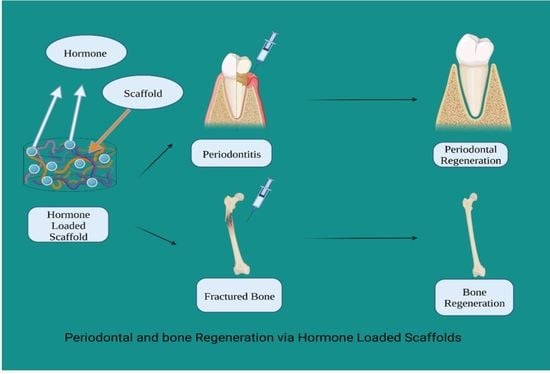
Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review
Abstract
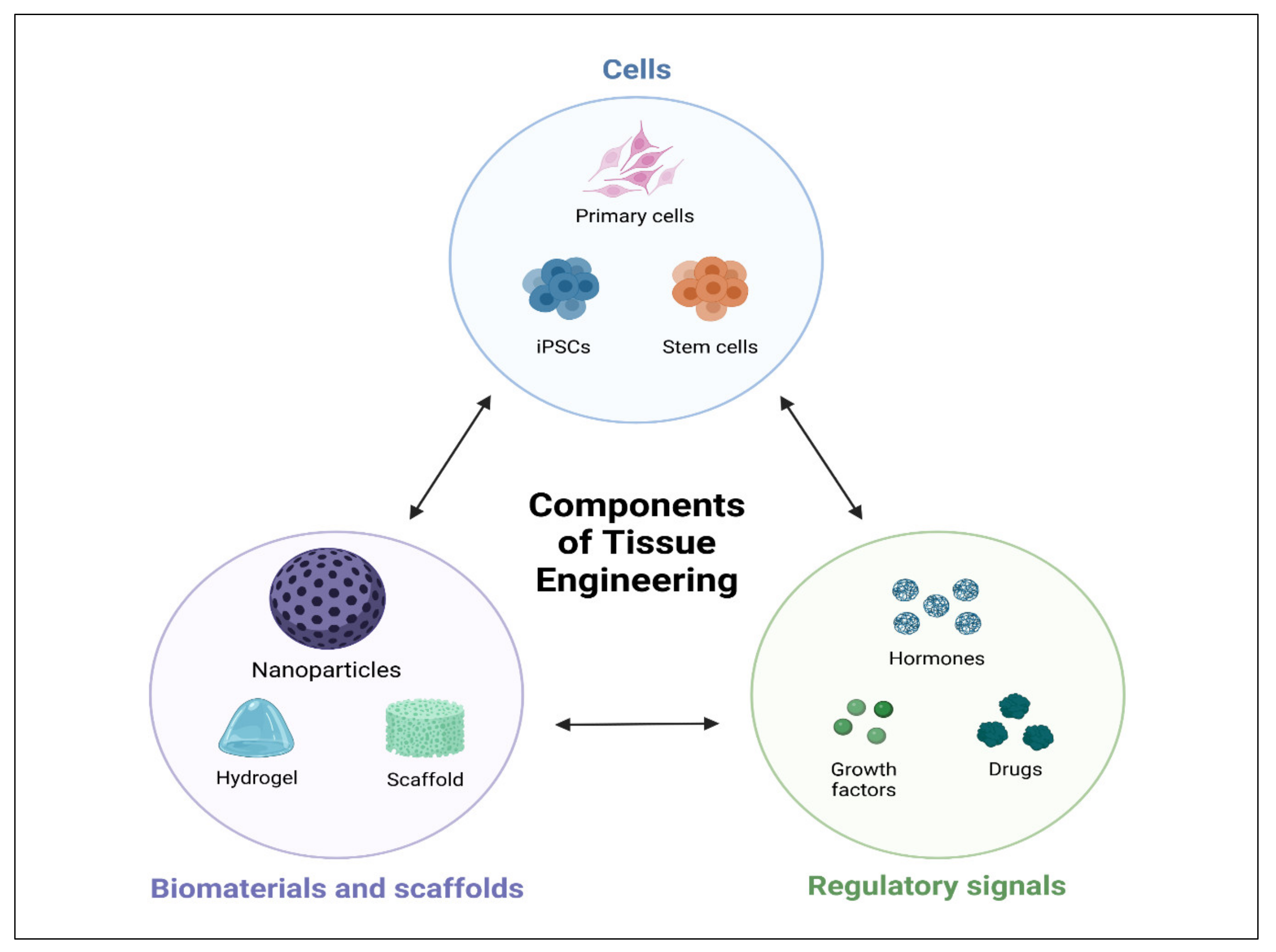
1. Introduction
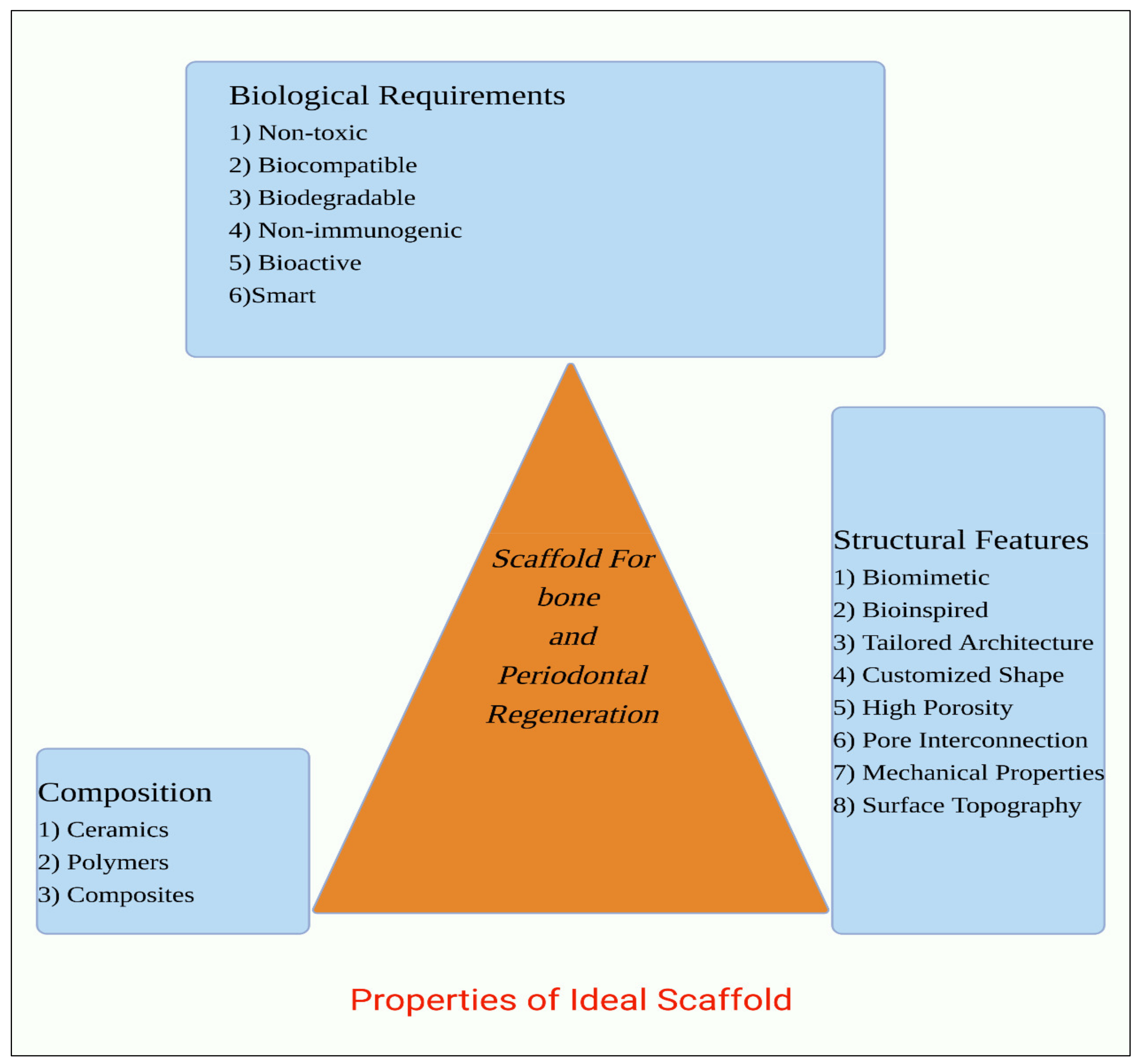
2. Properties of Scaffolds for Periodontal and Bone Regeneration
2.1. Biological Requirements
2.2. Structural Features
2.3. Biomaterial Composition
3. Growth Factors
4. Drug Repurposing
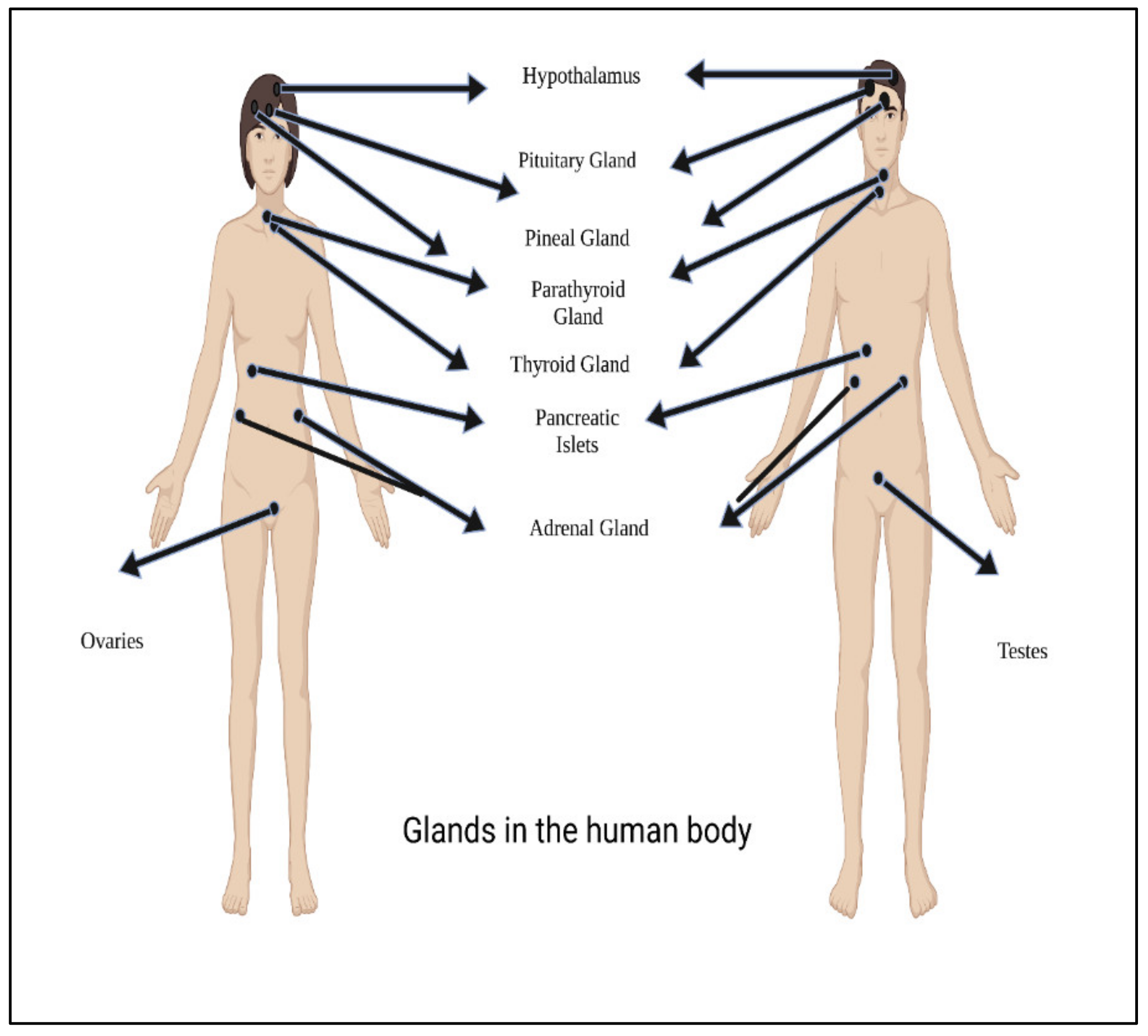
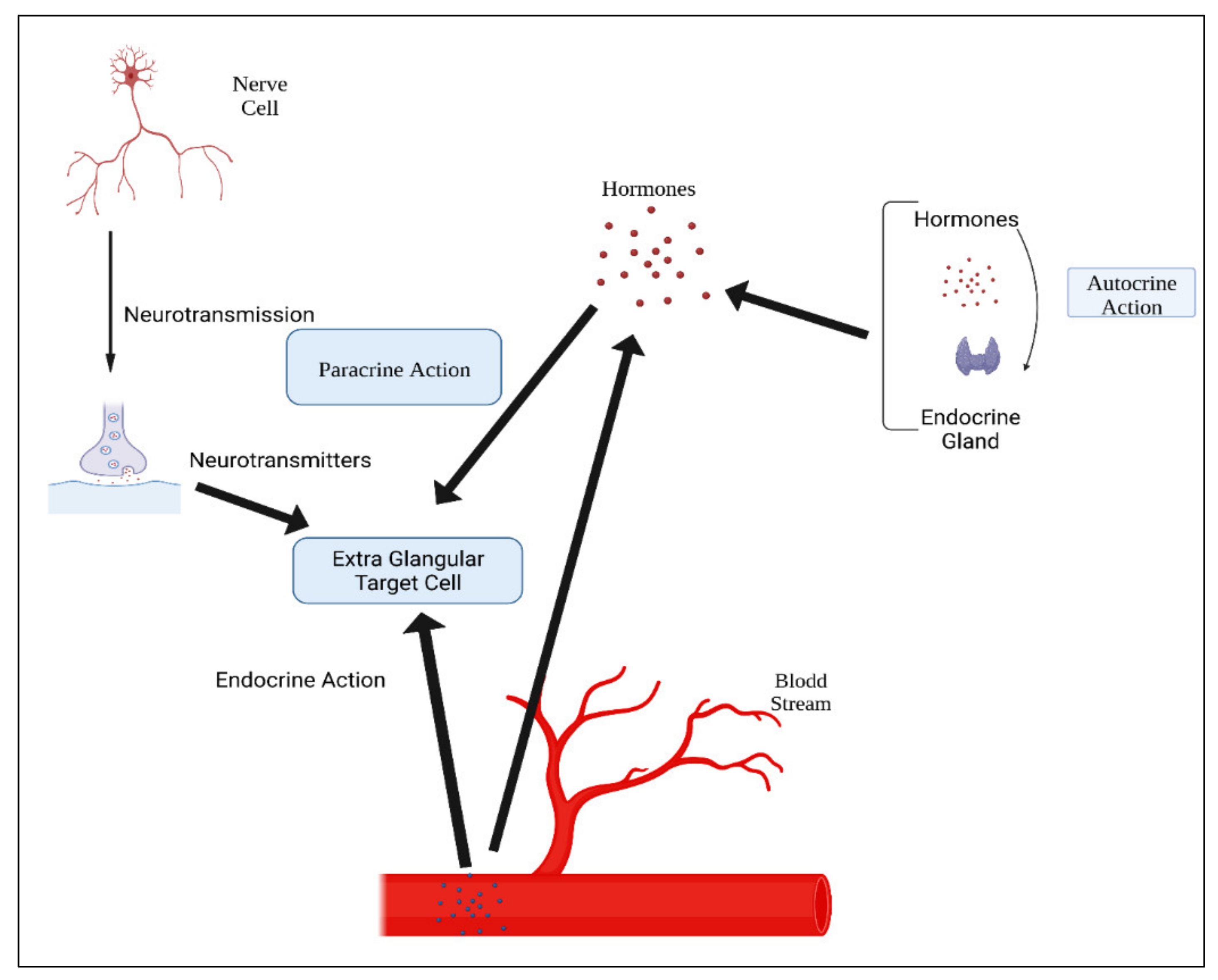
5. Hormones
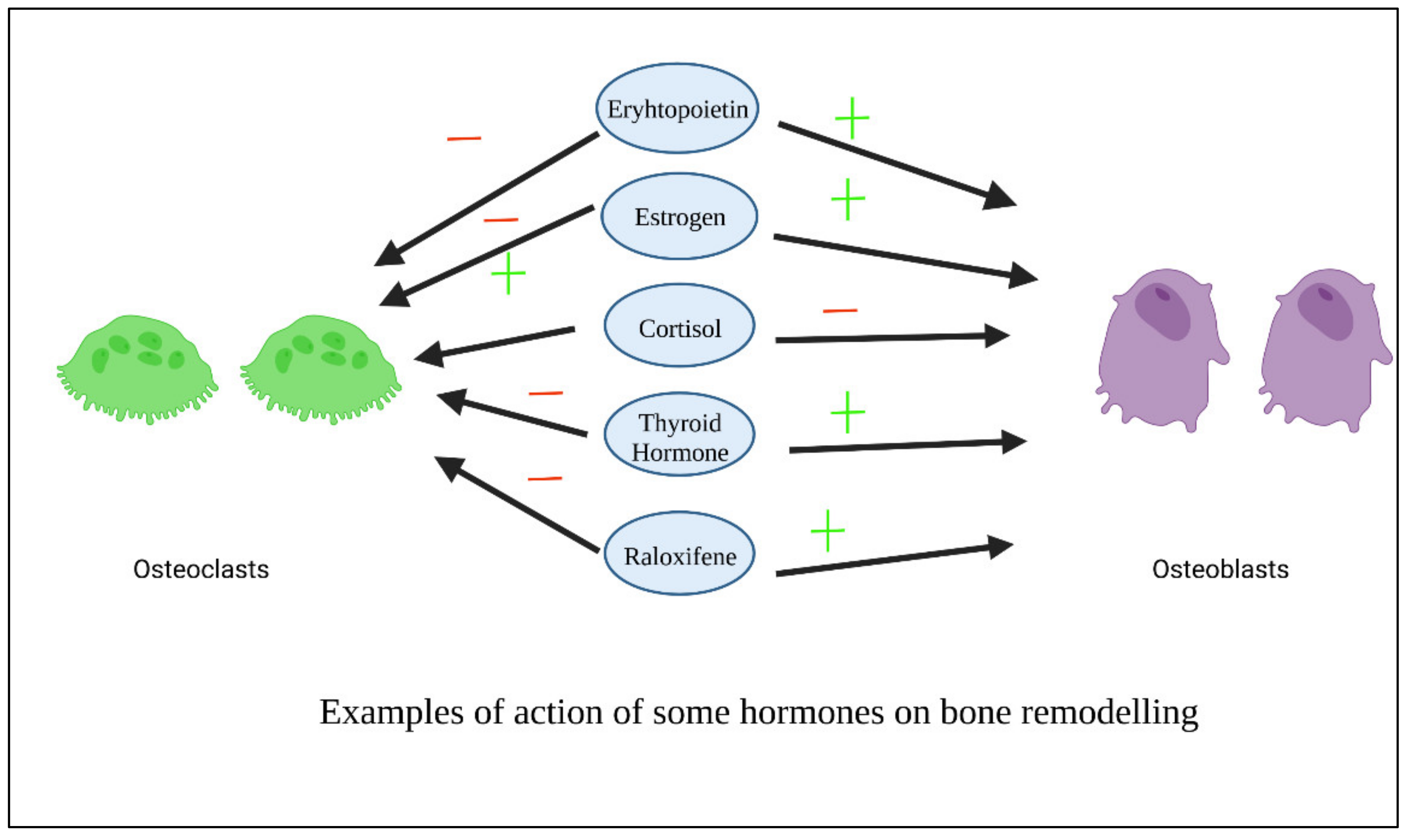
6. Examples of Repositioned Hormones for Bone and Periodontal Tissue Engineering
6.1. Thyroxin
6.2. Oxytocin
6.3. Dexamethasone
6.4. Androgens
6.5. Parathyroid Hormone (PTH)
6.6. Insulin
6.7. Estrogen
6.8. Selective Estrogen Receptor Modulators (SERMs)
Raloxifene
6.9. 1, 25(OH) 2 Vitamin D3
6.10. Melatonin
6.11. Erythropoietin
6.12. Calcitonin (CTN)
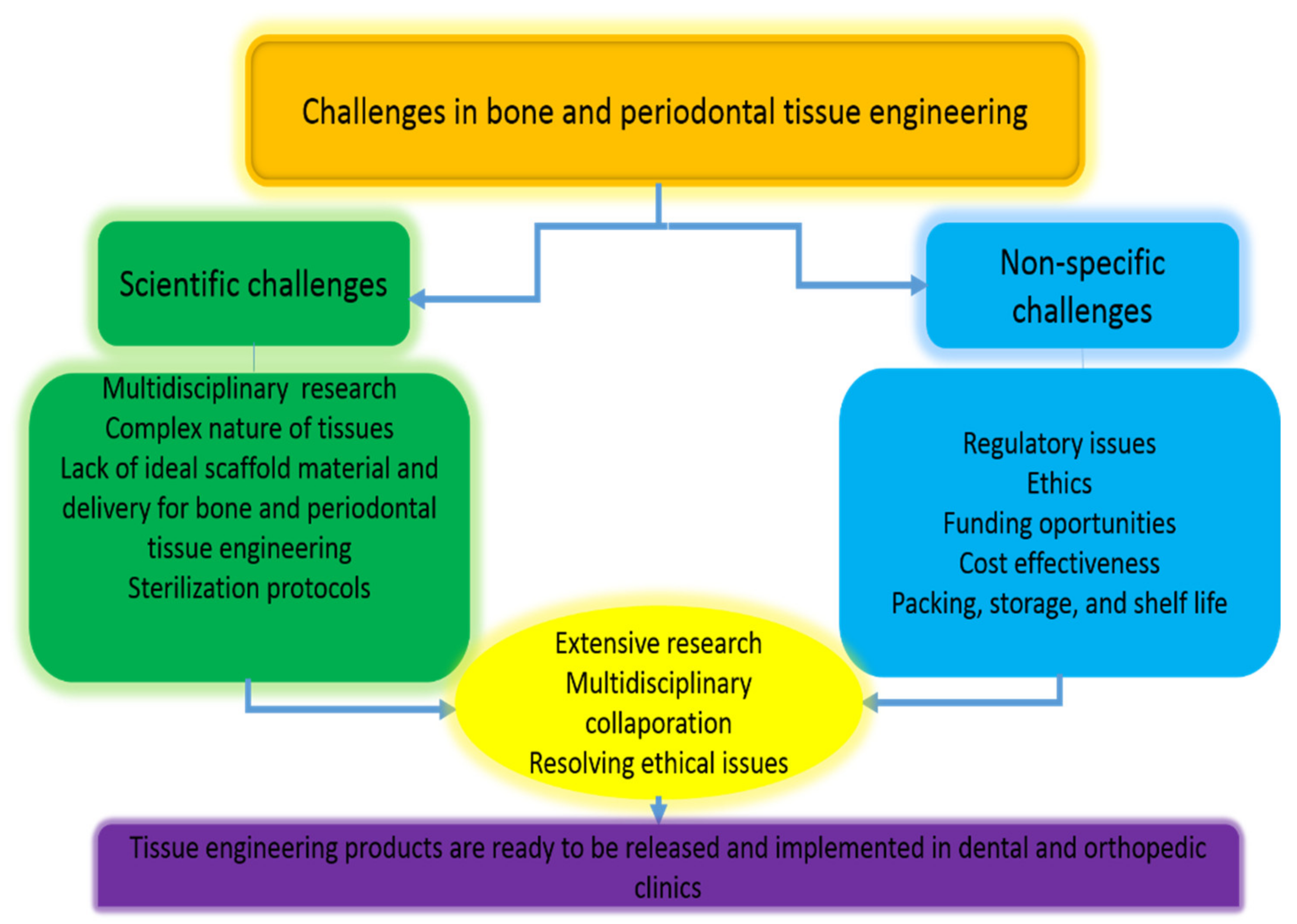
7. Limitations of Local Hormone Delivery Systems in Bone and Periodontal Tissue Engineering
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariotti, A. Sex steroid hormones and cell dynamics in the periodontium. Crit. Rev. Oral. Biol. Med. 1994, 5, 27–53. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.K.; Tozum, T.; Rosol, T. Estrogen Receptors in Skeletal Metabolism: Lessons from Genetically Modified Models of Receptor Function. Crit. Rev. Eukaryot. Gene Expr. 2002, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J. A new hypothesis for how sex steroid hormones regulate bone mass. J. Clin. Investig. 2003, 111, 1641–1643. [Google Scholar] [CrossRef]
- Wang, Q.; Alén, M.; Nicholson, P.H.F.; Halleen, J.M.; Alatalo, S.L.; Ohlsson, C.; Suominen, H.; Cheng, S. Differential Effects of Sex Hormones on Peri- and Endocortical Bone Surfaces in Pubertal Girls. J. Clin. Endocrinol. Metab. 2006, 91, 277–282. [Google Scholar] [CrossRef]
- Mascarenhas, P.; Gapski, R.; Al-Shammari, K.; Wang, H.-L. Influence of sex hormones on the periodontium. J. Clin. Periodontol. 2003, 30, 671–681. [Google Scholar] [CrossRef]
- Nagai, N.; Yunoki, S.; Suzuki, T.; Sakata, M.; Tajima, K.; Munekata, M. Application of cross-linked salmon atelocollagen to the scaffold of human periodontal ligament cells. J. Biosci. Bioeng. 2004, 97, 389–394. [Google Scholar] [CrossRef]
