Polymer 2016, 98, 516–535. [Google Scholar] [CrossRef]
- Saul, J.M.; Williams, D.F. Hydrogels in Regenerative Medicine. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2011; pp. 279–302. ISBN 978-0-323-22805-3. [Google Scholar]
- Fan, R.; Sun, W.; Zhang, T.; Wang, R.; Tian, Y.; Zhang, H.; Li, J.; Zheng, A.; Song, S. Paclitaxel-Nanocrystals-Loaded Network Thermosensitive Hydrogel for Localised Postsurgical Recurrent of Breast Cancer after Surgical Resection. Biomed. Pharmacother. 2022, 150, 113017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.; Wu, C. Injectable and Body Temperature Sensitive Hydrogels Based on Chitosan and Hyaluronic Acid for PH Sensitive Drug Release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic Imine Bond Cross-Linked Self-Healing Thermosensitive Hydrogels for Sustained Anticancer Therapy via Intratumoral Injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Geng, S.; Zhao, H.; Peng, X.; Zhou, Q.; Li, H.; He, M.; Zhao, Y.; Yang, X.; Xu, H. Doxorubicin-Induced Co-Assembling Nanomedicines with Temperature-Sensitive Acidic Polymer and Their in-Situ -Forming Hydrogels for Intratumoral Administration. J. Control. Release 2016, 235, 328–336. [Google Scholar] [CrossRef]
- Park, K.S.; Bazzill, J.D.; Son, S.; Nam, J.; Shin, S.W.; Ochyl, L.J.; Stuckey, J.A.; Meagher, J.L.; Chang, L.; Song, J.; et al. Lipid-Based Vaccine Nanoparticles for Induction of Humoral Immune Responses against HIV-1 and SARS-CoV-2. J. Control. Release 2021, 330, 529–539. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Thambi, T.; Gil, M.S.; Lee, D.S. Temperature and PH-Sensitive Injectable Hydrogels Based on Poly(Sulfamethazine Carbonate Urethane) for Sustained Delivery of Cationic Proteins. Polymer 2017, 109, 38–48. [Google Scholar] [CrossRef]
- Song, K.; Li, L.; Yan, X.; Zhang, W.; Zhang, Y.; Wang, Y.; Liu, T. Characterization of Human Adipose Tissue-Derived Stem Cells in Vitro Culture and in Vivo Differentiation in a Temperature-Sensitive Chitosan/β- Glycerophosphate/Collagen Hybrid Hydrogel. Mater. Sci. Eng. C 2017, 70, 231–240. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, X.; Xiao, X.; Chai, Y.; Chen, Z.; Zhou, G.; Fan, Y. Near-Infrared Triggered Injectable Ferrimagnetic Chitosan Thermosensitive Hydrogel for Photo Hyperthermia and Precisely Controlled Drug Release in Tumor Ablation. Eur. Polym. J. 2022, 162, 110879. [Google Scholar] [CrossRef]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.-E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.K.A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.; Dong, T.; Wang, X.; Zhang, L.; Yang, W. Dual Physically Cross-Linked κ-Carrageenan-Based Double Network Hydrogels with Superior Self-Healing Performance for Biomedical Application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef] [PubMed]
- Ghanian, M.H.; Mirzadeh, H.; Baharvand, H. In Situ Forming, Cytocompatible, and Self-Recoverable Tough Hydrogels Based on Dual Ionic and Click Cross-Linked Alginate. Biomacromolecules 2018, 19, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, G.; Wang, Y.; Ding, F. Thermosensitive Chitosan Hydrogel for Implantable Drug Delivery: Blending PVA to Mitigate Body Response and Promote Bioavailability. J. Appl. Polym. Sci. 2012, 125, 2092–2101. [Google Scholar] [CrossRef]
- Khan, M.; Koivisto, J.T.; Hukka, T.I.; Hokka, M.; Kellomäki, M. Composite Hydrogels Using Bioinspired Approach with in Situ Fast Gelation and Self-Healing Ability as Future Injectable Biomaterial. ACS Appl. Mater. Interfaces 2018, 10, 11950–11960. [Google Scholar] [CrossRef]
- Sharma, P.K.; Taneja, S.; Singh, Y. Hydrazone-Linkage-Based Self-Healing and Injectable Xanthan–Poly(Ethylene Glycol) Hydrogels for Controlled Drug Release and 3D Cell Culture. ACS Appl. Mater. Interfaces 2018, 10, 30936–30945. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Thorat, K.; Kaur, S.; Chandan, R.; Banerjee, R. A Tumor Responsive Self Healing Prodrug Hydrogel Enables Synergistic Action of Doxorubicin and Miltefosine for Focal Combination Chemotherapy. J. Mater. Chem. B 2019, 7, 2920–2925. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.; An, H.; Chang, L.; Wang, Y.; Li, W.; Qin, J. Cross-Linking Induced Thermoresponsive Hydrogel with Light Emitting and Self-Healing Property. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 869–877. [Google Scholar] [CrossRef]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and Characterization of Lyophilized Chitosan-Based Hydrogels Cross-Linked with Benzaldehyde for Controlled Drug Release. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Nejadnik, M.R.; Yang, X.; Bongio, M.; Alghamdi, H.S.; van den Beucken, J.J.J.P.; Huysmans, M.C.; Jansen, J.A.; Hilborn, J.; Ossipov, D.; Leeuwenburgh, S.C.G. Self-Healing Hybrid Nanocomposites Consisting of Bisphosphonated Hyaluronan and Calcium Phosphate Nanoparticles. Biomaterials 2014, 35, 6918–6929. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An Injectable Self-Healing Hydrogel with Adhesive and Antibacterial Properties Effectively Promotes Wound Healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S. Novel Chitosan–Cellulose Nanofiber Self-Healing Hydrogels to Correlate Self-Healing Properties of Hydrogels with Neural Regeneration Effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
