Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. QbD Using Box–Behnken Experimental Design
2.3. Preparation of PGZ-Loaded NE
2.4. Particle Size Measurement
2.5. Drug Release Study
2.6. Kinetic Study
2.7. Drug Content
2.8. Scanning Electron Microscopy
2.9. Stability Study
2.10. Handling of Animals
2.11. Ethical Approval
2.12. In Vivo Hypoglycemic Activity
2.12.1. Induction of Diabetes
2.12.2. Study Design
- Group I:
- negative control group received saline orally (non-treated).
- Group I:
- positive control group received marketed PGZ product (ACTOS®) (equivalent to 30 mg/kg).
- Group III:
- rats treated with NE free from PGZ (blank NE).
- Group IV:
- rats treated with optimized PGZ-loaded NE (equivalent to 30 mg/kg) [28].
2.13. Statistical Analysis
3. Result and Discussion
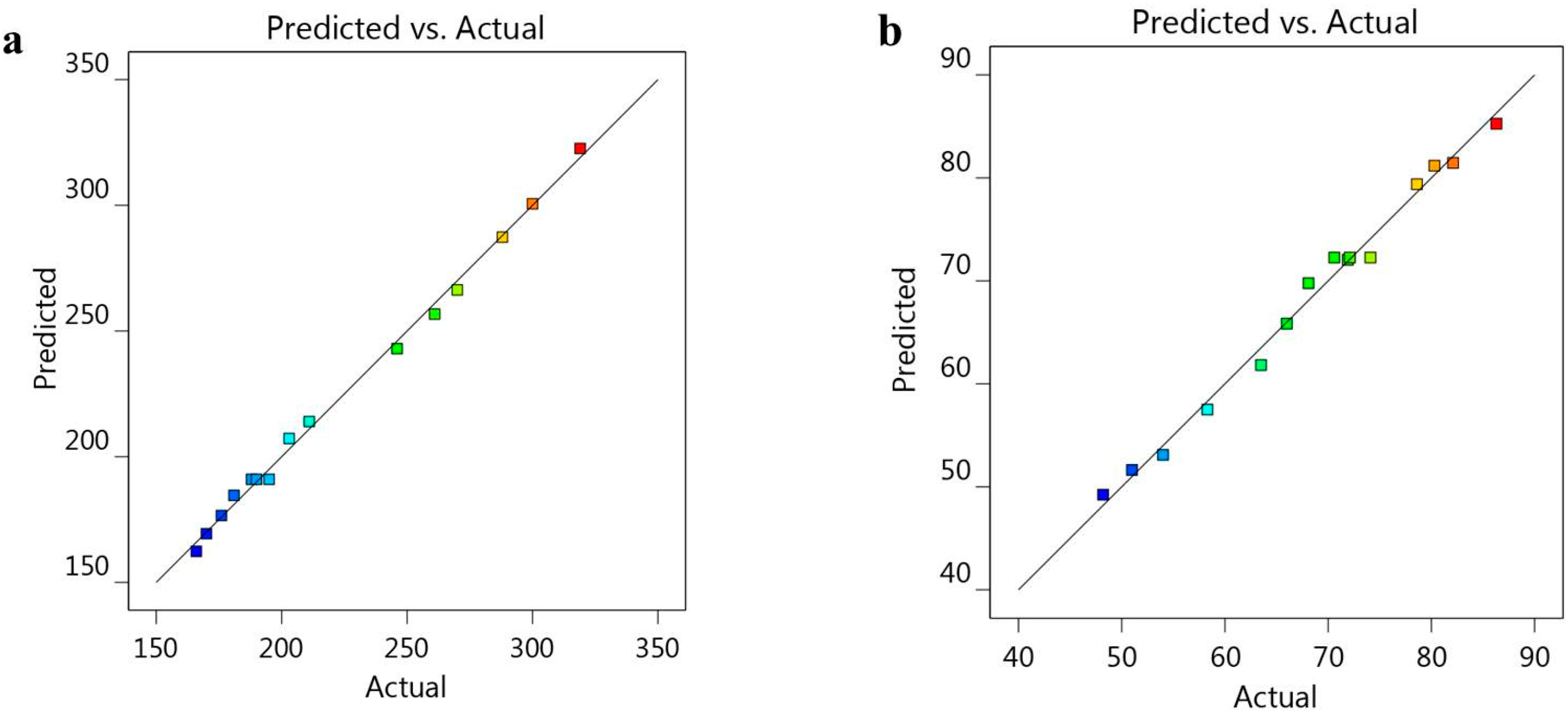
3.1. Validation of BBD Data
3.2. Analysis of Response
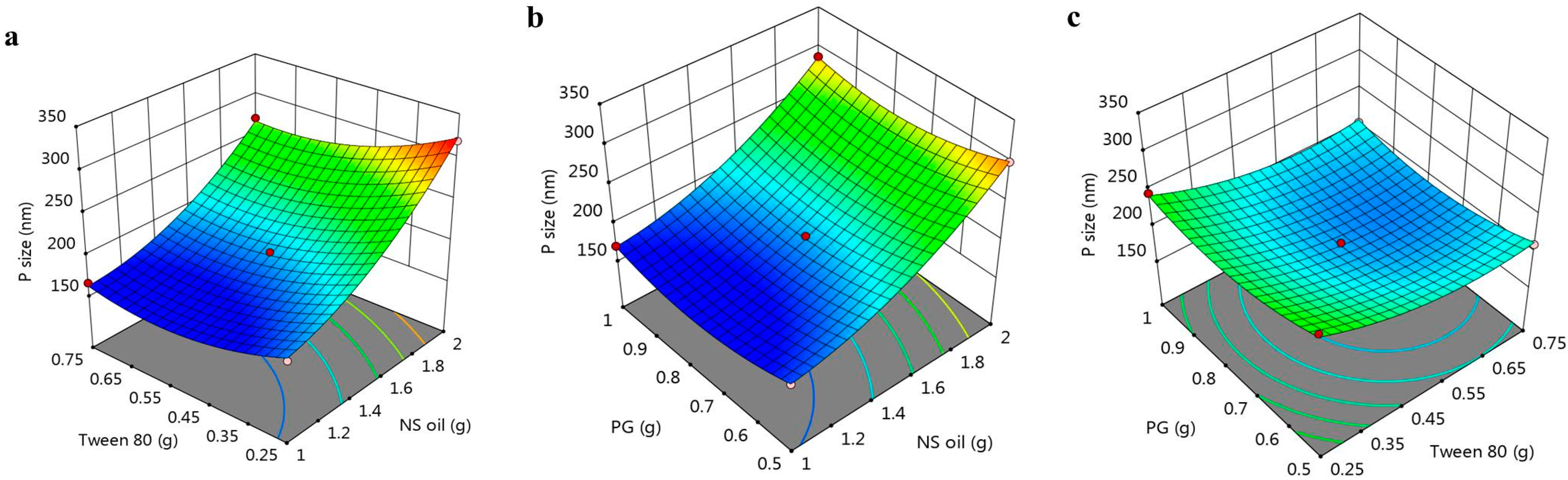
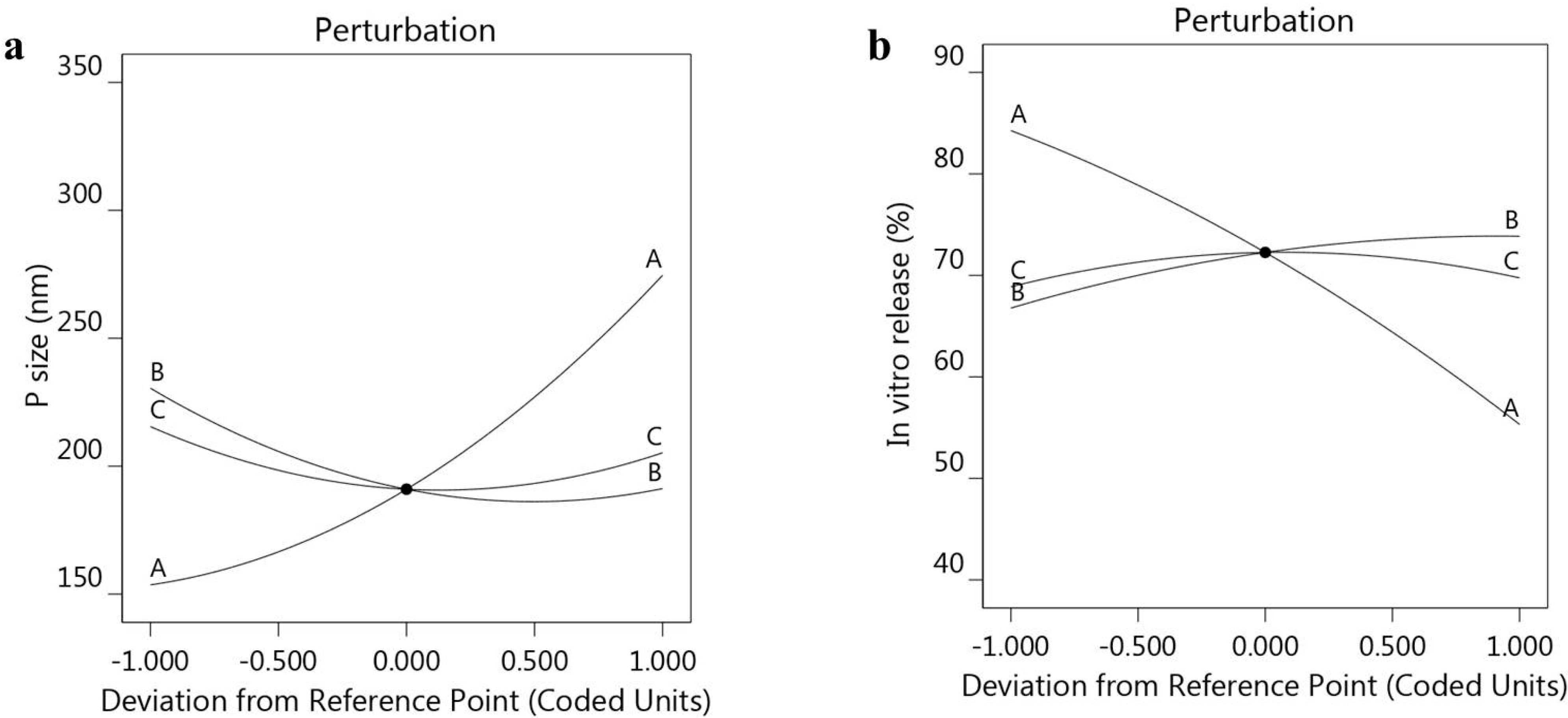
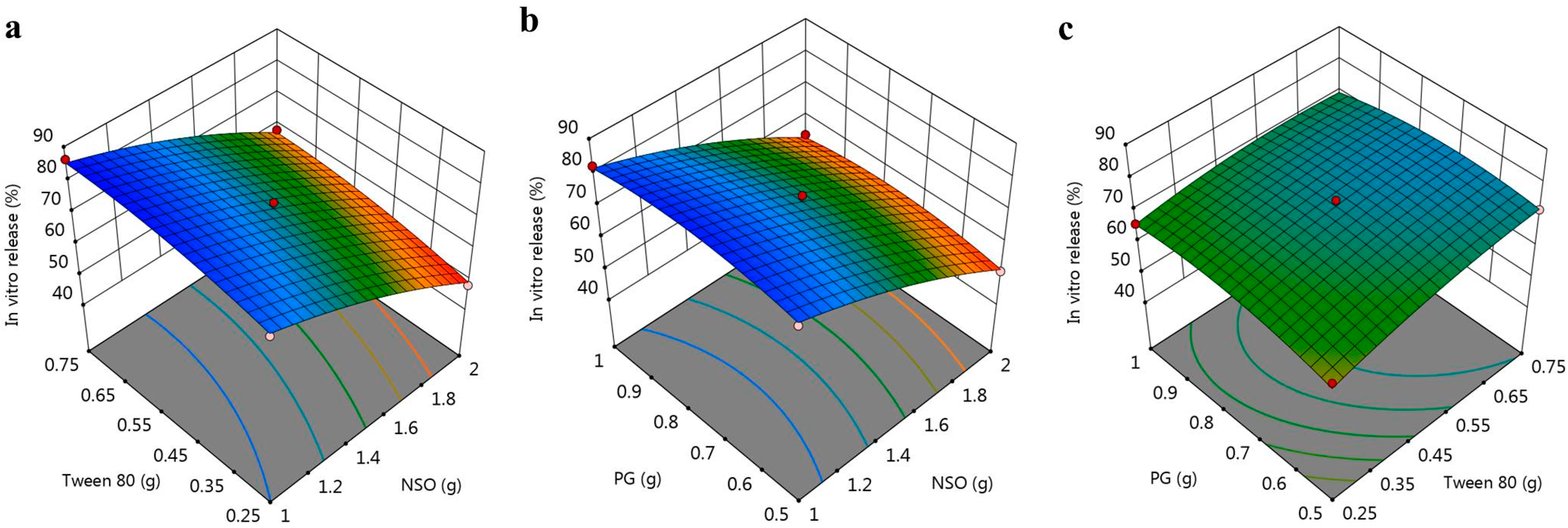
3.2.1. Response 1: Influence of the Independent Variables on R1
3.2.2. Response 2: Influence of the Independent Variables on R2
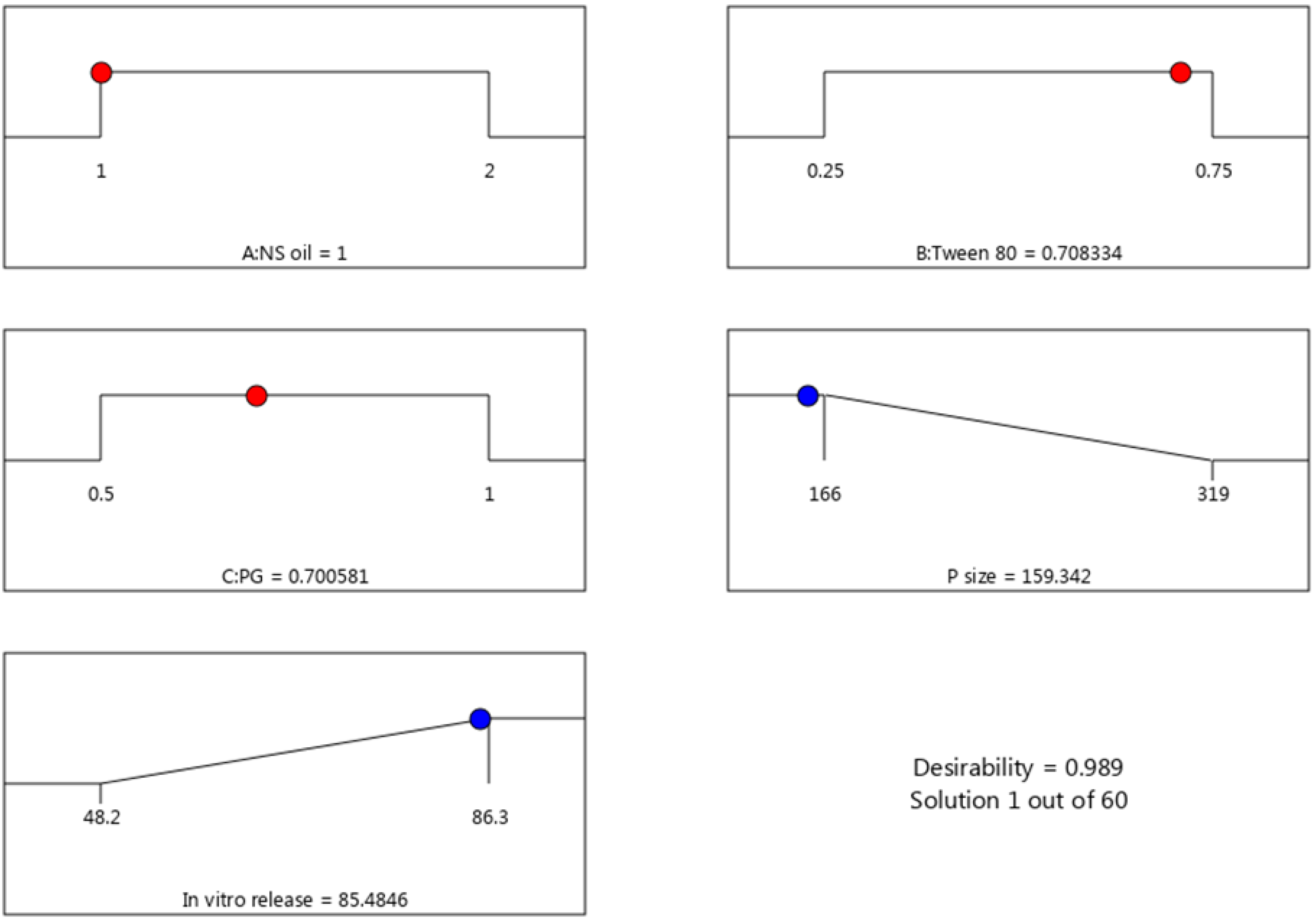
3.3. Selection of Optimized Formulation
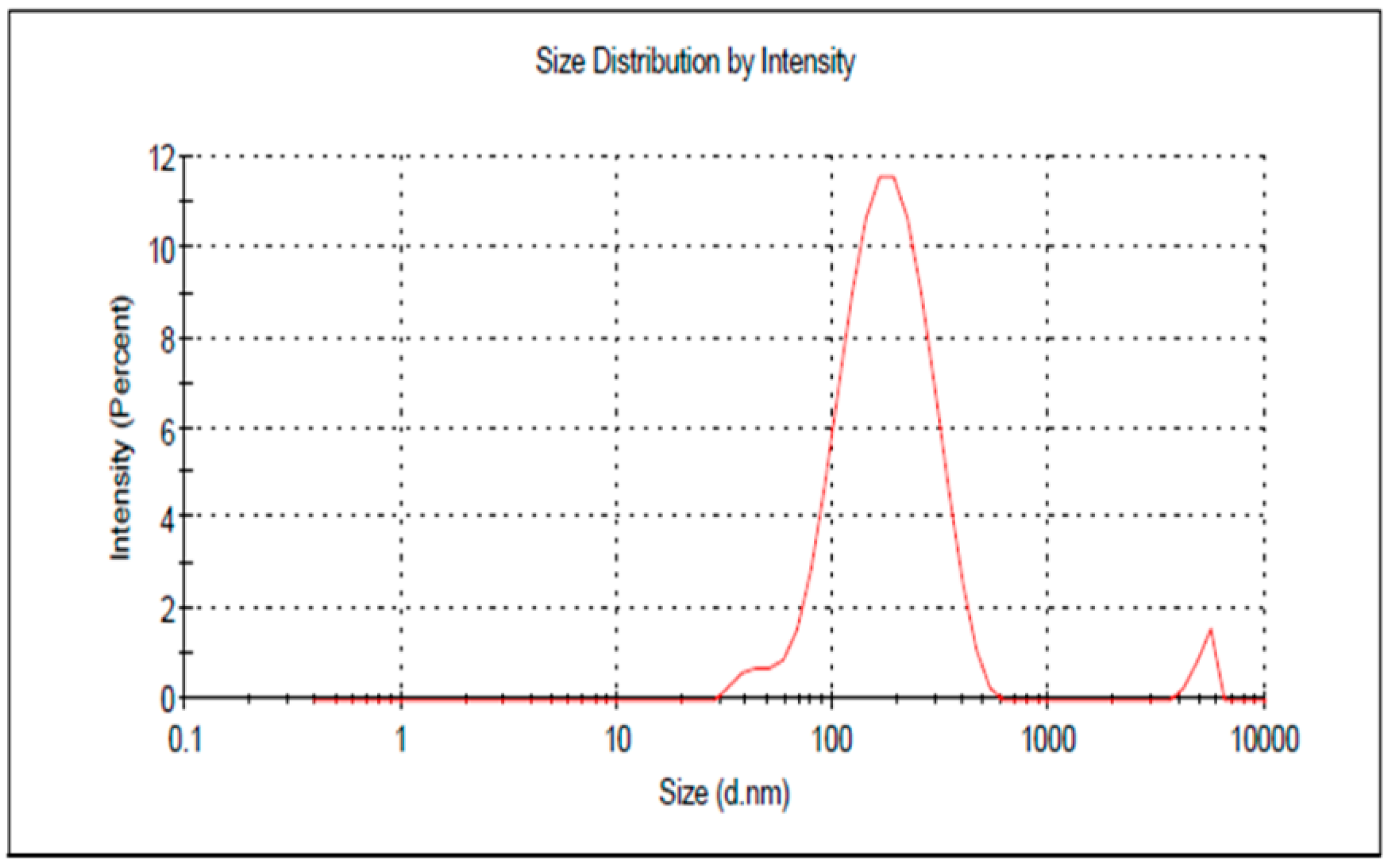
3.4. Characterization of the Optimized PGZ-Loaded NE
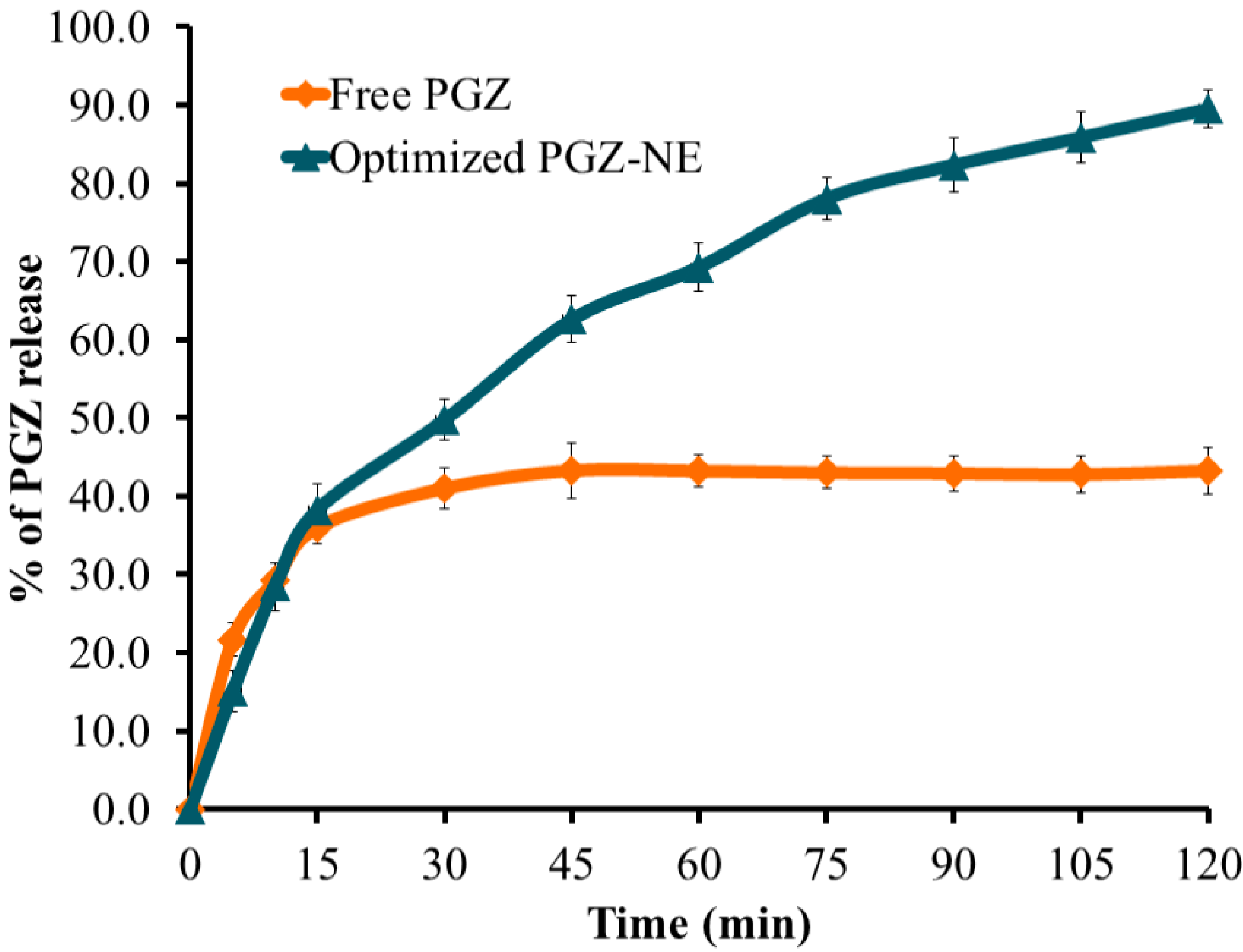
3.5. Kinetic Study
3.6. Drug Content
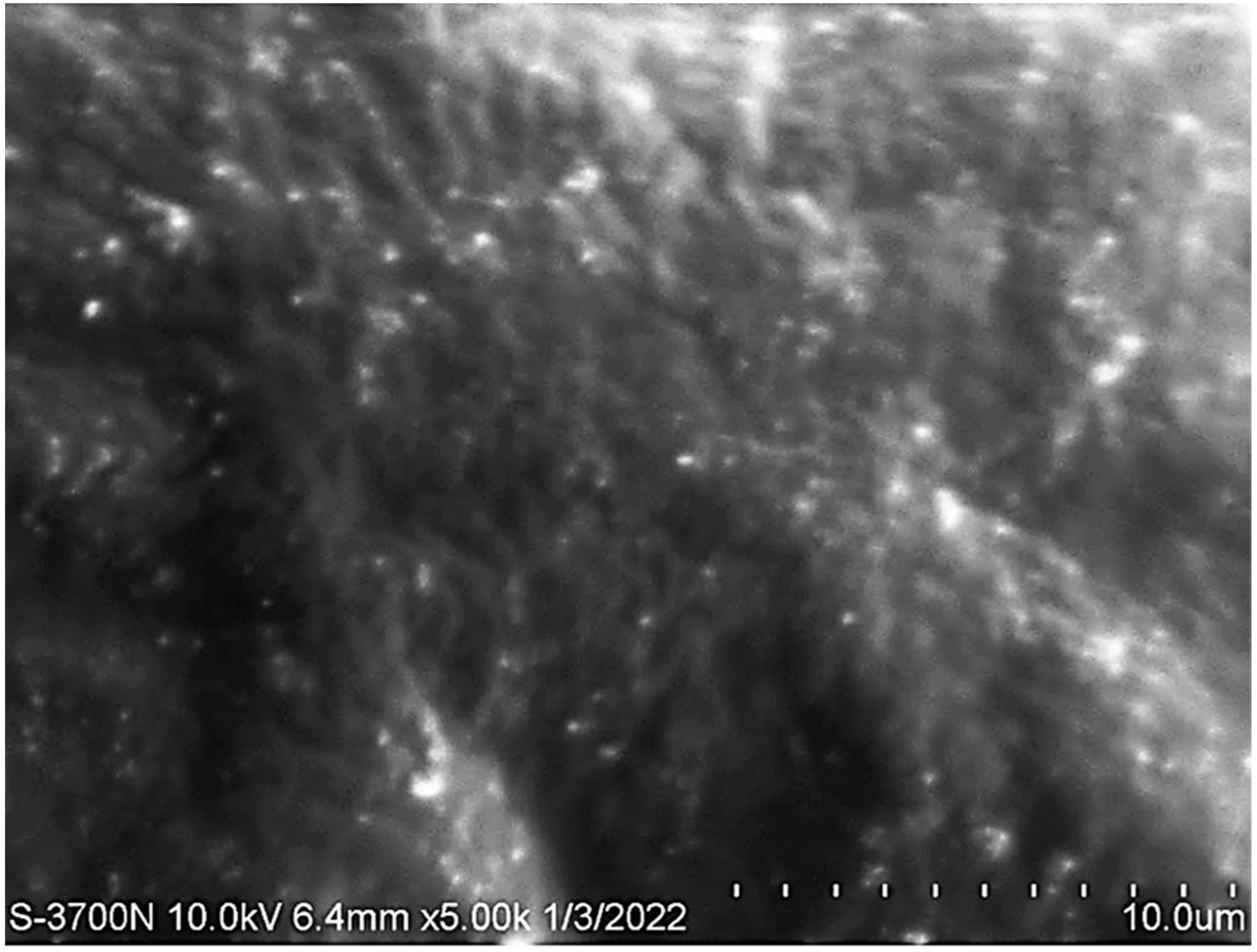
3.7. Scanning Electron Microscopy
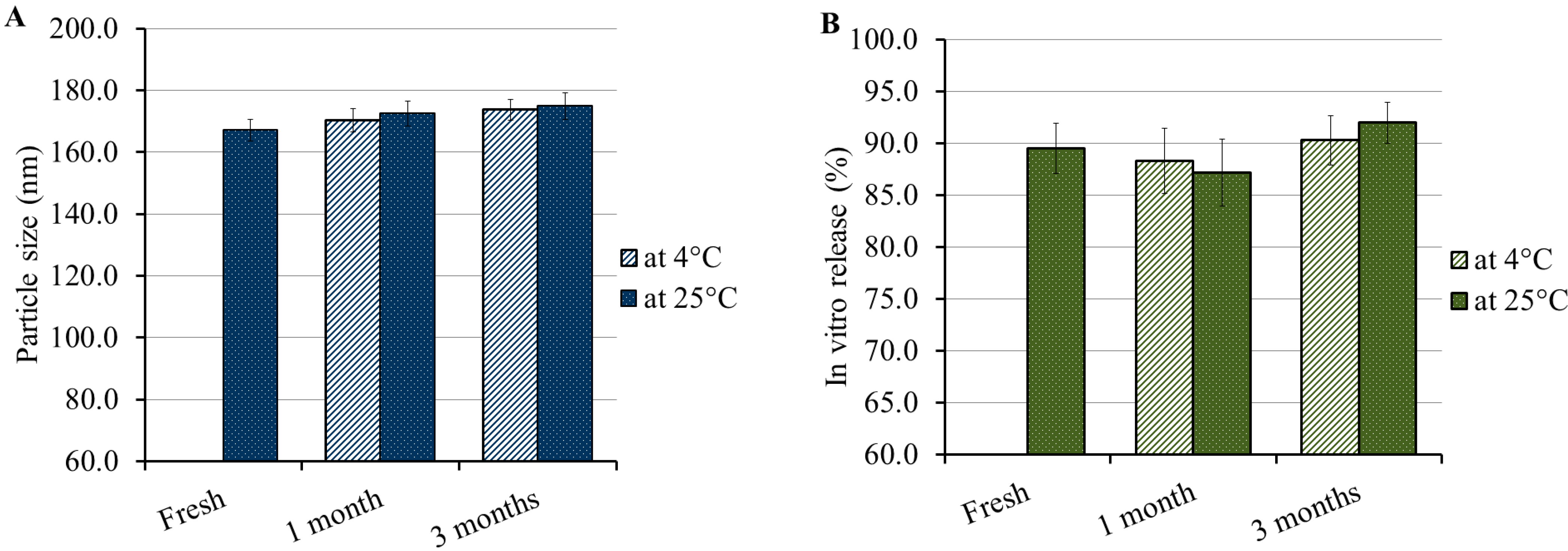
3.8. Stability Study
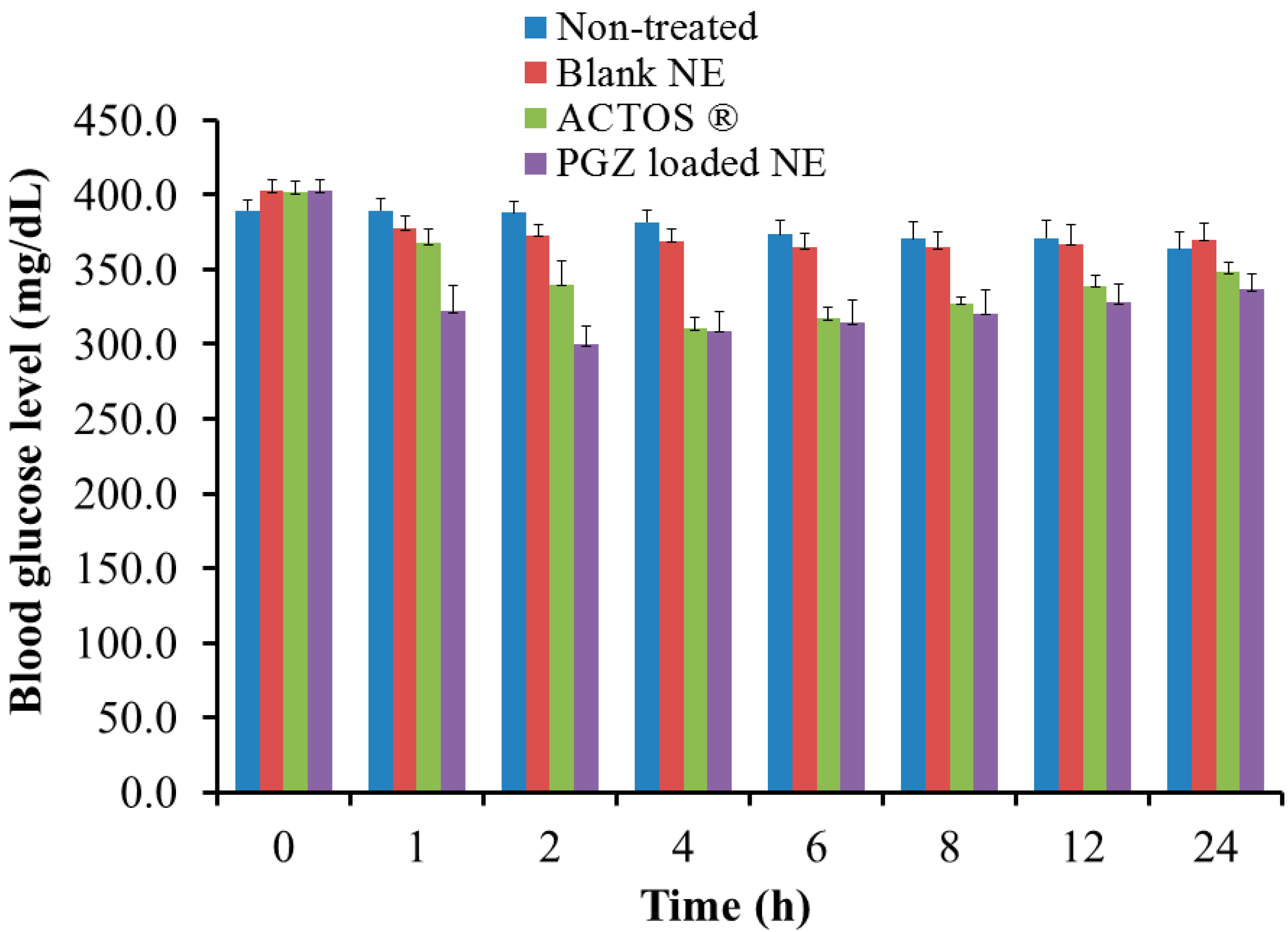