- AlRowis, R.; AlMoharib, H.S.; AlMubarak, A.; Bhaskardoss, J.; Preethanath, R.S.; Anil, S. Oral fluid-based biomarkers in periodontal disease–Part 2. Gingival crevicular fluid. J. Int. Oral Health 2014, 6, 126. [Google Scholar]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. [Google Scholar] [CrossRef]
- Kini, U.; Nandeesh, B. Physiology of bone formation, remodeling, and metabolism. In Radionuclide and Hybrid Bone Imaging; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–57. [Google Scholar]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials 2020, 13, 2077. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, X.; Gu, L. Periodontal Bifunctional Biomaterials: Progress and Perspectives. Materials 2021, 14, 7588. [Google Scholar] [CrossRef]
- Zang, S.; Mu, R.; Chen, F.; Wei, X.; Zhu, L.; Han, B.; Yu, H.; Bi, B.; Chen, B.; Wang, Q.; et al. Injectable chitosan/β-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects. Mater. Sci. Eng. C 2019, 99, 919–928. [Google Scholar] [CrossRef]
- Radulescu, D.-E.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers 2022, 14, 899. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2018, 64, 91–126. [Google Scholar] [CrossRef]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Mi, F.-L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef]
- Yadegari, A.; Fahimipour, F.; Rasoulianboroujeni, M.; Dashtimoghadarm, E.; Omidi, M.; Golzar, H.; Tahriri, M.; Tayebi, L. 10—Specific considerations in scaffold design for oral tissue engineering. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 157–183. ISBN 9780081009611. [Google Scholar]
- Zhao, H.; Chai, Y. Stem cells in teeth and craniofacial bones. J. Dent. Res. 2015, 94, 1495–1501. [Google Scholar] [CrossRef]
- Hughes, D.; Song, B. Dental and Nondental Stem Cell Based Regeneration of the Craniofacial Region: A Tissue Based Approach. Stem Cells Int. 2016, 2016, 1–20. [Google Scholar] [CrossRef]
- El-Sayed, K.M.F.; Dörfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef]
- Najeeb, Z.S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The role of nutrition in periodontal health: An update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
- Péault, B.; Asatrian, G.; Pham, D.; Hardy, W.R.; James, A.W. Stem cell technology for bone regeneration: Current status and potential applications. Stem Cells Cloning Adv. Appl. 2015, 8, 39–48. [Google Scholar] [CrossRef]