- Chouhan, G.; Moakes, R.J.A.; Esmaeili, M.; Hill, L.J.; deCogan, F.; Hardwicke, J.; Rauz, S.; Logan, A.; Grover, L.M. A Self-Healing Hydrogel Eye Drop for the Sustained Delivery of Decorin to Prevent Corneal Scarring. Biomaterials 2019, 210, 41–50. [Google Scholar] [CrossRef]
- Jiang, X.; Li, M.; Guo, X.; Chen, H.; Yang, M.; Rasooly, A. Self-Assembled DNA-THPS Hydrogel as a Topical Antibacterial Agent for Wound Healing. ACS Appl. Bio Mater. 2019, 2, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.G.S.S.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Gavel, P.K.; Dev, D.; Parmar, H.S.; Bhasin, S.; Das, A.K. Investigations of Peptide-Based Biocompatible Injectable Shape-Memory Hydrogels: Differential Biological Effects on Bacterial and Human Blood Cells. ACS Appl. Mater. Interfaces 2018, 10, 10729–10740. [Google Scholar] [CrossRef]
- Tang, L.; Liao, S.; Qu, J. Self-Healing and Multistimuli-Responsive Hydrogels Formed via a Cooperation Strategy and Their Application in Detecting Biogenic Amines. ACS Appl. Mater. Interfaces 2018, 10, 27365–27373. [Google Scholar] [CrossRef]
- Huang, S.; Wan, F.; Bi, S.; Zhu, J.; Niu, Z.; Chen, J. A Self-Healing Integrated All-in-One Zinc-Ion Battery. Angew. Chem. Int. Ed. 2019, 58, 4313–4317. [Google Scholar] [CrossRef]
- Kuddushi, M.; Rajput, S.; Shah, A.; Mata, J.; Aswal, V.K.; El Seoud, O.; Kumar, A.; Malek, N.I. Stimuli Responsive, Self-Sustainable, and Self-Healable Functionalized Hydrogel with Dual Gelation, Load-Bearing, and Dye-Absorbing Properties. ACS Appl. Mater. Interfaces 2019, 11, 19572–19583. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Kang, B.; Zhao, Z.; Song, S. Stretchable Self-Healing Hydrogels Capable of Heavy Metal Ion Scavenging. RSC Adv. 2019, 9, 19039–19047. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Rahman, M.H.; Zehravi, M.; Awaji, A.A.; Nasrullah, M.Z.; Gad, H.A.; Bani-Fwaz, M.Z.; Varma, R.S.; Germoush, M.O.; Al-malky, H.S.; et al. MXene (Ti3C2Tx)-Embedded Nanocomposite Hydrogels for Biomedical Applications: A Review. Materials 2022, 15, 1666. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Bachra, Y.; Berrada, M. Synthesis and Characterization of 4-Formylphenylboronic Acid Cross-Linked Chitosan Hydrogel with Dual Action: Glucose-Sensitivity and Controlled Insulin Release. Chin. J. Anal. Chem. 2022, 50, 100092. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Raščan, I.M.; Smrke, D.M. Advanced Therapies of Skin Injuries. Wien Klin Wochenschr 2015, 127, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A Review of Tissue-Engineered Skin Bioconstructs Available for Skin Reconstruction. J. R. Soc. Interface. 2010, 7, 229–258. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, B.; Zhang, X.; Xu, L.; Tao, L.; Li, S.; Wei, Y. A Magnetic Self-Healing Hydrogel. Chem. Commun. 2012, 48, 9305. [Google Scholar] [CrossRef]
- Kabir, M.T.; Ferdous Mitu, J.; Akter, R.; Akhtar, M.F.; Saleem, A.; Al-Harrasi, A.; Bhatia, S.; Rahman, M.S.; Damiri, F.; Berrada, M.; et al. Therapeutic Potential of Dopamine Agonists in the Treatment of Type 2 Diabetes Mellitus. Environ. Sci. Pollut. Res. 2022, 29, 46385–46404. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Naficy, S.; Brown, H.R.; Razal, J.M.; Spinks, G.M.; Whitten, P.G. Progress Toward Robust Polymer Hydrogels. Aust. J. Chem. 2011, 64, 1007. [Google Scholar] [CrossRef]
- Gupta, D.; Gangwar, A.; Jyoti, K.; Sainaga Jyothi, V.G.S.; Sodhi, R.K.; Mehra, N.K.; Singh, S.B.; Madan, J. Self Healing Hydrogels: A New Paradigm Immunoadjuvant for Delivering Peptide Vaccine. Colloids Surf. B Biointerfaces 2020, 194, 111171. [Google Scholar] [CrossRef]
- Lai, W.-F. Development of Hydrogels with Self-Healing Properties for Delivery of Bioactive Agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, V.; Damiri, F.; Talreja, N.; Chauhan, D.; Mangalaraja, R.V.; Berrada, M.; Ashfaq, M. Carbon-Based Nanomaterials: An Efficient Tool for Improving the Nutritional Quality of Crops. In Metabolic Engineering in Plants; Aftab, T., Hakeem, K.R., Eds.; Springer Nature: Singapore, 2022; pp. 375–389. ISBN 9789811672613. [Google Scholar]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Tyne, C.; Blayney, D.W.; Blum, D.; Dicker, A.P.; Ganz, P.A.; Hoverman, J.R.; Langdon, R.; et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. JCO 2015, 33, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Noone, A.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Henley, S.J.; Jemal, A.; Cho, H.; Anderson, R.N.; Kohler, B.A.