3.9. In Vivo Hypoglycemic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus–Atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 8–16. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Vera-García, R.; Silva-Abreu, M.; Domènech, Ò.; Badia, J.; Rodríguez-Lagunas, M.J.; Clares, B.; Calpena, A.C. Topical pioglitazone nanoformulation for the treatment of atopic dermatitis: Design, characterization and efficacy in hairless mouse model. Pharmaceutics 2020, 12, 255. [Google Scholar] [CrossRef] [Green Version]
- Pfützner, A.; Forst, T. Pioglitazone: An antidiabetic drug with the potency to reduce cardiovascular mortality. Expert Opin. Pharmacother. 2006, 7, 463–476. [Google Scholar] [CrossRef]
- Teaima, M.; Hababeh, S.; Khanfar, M.; Alanazi, F.; Alshora, D.; El-Nabarawi, M. Design and optimization of pioglitazone hydrochloride self-nanoemulsifying drug delivery system (SNEDDS) incorporated into an orally disintegrating tablet. Pharmaceutics 2022, 14, 425. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for poorly water-soluble anticancer drugs—Barriers of translation and solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Al-Dhubiab, B.E.; Mahdy, M.A.; Elnahas, H.M. Basic concepts of nanoemulsion and its potential application in pharmaceutical, cosmeceutical and nutraceutical fields. Res. J. Pharm. Technol. 2021, 14, 3938–3946. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar] [PubMed]
- Elsewedy, H.S.; Younis, N.S.; Shehata, T.M.; Mohamed, M.E.; Soliman, W.E. Enhancement of anti-inflammatory activity of optimized niosomal colchicine loaded into jojoba oil-based emulgel using response surface methodology. Gels 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (black cumin): A promising natural remedy for wide range of illnesses. Evid.-Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakkoli, A.; Ahmadi, A.; Razavi, B.M.; Hosseinzadeh, H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran. J. Pharm. Res. 2017, 16, 2–23. [Google Scholar] [PubMed]