- Szulc, M.; Zakrzewska, A.; Zborowski, J. Local drug delivery in periodontitis treatment: A review of contemporary literature. Dent. Med Probl. 2018, 55, 333–342. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Greenstein, G.; Polson, A. The Role of Local Drug Delivery in the Management of Periodontal Diseases: A Comprehensive Review. J. Periodontol. 1998, 69, 507–520. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Hayei, N.A.A.; Sabarudin, M.A.; Baharin, N.H.M. Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes 2022, 12, 444. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Materialstoday 2011, 14, 88–95. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions–A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Bouet, G.; Marchat, D.; Cruel, M.; Malaval, L.; Vico, L. In Vitro Three-Dimensional Bone Tissue Models: From Cells to Controlled and Dynamic Environment. Tissue Eng. Part B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Wang, H.-L.; Miyauchi, M. Attachment, proliferation and differentiation of periodontal ligament cells on various guided tissue regeneration membranes. J. Periodontal Res. 2001, 36, 322–327. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Rutkowski, J.L. Regenerative Medicine for Dentistry—“Times are A-Changin”. J. Oral Implantol. 2015, 41, 234. [Google Scholar] [CrossRef]
- Hollister, S.; Lin, C.; Saito, E.; Schek, R.; Taboas, J.; Williams, J.; Partee, B.; Flanagan, C.; Diggs, A.; Wilke, E.; et al. Engineering craniofacial scaffolds. Orthod. Craniofacial Res. 2005, 8, 162–173. [Google Scholar] [CrossRef]
- Matassi, F.; Nistri, L.; Paez, D.C.; Innocenti, M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar]
- Zhu, N.; Chen, X. Biofabrication of Tissue Scaffolds. In Advances in Biomaterials Science and Biomedical Applications; Intech Open: London, UK, 2013. [Google Scholar] [CrossRef]
- Fan, J.; Abedi-Dorcheh, K.; Sadat Vaziri, A.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Rastegar Adib, F.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2016, 45, 185–192. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Gamma radiation synthesis of a novel amphiphilic terpolymer hydrogel pH-responsive based chitosan for colon cancer drug delivery. Carbohydr. Polym. 2021, 263, 117975. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Alshangiti, D.M.; Alkhursani, S.A.; Al-Gahtany, S.A.; Shokr, F.S.; Madani, M. Improvement of In Vitro Dissolution of the Poor Water-Soluble Amlodipine Drug by Solid Dispersion with Irradiated Polyvinylpyrrolidone. ACS Omega 2020, 5, 21476–21487. [Google Scholar] [CrossRef]