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2010, Featuring Prevalence of Comorbidity and Impact on Survival among Persons with Lung, Colorectal, Breast, or Prostate Cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High Interstitial Fluid Pressure — An Obstacle in Cancer Therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-Responsive Injectable Hydrogels Encapsulating Drug-Loaded Micelles for on-Demand Antimicrobial Activity and Accelerated Wound Healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-Rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Priya, S.G.; Jungvid, H.; Kumar, A. Skin Tissue Engineering for Tissue Repair and Regeneration. Tissue Eng. B Rev. 2008, 14, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Pixley, S.K.; Hopkins, T.M.; Little, K.J.; Hom, D.B. Evaluation of Peripheral Nerve Regeneration through Biomaterial Conduits via Micro-CT Imaging. Laryngoscope Investig. Otolaryngol. 2016, 1, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic Bonds between Boronic Acid and Alginate: Hydrogels with Stretchable, Self-Healing, Stimuli-Responsive, Remoldable, and Adhesive Properties. Biomacromolecules 2018, 19, 2053–2061. [Google Scholar] [CrossRef]
- Dankers, P.Y.W.; Hermans, T.M.; Baughman, T.W.; Kamikawa, Y.; Kieltyka, R.E.; Bastings, M.M.C.; Janssen, H.M.; Sommerdijk, N.A.J.M.; Larsen, A.; van Luyn, M.J.A.; et al. Hierarchical Formation of Supramolecular Transient Networks in Water: A Modular Injectable Delivery System. Adv. Mater. 2012, 24, 2703–2709. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.Y.; Peppas, N.A. Feedback Control Systems Using Environmentally and Enzymatically Sensitive Hydrogels. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010; pp. 45–64. ISBN 978-1-4419-5918-8. [Google Scholar]
- Feksa, L.R.; Troian, E.A.; Muller, C.D.; Viegas, F.; Machado, A.B.; Rech, V.C. Hydrogels for Biomedical Applications. In Nanostructures for the Engineering of Cells, Tissues and Organs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 403–438. ISBN 978-0-12-813665-2. [Google Scholar]
- Lu, W.; Ling, M.; Jia, M.; Huang, P.; Li, C.; Yan, B. Facile Synthesis and Characterization of Polyethylenimine-Coated Fe3O4 Superparamagnetic Nanoparticles for Cancer Cell Separation. Mol. Med. Rep. 2014, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Vatta, L.L.; Sanderson, R.D.; Koch, K.R. Magnetic Nanoparticles: Properties and Potential Applications. Pure Appl. Chem. 2006, 78, 1793–1801. [Google Scholar] [CrossRef]
- Cezar, C.A.; Kennedy, S.M.; Mehta, M.; Weaver, J.C.; Gu, L.; Vandenburgh, H.; Mooney, D.J. Biphasic Ferrogels for Triggered Drug and Cell Delivery. Adv. Health. Mater. 2014, 3, 1869–1876. [Google Scholar] [CrossRef]
- Weeber, R.; Kantorovich, S.; Holm, C. Ferrogels Cross-Linked by Magnetic Particles: Field-Driven Deformation and Elasticity Studied Using Computer Simulations. J. Chem. Phys. 2015, 143, 154901. [Google Scholar] [CrossRef]
- Balu, S.K.; Andra, S.; Damiri, F.; Sivaramalingam, A.; Sudandaradoss, M.V.; Kumarasamy, K.; Bhakthavachalam, K.; Ali, F.; Kundu, M.K.; Rahman, M.H.; et al. Size-Dependent Antibacterial, Antidiabetic, and Toxicity of Silver Nanoparticles Synthesized Using Solvent Extraction of Rosa Indica L. Petals. Pharmaceuticals 2022, 15, 689. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Day, J.; Shikanov, A. Immunoisolation to Prevent Tissue Graft Rejection: Current Knowledge and Future Use. Exp. Biol. Med. 2016, 241, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Regmi, S.; Jeong, J.-H. Injectable Hydrogels for Islet Transplantation: A Concise Review. J. Pharm. Investig. 2020, 50, 29–45. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and nanostructured surface fabrication for innovative cranial and maxillofacial surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef]
- Dang, J.M.; Sun, D.D.N.; Shin-Ya, Y.; Sieber, A.N.; Kostuik, J.P.; Leong, K.W. Temperature-Responsive Hydroxybutyl Chitosan for the Culture of Mesenchymal Stem Cells and Intervertebral Disk Cells. Biomaterials 2006, 27, 406–418. [Google Scholar] [CrossRef]
- Jiang, H.; Wagner, W.R.; Yoshizumi, T.; Zhu, Y. Biodegradable, Thermally Responsive Injectable Hydrogel for Treatment of Ischemic Cardiomyopathy. U.S. Patent No. 10,589,002, 17 March 2020. [Google Scholar]
- Brey, E.; Kang-Mieler, J.J.; Perez-Luna, V.; Jiang, B.; Drapala, P.; Schäfer, R.; Hitz, H. Thermo-Responsive Hydrogel Compositions. U.S. Patent No. 13,261,732, 5 March 2012. [Google Scholar]
- Fedorchak, M.V.; Little, S.R.; Schuman, J.S.; Cugini, A. Thermoresponsive Hydrogel Containing Polymer Microparticles for Noninvasive Ocular Drug Delivery. U.S. Patent No. 11,246,838, 15 February 2022. [Google Scholar]
- Kelly, H.; Duffy, G.; Rossi, S.; Hastings, C. A Thermo-Responsive Hydrogel For Intratumoral Administration as a Treatment in Solid Tumor Cancers. U.S. Patent No. 16/762,138, 7 November 2018. [Google Scholar]
- Hu, Z.; Xia, X. Aqueous Dispersion of Hydrogel Nanoparticles with Inverse Thermoreversible Gelation. U.S. Patent No. 8,048,450, 1 November 2011. [Google Scholar]
- Deming, T.J.; Michael, V.S.; Zhang, S. Non-Ionic and Thermoresponsive Diblock Copolypeptide Hydrogels for Delivery of Molecules and Cells. U.S. Patent No. 11,298,424, 12 April 2022. [Google Scholar]
- Thermoresponsive Arginine-Based Hydrogels as Biologic Carriers. Available online: https://portal.unifiedpatents.com/patents/patent/US-8858998-B2 (accessed on 7 May 2022).