- Razavi, B.; Hosseinzadeh, H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J. Endocrinol. Investig. 2014, 37, 1031–1040. [Google Scholar] [CrossRef]
- Khalil, H.E.; Shaikh, S.; Rizvi, S.M.D.; Moin, A.; Abu Lila, A.S.; Shehata, T.M.; Elsewedy, H.S. Dual-targeting potential of active constituents of Nigella sativa against FimH and CTX-M-15: A plausible therapeutic strategy against drugresistant uropathogenic strains. Pak. J. Pharm. Sci. 2020, 33, 2847–2857. [Google Scholar]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Ustun-Argon, Z.; Gumus, Z.P.; Celenk, V.U.; Ramadan, M.F. Bioactive phytochemicals from Nigella sativa oil processing by-products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Springer: Manhattan, NY, USA, 2022; pp. 1–21. [Google Scholar]
- Haseena, S.; Aithal, M.; Das, K.K.; Saheb, S.H. Phytochemical analysis of Nigella sativa and its effect on reproductive system. J. Pharm. Sci. Res. 2015, 7, 514. [Google Scholar]
- Fatima Shad, K.; Soubra, W.; Cordato, D.J. The role of thymoquinone, a major constituent of Nigella sativa, in the treatment of inflammatory and infectious diseases. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Best herbs for managing diabetes: A review of clinical studies. Braz. J. Pharm. Sci. 2013, 49, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.A.; Shehata, T.M.; Mohamed, D.I.; Elsewedy, H.S.; Soliman, W.E. Quality by design for development, optimization and characterization of brucine ethosomal gel for skin cancer delivery. Molecules 2021, 26, 3454. [Google Scholar] [CrossRef] [PubMed]