- Younis, S.A.; Ghobashy, M.M.; Samy, M. Development of aminated poly(glycidyl methacrylate) nanosorbent by green gamma radiation for phenol and malathion contaminated wastewater treatment. J. Environ. Chem. Eng. 2017, 5, 2325–2336. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Damhougy, B.K.; Nady, N.; El-Wahab, H.A.; Naser, A.M.; Abdelhai, F. Radiation Crosslinking of Modifying Super Absorbent (Polyacrylamide/Gelatin) Hydrogel as Fertilizers Carrier and Soil Conditioner. J. Polym. Environ. 2018, 26, 3981–3994. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Sawy, N.M.; Kodous, A.S. Nanocomposite of cosubstituted carbonated hydroxyapatite fabricated inside Poly(sodium hyaluronate-acrylamide) hydrogel template prepared by gamma radiation for osteoblast cell regeneration. Radiat. Phys. Chem. 2021, 183, 109408. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Sánchez, M.; Landin, M. Drug-Loaded Biomimetic Ceramics for Tissue Engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef]
- Ormanci, O.; Akin, I.; Sahin, F.; Yucel, O.; Simon, V.; Cavalu, S.; Goller, G. Spark plasma sintered Al2O3-YSZ-TiO2 composites: Processing, characterization and in vivo evaluation. Mater. Sci. Eng. C 2014, 40, 16–23. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Serban, A.P.; Nicoara, A.I.; Trusca, R.; Ene, V.L.; Iordache, F. Biomimetic Composite Scaffold Based on Naturally Derived Biomaterials. Polymers 2020, 12, 1161. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Sattar, N.E.A.A. Physics. Radiation synthesis of rapidly self-healing hydrogel derived from poly (acrylic acid) with good mechanical strength. Macromol. Chem. Phys. 2020, 221, 2000218. [Google Scholar] [CrossRef]
- Yunos, D.M.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Stahl, B.; Eblenkamp, M.; Wintermantel, E.; Ramakrishna, S.J.N. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology 2012, 23, 095705. [Google Scholar] [CrossRef]
- Kinard, L.A.; Dahlin, R.L.; Lam, J.; Lu, S.; Lee, E.J.; Kasper, F.K.; Mikos, A.G. Synthetic biodegradable hydrogel delivery of demineralized bone matrix for bone augmentation in a rat model. Acta Biomater. 2014, 10, 4574–4582. [Google Scholar] [CrossRef]