- Hakim, G.; Friedman, A.; Konorty, M.; Strauss-Ayali, D. Immunomodulating Treatments of Body Cavities. U.S. Patent No. 10,758,482, 1 September 2020. [Google Scholar]
- Czaplewski, L.; Guest, S. Antibiotic Formulations for Lower Back Pain. U.S. Patent No. 17,142,559, 6 January 2021. [Google Scholar]
- NAM, J.; Brunelle, A. Hydrogel Scaffold for Three Dimensional Cell Culture. U.S. Patent Application No. 16,050,510, 31 January 2019. [Google Scholar]
- Ameer, G.A.; Morochnik, S. Bone-Promoting Thermoresponsive Macromolecules. U.S. Patent Application No. 16,468,224, 7 November 2019. [Google Scholar]
- Benhabbour, S.R.; Maturavongsadit, P. Injectable Thermoresponsive Hydrogels as a Combinatory Mo-dality for Controlled Drug Delivery, Biomaterial Implant and 3D Printing Bioink. International Patent Application No. WO2019232114, 30 May 2019. [Google Scholar]
- Schoess, J.N.; Sivaprakasam, K. Miniature Pumps. U.S. Patent No. 9046085, 30 May 2013. [Google Scholar]
- Lorson, T.; Luxenhofer, R. Thermogelling Supramolecular Sponge as Self-Healing and Biocompatible Hydrogel. U.S. Patent Application No. 16,253,897, 23 May 2019. [Google Scholar]
- Samia, A.C.S.; Frangiamore, S.J.; Higuera Rueda, C.A.; Klika, A.K.; Barsoum, W.K. Thermoresponsive Compositions and Methods for Preventing and Disrupting Biofilms. U.S. Patent Application No. 16,982,090, 15 April 2021. [Google Scholar]
- Maudens, P.; Allemann, E.; Jordan, O. Hyaluronic Acid Conjugates and Uses Thereof. U.S. Patent Application No. 16,094,559, 18 April 2017. [Google Scholar]
- Lu, S.X.; Lu, J.; Liu, L. Water-Soluble Supramolecular Complexes. International Patent Application No. PCT/US2015/061553, 19 November 2015. [Google Scholar]
- Han, D.K.; Park, K.; Kim, J.-J. Thermosensitive pluronic Derivative Hydrogels With High Biodegradability And Biocompatibility For Tissue Regeneration and Preparation Method Thereof. U.S. Patent Application No. 12,573,037, 22 October 2008. [Google Scholar]
- Wang, W.; Kennedy, R.; McMahon, S. Multifunctional Hyperbranched Polymers. U.S. Patent Application No. 14/221,106, 20 March 2014. [Google Scholar]
- Ben-Shalom, N.; Nevo, Z.; Patchornik, A.; Robinson, D. Injectable Chitosan Mixtures Forming Hydrogels. U.S. Patent No. 9,034,348, 19 May 2015. [Google Scholar]
- Kang-Mieler, J.J.; Brey, E.; Perez-Luna, V.; Jiang, B.; Drapala, P.; Hitz, H.; Schäfer, R. Thermo-Responsive Hydrogel Compositions. U.S. Patent Application No. 13/045,643, 11 March 2011. [Google Scholar]
- Richard, R.E. Injectable Hydrogel Compositions. International Patent Application No. PCT/US96/09065, 5 June 1996. [Google Scholar]
- Chausson, M.; Lecler, R. Thermo-Gelling Composition. International Patent Application No. PCT/EP2015/067754, 31 July 2015. [Google Scholar]
- Yao, J.Q.; Gao, J.; Huang, X.; Sanghvi, A. Thermosensitive Hydrogel Composition and. Method. Patent Application: RU2013143401A, Russia, 5 March 2012. [Google Scholar]
- Han, D.K.; Park, K.; Kim, J.-J. Thermosensitive Pluronic Derivative Hydrogels With High Biodegradability And Biocompatibility For Tissue Regeneration And Preparation Method. Thereof. Patent Application: US20100098762A1, 22 April 2010. [Google Scholar]
- Talaab, M. Nitazoxanide as a New Local Adjunctive to Nonsurgical Treatment of Moderate Periodontitis: Clinical and Biochemical Evaluation. Available online: https://clinicaltrials.gov/ct2/show/NCT04768530 (accessed on 7 May 2022).
- SocraTec R&D GmbH. Characterisation of Relative Bioavailability of a Newly Developed S-Flurbiprofen Containing Patch Formulation in Comparison With a Marketed Oral Flurbiprofen Containing Tablet Formulation - a Multiple Dose, Randomised, 2-Period Crossover. Available online: https://clinicaltrials.gov/ct2/show/NCT04505787 (accessed on 7 May 2022).
- Bayoumi, M. Evaluation of Diabetic Foot Wound Healing Using Hydrogel/Nano Silver-Based Dressing vs. Traditional Dressing: A Prospective Randomized Control Study. Available online: https://clinicaltrials.gov/ct2/show/NCT04834245 (accessed on 7 May 2022).
- Marchetti, E. Evaluation of Effects of Subgingival Administration of Metronidazole Hydrogel 25% in Stage II and III Periodontitis: Randomized, Split Mouth, Single-Blind Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT04983849 (accessed on 7 May 2022).
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 7 May 2022).
- History of Changes for Study: NCT04061733. Available online: https://clinicaltrials.gov/ct2/history/NCT04061733 (accessed on 7 May 2022).
- Polispecialistico, C.M. Prospective, Controlled, Randomized, Blinded Evaluator, Split-Face Clinical Study to Evaluate the Efficacy and Safety of Hyaluronic Acid Hydrogel With Lidocaine Lidocaine for the Treatment of Moderate to Severe Nasolabial Folds. Available online: https://clinicaltrials.gov/ct2/show/NCT05252325 (accessed on 7 May 2022).
- AquatrixTM II Cosmetic Hydrogels. Available online: https://www.knowde.com/stores/hydromer-inc/products/aquatrix-ii-cosmetic-hydrogels/ (accessed on 7 May 2022).
- Hydrogel Lip Patch, Private Label Manufacturer. Available online: https://www.taikicosmetics.com/en/masks-patches/31-lip-patch.html (accessed on 7 May 2022).
- Bio-Connect Mebiol® Gel, PNIPAAm-PEG 3D Thermoreversible Hydrogel. Available online: https://www.corning.com/worldwide/en/products/life-sciences/resources/digital-campaign/corning-matribot-bioprinter.html?_bt=575217572832&_bk=3d%20bio&_bm=b&_bn=g&_bg=133947935444&gclid=Cj0KCQjwviWBhD8ARIsAH1mCd4JnBhKObRZb5o4N9R-CjRixOwxI54V7_a0QS7iGYT_23ZBF10XJpkaApgDEALw_wcB, (accessed on 7 May 2022).
- Avastin® (Bevacizumab) Safety, Side Effects & Patient Financial Support. Available online: https://www.avastin.com/patient/mcrc.html (accessed on 7 May 2022).
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef]
- Purilon® Gel. Available online: https://www.coloplast.us/purilon-gel-en-us.aspx (accessed on 7 May 2022).
- INTRASITE Gel, Hydrogel Wound Dressing Range. Available online: https://www.smith-nephew.com/professional/products/advanced-wound-management/intrasite-gel/ (accessed on 7 May 2022).
- Pluromed, Inc. Receives FDA Approval for Its LeGoo(R) Endovascular Occlusion Gel. Available online: https://www.biospace.com/article/pluromed-inc-receives-fda-approval-for-its-legoo-r-endovascular-occlusion-gel-/ (accessed on 8 May 2022).
- Ashland GantrezTM Polymers. Available online: https://www.ashland.com/industries/personal-and-home-care/hair-care/gantrez-polymers (accessed on 8 May 2022).
- BTG International Inc. A Randomized Study of the Efficacy and Safety of OncoGelTM Treatment as an Adjunctive Therapy to Systemic Chemotherapy and Concurrent External Beam Radiation Prior to Surgery in Subjects With Localized or Loco-Regional Esophageal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00573131, (accessed on 8 May 2022).