- Almostafa, M.M.; Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Novel formulation of fusidic acid incorporated into a myrrh-oil-based nanoemulgel for the enhancement of skin bacterial infection treatment. Gels 2022, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.-I.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date palm extract (Phoenix dactylifera) PEGylated nanoemulsion: Development, optimization and cytotoxicity evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Shaveta, S.; Singh, J.; Afzal, M.; Kaur, R.; Imam, S.S.; Alruwaili, N.K.; Alharbi, K.S.; Alotaibi, N.H.; Alshammari, M.S.; Kazmi, I.; et al. Development of solid lipid nanoparticle as carrier of pioglitazone for amplification of oral efficacy: Formulation design optimization, in-vitro characterization and in-vivo biological evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101674. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; e Silva, K.G.D.H.; Mansur, C.R.E. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater. Sci. Eng. C 2018, 92, 245–253. [Google Scholar] [CrossRef]
- Soliman, W.E.; Shehata, T.M.; Mohamed, M.E.; Younis, N.S.; Elsewedy, H.S. Enhancement of curcumin anti-inflammatory effect via formulation into myrrh oil-based nanoemulgel. Polymers 2021, 13, 577. [Google Scholar] [CrossRef]
- Shehata, T.M.; Ibrahima, M.M. BÜCHI nano spray dryer B-90: A promising technology for the production of metformin hydrochloride-loaded alginate-gelatin nanoparticles. Drug Dev. Ind. Pharm. 2019, 45, 1907–1914. [Google Scholar] [CrossRef]
- Shukr, M.H.; Eltablawy, N.A. Development and optimization of novel controlled-release pioglitazone provesicular powders using 32 factorial design. Drug Deliv. Transl. Res. 2015, 5, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elsewedy, H.S.; AbuLila, A.S.; Almansour, K.; Unissa, R.; Elghamry, H.A.; Soliman, M.S. Quality by design for optimizing a novel liposomal jojoba oil-based emulgel to ameliorate the anti-inflammatory effect of brucine. Gels 2021, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Zainol, S.; Basri, M.; Basri, H.B.; Shamsuddin, A.F.; Abdul-Gani, S.S.; Karjiban, R.A.; Abdul-Malek, E. Formulation optimization of a palm-based nanoemulsion system containing levodopa. Int. J. Mol. Sci. 2012, 13, 13049–13064. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, M.; Li, X.; Wang, L.; Liang, S.; Guo, X. Effects of surfactant and hydrophobic nanoparticles on the crude oil-water interfacial tension. Energies 2021, 14, 6234. [Google Scholar] [CrossRef]
- Ghanem, H.; Nasr, A.; Hassan, T.; Elkhoudary, M.; Alshaman, R.; Alattar, A.; Gad, S. Comprehensive study of Atorvastatin nanostructured lipid carriers through multivariate conceptualization and optimization. Pharmaceutics 2021, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Elnahas, H.M.; Elsewedy, H.S. Development, characterization and optimization of the anti-inflammatory influence of meloxicam loaded into a eucalyptus oil-based nanoemulgel. Gels 2022, 8, 262. [Google Scholar] [CrossRef]
- Raval, A.; Bahadur, P.; Raval, A. Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating. J. Pharm. Anal. 2018, 8, 45–54. [Google Scholar] [CrossRef]
- Mander, A. 3D surface plots. Stata Tech. Bull. 2000, 9, gr39. [Google Scholar]
- Shehata, T.M.; Khalil, H.E.; Elsewedy, H.S.; Soliman, W.E. Myrrh essential oil-based nanolipid formulation for enhancement of the antihyperlipidemic effect of atorvastatin. J. Drug Deliv. Sci. Technol. 2021, 61, 102277. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Aldhubiab, B.E.; Mahdy, M.A.; Elnahas, H.M. Brucine PEGylated nanoemulsion: In vitro and in vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125618. [Google Scholar] [CrossRef]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif. Cells Nanomed. Biotechnol. 