- Wei, Q.; Pohl, T.L.M.; Seckinger, A.; Spatz, J.P.; Cavalcanti-Adam, E.A. Regulation of integrin and growth factor signaling in biomaterials for osteodifferentiation. Beilstein J. Org. Chem. 2015, 11, 773–783. [Google Scholar] [CrossRef]
- Raja, S.; Byakod, G.; Pudakalkatti, P. Growth factors in periodontal regeneration. Int. J. Dent. Hyg. 2009, 7, 82–89. [Google Scholar] [CrossRef]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J. Bone. Jt. Surg. Am. 2013, 95, 1537–1545. [Google Scholar] [CrossRef]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A.; et al. Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Delavan, B.; Roberts, R.; Huang, R.; Bao, W.; Tong, W.; Liu, Z. Computational drug repositioning for rare diseases in the era of precision medicine. Drug Discov. Today 2018, 23, 382–394. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Diabetes & Endocrinology. Spotlight on rare diseases. Lancet Diabetes Endocrinol. 2019, 7, 75. [Google Scholar] [CrossRef]
- Zhu, Q.; Nguyen, D.-T.; Grishagin, I.; Southall, N.; Sid, E.; Pariser, A. An integrative knowledge graph for rare diseases, derived from the Genetic and Rare Diseases Information Center (GARD). J. Biomed. Semant. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nosengo, N. New tricks for old drugs: Faced with skyrocketing costs for developing new drugs, researchers are looking at ways to repurpose older ones–and even some that failed in initial trials. Nature 2016, 534, 314317. [Google Scholar]
- Arrowsmith, J. Phase II failures: 2008–2010. Nat. Rev. Drug Discov. 2011, 10, 328–329. [Google Scholar] [CrossRef]
- Arrrowsmith, J. Phase III and submission failures 2007–2010. Nat. Rev. Drug Discov. 2011, 10, 87. [Google Scholar] [CrossRef]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Gatta, G.; van der Zwan, J.M.; Casali, P.G.; Siesling, S.; Tos, A.P.D.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef]
- Padhy, B.M.; Gupta, Y.K. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153. [Google Scholar] [CrossRef]
- Shineman, D.W.; Alam, J.; Anderson, M.; Black, S.E.; Carman, A.J.; Cummings, J.L.; Dacks, P.A.; Dudley, J.T.; Frail, D.E.; Green, A.; et al. Overcoming obstacles to repurposing for neurodegenerative disease. Ann. Clin. Transl. Neurol. 2014, 1, 512–518. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Novac, N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013, 34, 267–272. [Google Scholar] [CrossRef]