- Shive, M.S.; Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Méthot, S.; Vehik, K.; Restrepo, A. BST-CarGel® Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. Cartilage 2015, 6, 62–72. [Google Scholar] [CrossRef]

| Matrix/Geometry Type | Diffusion-Controlled Drug Delivery System (Case I) | Swelling-Controlled Drug Delivery System (Case II) |
|---|---|---|
| Slab | n = 0.5 | n = 1 |
| Cylinder | n = 0.45 | n = 0.89 |
| Sphere | n = 0.43 | n = 0.85 |
| System Type | Subtype | Mechanism | Example | References |
|---|---|---|---|---|
| Kinetically controlled systems | Pendant chain | The drug is covalently bound to the hydrogel through breakable spacers, and the rate of the spacer-bond breakage controls drug release | Fibrin matrix bounded with pendant VEGF factors variants attached through the plasmin sensitive peptidyl substrates | [125] |
| Surface eroding | Drug release is facilitated through surface erosion of polymer matrices | In-vitro enzymatic surface erosion of the degradable poly (ethylene glycol)-poly-caprolactone (PCL-b-PEG-b-PCL) block-copolymer hydrogel systems in the presence of a high concentration of the lipase | [126] | |
| Reaction controlled systems | Bulk degrading | The drug release profile is facilitated through both network degradation (chemical reaction) and drug diffusion | PEG–PLA block co-polymers could be polymerized to form hydrolytically degradable hydrogel drug delivery systems | [127] |
| Affinity type | Reaction type reversible drug release mechanism works on the principle of affinity of the hydrogel systems, mainly used for therapeutic proteins-ligand delivery | The Heparin-loaded hydrogel matrix controlled the release rate of these growth factors by affinity binding. | [128,129] |
| System Type | Subtype | Mechanism | Example | References |
|---|---|---|---|---|
| Dynamic hydrogel drug delivery systems | Degradable | The drug release rate depends upon the matrix swelling, degradation and the diffusion | In-vitro enzymatic surface erosion or degradation of hydrogel systems and polymer-based microneedle systems or plasma coated drug delivery systems | [97,130,131,132,133] |
| Stimuli-sensitive | Drug release is controlled by external stimuli such as temperature, pH and enzymes. | pH-responsive poly (methacrylic acid) (PMAA) hydrogel system for delivery application | [134] | |
| Composite hydrogel drug delivery systems | Multi-layer type | The different layers were formed as per the release requirement; at a time, multiple drugs could be released or if required release of a single drug or molecule could be tuned | The multi-laminated hydrogel system developed through the photo-polymerization for multiple protein drug delivery | [135] |
| Multi-phase type | The drug release could be controlled by the multi-phase systems, such as the microsphere system in the hydrogel system (several viscosities) for multiple drug deliveries of biologics | Multiple protein drug delivery using the protein loaded microsphere and other protein-loaded hydrogel systems, the microsphere could be placed in the different viscosity of the hydrogel to tunned the drug release from the two phases. E.g., protein-loaded PLGA microspheres in hydrogel | [136] | |
| Micro/nano-scaled hydrogel drug delivery systems | This hydrogel system is prepared from the hydrophilic polymer. Generally, nano or microparticles were developed and loaded in the gel for single or multiple drug delivery; the type of polymer could control the drug release of the drug. And drug release could be predicted using diffusion or the monte Carlo model. | Protein-loaded PLGA microspheres in hydrogel | [137] | |
| In-situ forming hydrogels drug delivery systems | Drug release could depend upon the monomer/polymer used with different functionalities in this system. The solution form is converted into the gel form in-vivo, which regulates the drug release from the matrix; this could be based on the temperature or pH. | In-situ hydrogel-based delivery of the proteins, peptides | [138] | |
| Polymeric Carriers | Encapsulant | Gelling Temp | Comments | Ref. |
|---|---|---|---|---|
| Poloxamer 407, Poloxamer 188 and carbomer 974P | Paclitaxel (PTX) | 31–35 °C | Hydrogel has the adequate viscoelasticity and self-recovery. In vivo studies revealed that a PTX-nanocrystal laden gel suppressed both local and distant tumor growth. | [170] |
| Pluronic F127 and N, N, N-trimethyl chitosan | Docetaxel (DTX) | 30–35 °C | Pure DTX and DTX loaded PF127 hydrogel are less efficient at killing U87MG cells than DTX loaded PF127-TMC hydrogel. | [34] |
| Chitosan/hyaluronic acid/β-sodium glycerophosphate (CS/HA/GP) | Doxorubicin (DOX) | 31.2–37.2 °C | With increasing HA concentration, the gelling temperature of CS/HA/GP steadily declines and falls. | [171] |
| Chitosan/β-sodium glycerophosphate/polyethylene glycol (CGD) | Doxorubicin | 31–35 °C | Due to the development of Schiff base bonds among the amino groups in chitosan and the aldehyde groups in PEG, DOX-loaded CGD hydrogels had lower gelling temperatures and higher viscosity. | [172] |
| D-PNAx nanomedicines | Doxorubicin | 34–44 °C | Thermoresponsive sol-gel phase transitions of D-PNA100 nanoparticles observed in the range of 5.0 to 10.0% of D-PNA100 concentration, with CGTs decreasing from 38 °C at 5.0% to 32 °C at 10.0% as concentration rises. | [173] |
| Levan/N-isopropyl acrylamide | 5-aminosalicylic acid | 32.8–35.09 °C | The concentration of levan positively influenced the biocompatibility of the hydrogels. Moreover, when the amount of levan in the hydrogels increased, so did the amount of levan on the hydrogel surface. | [174] |
| Poly(ethylene glycol)-poly(sulfamethazine carbonate urethane | Lysozyme | 37 °C | Following subcutaneous administration in SD rats, lysozyme-loaded PEG-PSMCU composites produced an in-situ hydrogel, which significantly delayed the first burst and resulted in lysozyme release that lasted for 7 days. | [175] |
| Chitosan/b-glycerophosphate/collagen | Human adipose tissue-derived stem cells (ADSCs) | 36–38 °C | The capacity of ADSCs embedded hydrogel to develop into fatty tissue was also demonstrated in an in vivo investigation, indicating high histocompatibility and good adipogenesis potential. | [176] |