2013, 43, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Telny, T.; Ravi, T.; Kuppusamy, S. Role of surfactant and pH in dissolution of curcumin. Indian J. Pharm. Sci. 2009, 71, 139–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azhar, S.N.A.S.; Ashari, S.E.; Ahmad, S.; Salim, N. In vitro kinetic release study, antimicrobial activity and in vivo toxicity profile of a kojic acid ester-based nanoemulsion for topical application. RSC Adv. 2020, 10, 43894–43903. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A. Remington-Essentials of Pharmaceutics; Pharmaceutical Press: London, UK, 2013. [Google Scholar]
- Rodríguez-Burneo, N.; Busquets, M.A.; Estelrich, J. Magnetic nanoemulsions: Comparison between nanoemulsions formed by ultrasonication and by spontaneous emulsification. Nanomaterials 2017, 7, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunsirikongkon, A.; Pyo, Y.-C.; Kim, D.-H.; Lee, S.-E.; Park, J.-S. Optimization of polyarginine-conjugated PEG lipid grafted proliposome formulation for enhanced cellular association of a protein drug. Pharmaceutics 2019, 11, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, M.; Broniatowski, M.; Mach, M.; Płachta, Ł.; Wydro, P. The effect of the polyethylene glycol chain length of a lipopolymer (DSPE-PEGn) on the properties of DPPC monolayers and bilayers. J. Mol. Liq. 2021, 335, 116529. [Google Scholar] [CrossRef]
- Saeio, K.; Pongpaibul, Y.; Viernstein, H.; Okonogi, S. Factors influencing drug dissolution characteristic from hydrophilic polymer matrix tablet. Sci. Pharm. 2007, 75, 147–164. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.F.M.; Islam, M.S.; Hazrat, M.; Alam, K.; Rahman, A.; Rahman, A.; Sadik, M.; Rashid, M. Nigella sativa oil potentiates the effects of pioglitazone on long term alloxan-induced diabetic rats. Bangladesh Pharm. J. 2013, 16, 143–151. [Google Scholar] [CrossRef] [Green Version]