- Styne, D.M. Introduction to Pediatric Endocrinology: The Endocrine System; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. J. Control. Release 2016, 244, 122–135. [Google Scholar] [CrossRef]
- Malik, M.H.; Shahzadi, L.; Batool, R.; Safi, S.Z.; Khan, A.S.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Thyroxine-loaded chitosan/carboxymethyl cellulose/hydroxyapatite hydrogels enhance angiogenesis in in-ovo experiments. Int. J. Biol. Macromol. 2020, 145, 1162–1170. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, J.-M.; Lee, H.-J.; Jeong, S.-H.; Suh, J.-Y.; Hanawa, T. Bone healing with oxytocin-loaded microporous β-TCP bone substitute in ectopic bone formation model and critical-sized osseous defect of rat. J. Clin. Periodontol. 2014, 41, 181–190. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, P.; Nie, W.; Peng, C.; Li, T.; Qiang, L.; He, C.; Wang, J. Incorporation of dexamethasone-loaded mesoporous silica nanoparticles into mineralized porous biocomposite scaffolds for improving osteogenic activity. Int. J. Biol. Macromol. 2020, 149, 116–126. [Google Scholar] [CrossRef]
- Van de Ven, C.J.J.M.; Bakker, N.E.C.; Link, D.P.; Geven, E.J.W.; Gossen, J.A. Sustained release of ancillary amounts of testosterone and alendronate from PLGA coated pericard membranes and implants to improve bone healing. PLoS ONE 2021, 16, e0251864. [Google Scholar] [CrossRef]
- Ning, Z.; Tan, B.; Chen, B.; Lau, D.S.A.; Wong, T.M.; Sun, T.; Peng, S.; Li, Z.; Lu, W.W. Precisely Controlled Delivery of Abaloparatide through Injectable Hydrogel to Promote Bone Regeneration. Macromol. Biosci. 2019, 19, e1900020. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Qi, F.; Cheng, Y.; Lu, X.; Wang, L.; Zhao, J.; Zhao, B. Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/PLGA composite scaffold. Int. J. Nanomed. 2017, 13, 117–127. [Google Scholar] [CrossRef]