| Ferrimagnetic chitosan hydrogel (FCH) | Iron oxide Nanocubes (IONCs) | 37 °C | DOX-loaded ferrimagnetic chitosan hydrogel had a synergistic impact and provided long-term treatment for tumor cells. | [177] |
| Hydrogel Applications | References |
|---|---|
| Tissue engineering | [178,179,180,181] |
| Drug delivery | [178,179,182,183,184,185,186,187] |
| Wound management/healing | [131,178,179,188,189,190,191,192,193] |
| Miscellaneous applications | [35,51,52,53,54,55,170,171,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200] |
| Types of Hydrogel | Potential Application | References |
|---|---|---|
| Thermo-responsive | Tissue/Skin regeneration, wound healing, | [163,219,220] |
| Photoresponsive | Delivery of drugs, micro-fluidic devices | [163,221,222] |
| Electro responsive | Implant drug delivery | [178,223,224] |
| Magnetic responsive | Tissue repair, Diagnosis and targeting, Drug delivery, | [178,225,226,227,228,229] |
| pH-responsive | Protein and drug delivery, 3D cell culture | [178,221,222,230] |
| Glucose responsive | Devices, Immuno-isolation | [178,231,232] |
| Sr. No | Patent Number and Year | Title | Proposed Use | Findings of Invention/Summary | Inventors |
|---|---|---|---|---|---|
| 1 | US 20210361826, 2015 [235] | Biodegradable, Thermally Responsive Injectable Hydrogel for Treatment of Ischemic Cardiomyopathy | Ischemic Cardiomyopathy | Method of preparation and applications of biodegradable, thermoresponsive, elastomeric Material, especially copolymers of NIPAAm—N-isopropyl acrylamide (NIPAAm), N-vinylpyrrolidone and methacrylate-polylactide macromer residues are described. These have an LCST of less than 37ᴼC and degradation rate < 200 days in vivo. These compositions can be used for treating defects in heart muscle. | Hongbin Jiang, William R. Wagner, Tomo Yoshizumi, Yang Zhu |
| 2 | US20140065226A1, 2011 [236] | Thermo-responsive hydrogel compositions | Drug delivery for wound healing or Hydrogel loaded with Nanospheres for Ocular Application | The patent discloses the composition of thermoresponsive hydrogel synthesized by Radical polymerization, consisting of an acrylamide crosslinked with PEG -diacrylate and monomer containing amino acid. This thermoresponsive hydrogel shows a dual change in physicochemical characteristics when it comes in contact with the body temperature of mammal and releases embedded drug in a controlled manner | Eric Brey Jennifer J. Kang-Mieler, Victor Perez-Luna, Bin Jiang, Pawel Drapala, Rolf Schäfer, Hans Hitz |
| 3 | WO2014138085A1, 2014 [237] | The thermoresponsive hydrogel containing polymer microparticles for noninvasive ocular drug delivery | Ocular drug delivery | Self-administrable thermoresponsive hydrogel for ocular delivery of bioactive is discussed. The hydrogel consists of an elastin-like peptide, a polysaccharide. The drug is entrapped in polymeric microparticles, further embedded in the thermoresponsive hydrogel. | Morgan V. Fedorchak, Steven R. Little Joel S. Schuman Anthony Cugini |
| 4 | WO2019092049A1 WIPO (PCT) 2018 [238] | A thermo-responsive hydrogel for intratumoral administration as a treatment in solid tumor cancers | Solid tumors | A thermosensitive hydrogel that can be injected is formed using 15–25% poloxamer polymer along with chitosan, 2-Hydroxypropyl β-cyclodextrin and genipin. This hydrogel can be used to incorporate chemotherapeutic agents for treating solid tumors. | Helena Kelly, Garry Duffy, Seona Rossi, Conn Hastings |
| 5 | US20070116765A1, 2004 [239] | The aqueous dispersion of hydrogel nanoparticles with inverse thermoreversible gelation | Controlled drug delivery | Hydrogel nanoparticles have an interpenetrating polymer network with inverse thermogelation properties for drug delivery applications. An aqueous dispersion of hydrogel nanoparticles can release the drug in a time-dependent manner. Polymers used for preparation are poly(N-isopropyl acrylamide), and monomer comprises acrylic acid along with cross-linking agents such as N, N′-methylenebisacrylamide or N, N′-methylenebisacrylamide; potassium persulfate; ammonium persulfate are used as initiators; sodium dodecyl sulfate is used as a surfactant. | Zhibing Hu, Xiaohu Xia |
| 6 | US20170296672A1, 2015 [240] | Non-ionic and thermoresponsive diblock co-polypeptide hydrogels for delivery of molecules and cells | Delivery of drugs or cells and injecting cells into CNS. | The composition of co-polypeptide thermoresponsive hydrogel for delivery of the pharmaceutical substance, nucleic acid, peptide, hormone, or imaging agent is disclosed. The hydrogels are synthesized using a hydrophilic segment of poly methoxy ethoxy-ethyl-rac-glutamate for preparing nonionic diblock co-polypeptide hydrogels | Timothy J. Deming, Michael V. Sofroniew, Shanshan Zhang |
| 7 | US-8858998-B2, 2008 [241] | Thermoresponsive Arginine-based Hydrogels as Biologic Carriers | Biomedical applications for drug delivery | Cationic poly (ester amide) (PEA)-based hydrogels are fabricated using precursors such as unsaturated L-arginine based poly (ester amide) (UArg-PEA), pluronic DA or a combination. Hydrogels based on Pluronic DA/UArg-PEA combination and pure pluronicDA were thermosensitive, but pure UArg-PEA-based hydrogels were only biodegradable but not biodegradable thermoresponsive. These synthesized hydrogels can be utilized for various biomedical applications, especially drug delivery. | Chih-Chang Chu Hua Song |
| 8 | EP3708167A1, 2017 [242] | Immunomodulating treatments of body cavities | Cancer therapy | Treatment of cancer of internal body cavities (like cancer of the Urinary tract) and thus providing local drug delivery to the inaccessible regions in the body. It can also be used to deliver a combination of controlled drug delivery and immunomodulatory agents | Gil Hakim, Astar Friedman, Marina Konorty, Dalit Strauss-Ayali |
| 9 | US20190343761A1, 2017 [243] | Antibiotic formulations for lower back pain | relieve and treat low back pain | Discloses composition, methods of preparation of injectable, thermosensitive hydrogel containing a radio-contrast agent, a drug belonging to an antibiotic class, used for easing lower back pain | Lloyd Czaplewski, Sarah Guest |