| Independent Variable | Symbol | Level of Variation | ||
|---|---|---|---|---|
| Lowest (−1) | Central (0) | Highest (1) | ||
| NSO concentration (g) | A | 1.0 | 1.5 | 2.0 |
| Tween 80 concentration (g) | B | 0.25 | 0.5 | 0.75 |
| PG concentration (g) | C | 0.5 | 0.75 | 1.0 |
| Dependent variable | Symbol | Constraints | ||
| Particle size (nm) | R1 | Minimize | ||
| In Vitro release (%) | R2 | Maximize | ||
| Run | Selected Factors | Observed Responses | |||
|---|---|---|---|---|---|
| NSO Concentration (g) | Tween 80 Concentration (g) | PG Concentration (g) | P. Size (nm) | In Vitro (%) | |
| F1 | 1 | 0.25 | 0.75 | 181 ± 4.6 | 78.6 ± 3.8 |
| F2 | 1.5 | 0.5 | 0.75 | 188 ± 3.0 | 74.1 ± 2.8 |
| F3 | 2 | 0.5 | 1 | 288 ± 4.6 | 54.0 ± 3.2 |
| F4 | 1.5 | 0.5 | 0.75 | 195 ± 5.0 | 70.6 ± 2.2 |
| F5 | 1.5 | 0.75 | 0.5 | 211 ± 4.6 | 71.9 ± 3.1 |
| F6 | 1 | 0.75 | 0.75 | 166 ± 3.6 | 86.3 ± 2.9 |
| F7 | 2 | 0.25 | 0.75 | 319 ± 5.4 | 48.2 ± 3.1 |
| F8 | 1 | 0.5 | 1 | 170 ± 4.5 | 82.1 ± 3.4 |
| F9 | 1.5 | 0.75 | 1 | 203 ± 4.4 | 68.1 ± 3.6 |
| F10 | 1.5 | 0.5 | 0.75 | 190 ± 4.0 | 72.1 ± 2.9 |
| F11 | 1 | 0.5 | 0.5 | 176 ± 4.6 | 80.3 ± 3.3 |
| F12 | 2 | 0.5 | 0.5 | 300 ± 5.3 | 51.5 ± 2.4 |
| F13 | 1.5 | 0.25 | 0.5 | 261 ± 4.5 | 63.5 ± 3.1 |
| F14 | 2 | 0.75 | 0.75 | 270 ± 4.7 | 58.3 ± 2.6 |
| F15 | 1.5 | 0.25 | 1 | 246 ± 4.9 | 66.5 ± 2.7 |
| Source | R1 | R2 | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Model | 153.39 | <0.0001 | 58.36 | 0.0002 |
| A | 1090.58 | <0.0001 * | 476.08 | <0.0001 * |
| B | 114.75 | 0.0001 * | 28.43 | 0.0031 * |
| C | 7.83 | 0.0381 * | 0.4349 | 0.5387 |
| AB | 10.76 | 0.0219 * | 0.4090 | 0.5506 |
| AC | 0.3352 | 0.5877 | 0.1022 | 0.7621 |
| BC | 0.4562 | 0.5294 | 2.82 | 0.1540 |