- Mu, C.; Hu, Y.; Huang, L.; Shen, X.; Li, M.; Li, L.; Gu, H.; Yu, Y.; Xia, Z.; Cai, K. Sustained raloxifene release from hyaluronan-alendronate-functionalized titanium nanotube arrays capable of enhancing osseointegration in osteoporotic rabbits. Mater. Sci. Eng. C 2018, 82, 345–353. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Wang, D.; Steffi, C.; Wang, Z.; Kong, C.H.; Lim, P.N.; Shi, Z.; Thian, E.S.; Wang, W. Beta-cyclodextrin modified mesoporous bioactive glass nanoparticles/silk fibroin hybrid nanofibers as an implantable estradiol delivery system for the potential treatment of osteoporosis. Nanoscale 2018, 10, 18341–18353. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzadeh, M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 141–155. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Hsiao, P.-Y.; Mau, L.-P.; Tsai, Y.-W.C.; Cochran, D.L.; Weng, P.-W.; Cheng, W.-C.; Chung, C.-H.; Huang, Y.-C. Synthesis and Characterization of Melatonin-Loaded Chitosan Microparticles Promote Differentiation and Mineralization in Preosteoblastic Cells. J. Oral Implant. 2020, 46, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Wada-Mihara, C.; Seto, H.; Ohba, H.; Tokunaga, K.; Kido, J.-I.; Nagata, T.; Naruishi, K. Local administration of calcitonin inhibits alveolar bone loss in an experimental periodontitis in rats. Biomed. Pharmacother. 2018, 97, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.R.; Shahzadi, L.; Alvi, F.; Khan, A.F.; Chaudhry, A.A.; ur Rehman, I.; Yar, M. Thyroxin releasing chitosan/collagen based smart hydrogels to stimulate neovascularization. Mater. Des. 2017, 133, 416–425. [Google Scholar] [CrossRef]
- Luidens, M.K.; Mousa, S.A.; Davis, F.B.; Lin, H.-Y.; Davis, P.J. Thyroid hormone and angiogenesis. Vasc. Pharmacol. 2010, 52, 142–145. [Google Scholar] [CrossRef]
- Sirakov, M.; Skah, S.; Nadjar, J.; Plateroti, M. Thyroid hormone’s action on progenitor/stem cell biology: New challenge for a classic hormone? Biochim. Biophys. Acta 2013, 1830, 3917–3927. [Google Scholar] [CrossRef]
- Shahzadi, L.; Bashir, M.; Tehseen, S.; Zehra, M.; Mehmood, A.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Thyroxine impregnated chitosan-based dressings stimulate angiogenesis and support fast wounds healing in rats: Potential clinical candidates. Int. J. Biol. Macromol. 2020, 160, 296–306. [Google Scholar] [CrossRef]
- Colaianni, G.; Sun, L.; Di Benedetto, A.; Tamma, R.; Zhu, L.-L.; Cao, J.; Grano, M.; Yuen, T.; Colucci, S.; Cuscito, C.; et al. Bone Marrow Oxytocin Mediates the Anabolic Action of Estrogen on the Skeleton. J. Biol. Chem. 2012, 287, 29159–29167. [Google Scholar] [CrossRef]