| 10 | US20190030211A1, 2018 [244] | Hydrogel scaffold for three-dimensional cell culture | It encapsulates the cells in a 3D hydrogel scaffold that forms the engineered tissue. Methods of making engineered tissues. | This invention discusses the preparation and composition of an electrospun microfiber scaffold based on a combination of thermoresponsive polymer and biodegradable polymer for encapsulating cells for making engineered tissues. Thermoresponsive polymers (PEG)-poly(N-isopropyl acrylamide) and biodegradable polymers like PCL are mixed in the ratio of 65:35 | Jin Nam, Alexander Brunelle |
| 11 | US20190336648A1, 2017 [245] | Bone-promoting thermoresponsive macromolecules | Bone formation/repair and the treatment of bone diseases. | The thermoresponsive hydrogel formed via carbodiimide chemistry between peptide group covalently linked with the carboxyl group of citric acid monomers. The peptide is cyclic Arg-Gly-Asp (cRGD) which is conjugated covalently to carboxy groups of (Polyethyleneglycol citrate-co-N-isopropyl acrylamide) (PPCN). These are used for the delivery of bioactive agents. | Guillermo A. Ameer Simona Morochnik |
| 12 | US20210205459A1, 2019 [246] | Injectable thermoresponsive hydrogels as a combinatory modality for controlled drug delivery, biomaterial implant and 3d printing bio link | Drug delivery, implants, 3D printing bio link | Mechanical Stiffness and strength of Insitu thermoresponsive polymeric hydrogels formed using Polyethylene glycol, hyaluronic acid, polyvinyl chloride or methylcellulose were improved using cellulose derivatives such as Cellulose nanofibers/crystals. This combination can be used to control drug release or as an implant and bio ink for 3D printing and treating bone disorders, preventing cancer/infectious diseases. | Soumya Rahima Benhabbour, Panita Maturavongsadit |
| 13 | US20200100931A1 [247] | Thermoresponsive Skin Barrier Appliances | Wound healing | The patent discloses thermoresponsive ostomy (body wastes discharged through a surgically created opening in the body) and skin barrier appliances for wound healing. It describes an assembly consisting of a pump that expels biosealant to the pump output port upon being stimulated. It causes the collection of hydrogel beads (composed of NIPAAm) to vibrate with high energy. This friction causes localized heating, leading to the bead plug layer contracting or swelling in size. It can also sense wound leakage and absorb wound exudate | Jeffrey Norman, Schoess Kannan, Sivaprakasam |
| 14 | US2021317267A1, 2021 [248] | Thermogelling supramolecular sponge as self-healing and biocompatible hydrogel | Carrier materials for active ingredients such as drugs, cells, proteins and bioinks for 3D bioprinting in tissue engineering | Synthesis of block copolymers made up of poly (2-oxazine) and poly (2-oxazoline) is discussed. These hydrogels have advanced and efficient rheological and thermoresponsive characteristics due to specific structures [A].sub.n-[B].sub.m or [B].sub. N-[A].sub.m, where n and m have the approximately same value and range from 20 to 300. | Lorson, Thomas Luxenhofer, Robert |
| 15 | US 20210106708, 2019 [249] | THERMORESPONSIVE COMPOSITIONS AND METHODS FOR PREVENTING AND DISRUPTING BIOFILMS | Medical implant coated/impregnated with nanocomposite for disrupting or preventing biofilm formation. | A medical implant that is resistant to biofilm formation, wherein the medical implant is at least partially coated or impregnated with the nanocomposite A thermosensitive polymeric nanocomposite composed of one or more D-amino acids and one or more energy-actuatable particles is discussed. When the energy source is excited, it cause localized heat release from the nanocomposite leading to sol to gel transition of a glycol chitin-based hydrogel. | Anna Cristina S. Samias Alvatore J. Frangiamore Carlos A. Higuera Ruedaalison K. Klikawael K. Barsoum |
| 16 | WO2014138085A1, 2014 [237] | The thermoresponsive hydrogel containing polymer microparticles for noninvasive ocular drug delivery | Ocular drug delivery | This patent discloses the formulation method of drug-loaded polymeric microparticles embedded in thermoresponsive hydrogel for topical delivery to the ocular surface for treating glaucoma, conjunctivitis, chronic dry eye etc. Polymeric microparticles were composed of dextran, PLGA, PLA, PCL, alginate etc. Hydrogel comprises polyacrylamide, a silicon hydrogel, PEO/PPO, polyacrylic acid, N, N′-dimethyl aminoethyl methacrylate, which sustained release for up to 30 days. | Morgan V. Fedorchak Steven R. Little Joel S. Schuman Anthony Cugini |
| 17 | US10767037B2, 2016 [250] | Hyaluronic acid conjugates and uses thereof | Tissue engineering, cosmetics, drug delivery | Self-lubricating nano-ball-bearing (SLNBB) properties of Hyaluronic acid and N-isopropyl acrylamide-based polymer graft polymers are explored. Injectable, biocompatible, stable, biodegradable pH and thermo-sensitive polymeric hydrogels with long residence time at the injection site due to the formation of the spontaneous nanoparticles. These can be used as viscosupplementation/lubrication material for drug delivery or cosmetic applications | Pierre Maudens, Eric Allemann, Olivier Jordan |
| 18 | US9937254B2, 2011 [251] | Water-soluble supramolecular complexes | Solid dosage form for pharmaceutical, diagnosis or cosmetic use. | The water solubility of drugs can be improved when formulated as water-soluble supramolecular hydrogel complexes that form a transparent, thermoreversible gel upon the combination with water. They may be hydrated or dehydrated repeatedly for insoluble drugs. These are composed of at least two blocks of polyethylene oxide and at least one block of polypropylene oxide. | Shao Xiang, LuJeffrey LuLetian Liu |
| 19 | US20100098762A1, 2008 [252] | Thermosensitive Pluronic Derivative Hydrogels With High Biodegradability and Biocompatibility for Tissue Regeneration and Preparation Method Thereof | Tissue and organ regeneration | Pluronic-based thermoresponsive smart hydrogels are synthesized for tissue engineering applications. Pluronic is derivatized by conjugating it with biodegradable polymers. The drug/active ingredient is conjugated with methacryloxyethyl trimellitic acid anhydride that is conjugated to the biodegradable polymer | Dong Keun Han, Kwideok ParkJae-Jin Kim |