| A² | 73.54 | 0.0004 * | 6.40 | 0.0525 |
| B² | 54.32 | 0.0007 * | 3.97 | 0.1029 |
| C2 | 51.62 | 0.0008 * | 9.10 | 0.0295 * |
| Lack of Fit | 2.76 | 0.2759 | 1.24 | 0.4763 |
| R² | 0.9964 | 0.9906 | ||
| Adjusted R² | 0.9899 | 0.9736 | ||
| Predicted R² | 0.9519 | 0.8945 | ||
| Adequate Precision | 37.8766 | 23.5140 | ||
| Model | Quadratic | Quadratic | ||
| Remark | Suggested | Suggested | ||
| Dependent Variable | Symbol | Constraint |
|---|---|---|
| NSO concentration | A | In range |
| Tween 80 concentration | B | In range |
| PG concentration | C | In range |
| Response | Predicted values | Observed values |
| R1 (nm) | 159.49 ± 5.18 | 167.1 ± 3.43 |
| R2 (%) | 85.48 ± 1.87 | 89.5 ± 2.38 |
| Formula | Correlation Coefficient (R2) | Exponent (n) Value for Korsmeyer and Peppas | |||
|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Korsmeyer and Peppas | ||
| Free PGZ | 0.4729 | 0.5815 | 0.7619 | 0.9866 | 1.1188 |
| PGZ-loaded NE | 0.8853 | 0.7899 | 0.9674 | 0.9789 | 1.0292 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, T.M.; Almostafa, M.M.; Elsewedy, H.S. Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect. Polymers 2022, 14, 3021. https://doi.org/10.3390/polym14153021
Shehata TM, Almostafa MM, Elsewedy HS. Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect. Polymers. 2022; 14(15):3021. https://doi.org/10.3390/polym14153021
Chicago/Turabian StyleShehata, Tamer M., Mervt M. Almostafa, and Heba S. Elsewedy. 2022. "Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect" Polymers 14, no. 15: 3021. https://doi.org/10.3390/polym14153021
APA StyleShehata, T. M., Almostafa, M. M., & Elsewedy, H. S. (2022). Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect. Polymers, 14(15), 3021. https://doi.org/10.3390/polym14153021