| 20 | US20150266986A1, 2014 [253] | Multifunctional Hyperbranched Polymers | Biomedical applications- wound healing | RAFT (Reverse Addition-Fragmentation chain Transfer) polymerization technique synthesizes PEG-based hyperbranched copolymer. These can be used for delivering antimicrobial agents. These hydrogels are stable for one year, as seen from stability studies. RAFT agents can be Dithiobenzoates, Trithiocarbonates and Dithiocarbamates. | Wenxin WangRobert KennedySean McMahon |
| 21 | Indian patent 279339, 2017 [254] | “Injectable hydrogel-forming chitosan mixtures” | Biomedical applications | Aqueous solutions containing chitosan derivatives are synthesized, showing dual responsive behavior, i.e., temperature sensitivity and pH-dependent change in physicochemical characteristics. These can be utilized for various biomedical applications. | Ben-Shalom Noah, Nevo Zvi, Patchornik Avraham, Robinson Dror |
| 22 | US20120231072A1, 2012 [255] | Thermo-responsive hydrogel compositions | Wound healing, anti-microbial effect through drug or drug-loaded nanoparticle. | The synthesis method of smart, thermo-responsive hydrogel consists of monomer and polymer having an amino acid side chain (comprises an amino acid linked to an acrylic-, maleic-, or phthalic-derivative or N-isopropyl acrylamide). | Jennifer J. KANG-Mielereric Breyvictor PEREZ-Lunabin Jiangpawel Drapalahans Hitzrolf Schaefer |
| 23 | US20090053276A1, 2008 [256] | Injectable hydrogel compositions | Drug delivery | Thermosensitive hydrogels in dry form or hydrated form are synthesized in this invention. These injectable hydrogels swell in-vivo in the body because their UCST is below body temperature or their LCST is above average body temperature, i.e., these hydrogels contract when cooled below UCST and expand when heated. | Robert E. Richard |
| 24 | US7658947B2, 2010 [257] | Thermogelling composition patent. | Drug delivery | Thermoresponsive hydrogel consisting of methylcellulose and citric acid is described. The developed hydrogel can be utilized for diverse applications like drug delivery, cosmetics, adjuvants, and nutritional agents. Controlled release of pharmaceutical agents through body cavities, topically or subcutaneous injections are possible. | Yanbing, H. Thermogelling composition |
| 25 | US20120020932A1, 2012 [258] | Thermosensitive hydrogel composition and method patent. | Drug delivery | Drug-loaded injectable thermosensitive hydrogel composed of methylcellulose as the thermoresponsive polymer is synthesized. It also contains extracellular matrix protein and Hyaluronic acid. This can remain as a liquid at room temperature for ease of administration and gels; once it reaches the desired site in the body, it sets as a gel due to a change in the temperature. | Jian, Q.Y. |
| 26 | US20100098762A1, 2010 [259] | Thermosensitive pluronic derivative hydrogel with high biodegradability and biocompatibility for tissue regeneration and preparation method thereof | Tissue engineering | Biocompatible, thermosensitive and biodegradable hydrogels are synthesized using derivatization of Pluronic. Active constituents are conjugated with derivatized pluronic and utilized for tissue regeneration in tissue engineering | Dong, K.H. |
| Status of Clinical Trial | Outcome of Study | Use (Disease and Formulation) | Clinical Trial Identifier |
|---|---|---|---|
| Completed Phase II | Nonsurgical, local, adjunctive therapy for periodontitis treatment using Nitazoxanide loaded into thermoresponsive hydrogels. | Nitazoxanide hydrogel for periodontitis | ClinicalTrials.gov Identifier: Identifier: NCT04768530, 24 February 2021 [260] |
| Phase I | Hydrogel patch developed for S-flurbiprofen and its bioavailability is compared with the marketed tablet formulation | Flurbiprofen (Nonsteroidal anti-inflammatory drug) hydrogel patch for arthritis or dental pain. | ClinicalTrials.gov Identifier: NCT04505787, 10 August 2020 [261] |
| NA | Hydrogel based wound dressing for treating Diabetic Foot Wounds is formulated and evaluated, and its efficacy is checked against traditional wound dressing | Hydrogel/nano silver-based dressing for diabetic foot ulcers. | ClinicalTrials.gov Identifier: NCT04834245, 8 April 2021 [262] |
| Phase 4 | Metronidazole hydrogels are developed for sublingual administration to treat periodontitis in Stages I and II | Metronidazole hydrogels for periodontitis | NCT04983849, 30 July 2021 [263] |
| Phase 4 | Bulkamid is synthesized using polyacrylamide hydrogel as a transanal injection for the treatment of anal incontinence | Bulkamid for anal incontinence using transanal injection | ClinicalTrials.gov Identifier: NCT02550899, 12 January 2016 [264] |
| NA | The safety and efficacy of HEC-hydroxyethyl cellulose hydrogel (PROMGEL-OA) are studied to treat knee pain caused by osteoarthritis. | (PROMGEL-OA) Hydrogel injection for Osteoarthritis | NCT04061733, 4 May 2022 [265] |
| NA | Local injection for correction of nasolabial folds containing Hyaluronic Acid and Lidocaine | Hydrogel injection for nasolabial folds | ClinicalTrials.gov Identifier: NCT05252325, 23 February 2022 [266] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardeshi, S.; Damiri, F.; Zehravi, M.; Joshi, R.; Kapare, H.; Prajapati, M.K.; Munot, N.; Berrada, M.; Giram, P.S.; Rojekar, S.; et al. Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers 2022, 14, 3126. https://doi.org/10.3390/polym14153126
Pardeshi S, Damiri F, Zehravi M, Joshi R, Kapare H, Prajapati MK, Munot N, Berrada M, Giram PS, Rojekar S, et al. Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers. 2022; 14(15):3126. https://doi.org/10.3390/polym14153126
Chicago/Turabian StylePardeshi, Sagar, Fouad Damiri, Mehrukh Zehravi, Rohit Joshi, Harshad Kapare, Mahendra Kumar Prajapati, Neha Munot, Mohammed Berrada, Prabhanjan S. Giram, Satish Rojekar, and et al. 2022. "Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications" Polymers 14, no. 15: 3126. https://doi.org/10.3390/polym14153126
APA StylePardeshi, S., Damiri, F., Zehravi, M., Joshi, R., Kapare, H., Prajapati, M. K., Munot, N., Berrada, M., Giram, P. S., Rojekar, S., Ali, F., Rahman, M. H., & Barai, H. R. (2022). Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers, 14(15), 3126. https://doi.org/10.3390/polym14153126