- Colaianni, G.; Di Benedetto, A.; Zhu, L.-L.; Tamma, R.; Li, J.; Greco, G.; Peng, Y.; Dell’Endice, S.; Zhu, G.; Cuscito, C.; et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem. Biophys. Res. Commun. 2011, 411, 512–515. [Google Scholar] [CrossRef]
- Elnagar, A.; El-Dawy, K.; El-Belbasi, H.I.; Rehan, I.F.; Embark, H.; Al-Amgad, Z.; Shanab, O.; Mickdam, E.; Batiha, G.E.; Alamery, S.; et al. Ameliorative Effect of Oxytocin on FBN1 and PEPCK Gene Expression, and Behavioral Patterns in Rats’ Obesity-Induced Diabetes. Front. Public Health 2022, 10, 777129. [Google Scholar] [CrossRef]
- Dawood, M.Y. Novel approach to oxytocin induction-augmentation of labor. Application of oxytocin physiology during pregnancy. Single Mol. Single Cell Seq. 1995, 395, 585–594. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Akay, A.S.; Arısan, V.; Cevher, E.; Sessevmez, M.; Cam, B. Oxytocin-loaded sustained-release hydrogel graft provides accelerated bone formation: An experimental rat study. J. Orthop. Res. 2020, 38, 1676–1687. [Google Scholar] [CrossRef]
- Ge, B.; Liu, H.; Liang, Q.; Shang, L.; Wang, T.; Ge, S. Oxytocin facilitates the proliferation, migration and osteogenic differentiation of human periodontal stem cells in vitro. Arch. Oral. Biol. 2019, 99, 126–133. [Google Scholar] [CrossRef]
- Chen, Y.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018, 67, 341–353. [Google Scholar] [CrossRef]
- Jørgensen, N.; Henriksen, Z.; Sørensen, O.; Civitelli, R. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: Validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids 2004, 69, 219–226. [Google Scholar] [CrossRef]
- Martins, A.; Duarte, A.R.C.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. Osteogenic induction of hBMSCs by electrospun scaffolds with dexamethasone release functionality. Biomaterials 2010, 31, 5875–5885. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Wang, Y.; Yang, G.; Ding, S.; Zhou, S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials 2015, 37, 218–229. [Google Scholar] [CrossRef]
- Ren, H.; Chen, S.; Jin, Y.; Zhang, C.; Yang, X.; Ge, K.; Liang, X.-J.; Li, Z.; Zhang, J. A traceable and bone-targeted nanoassembly based on defect-related luminescent mesoporous silica for enhanced osteogenic differentiation. J. Mater. Chem. B 2017, 5, 1585–1593. [Google Scholar] [CrossRef]
- Porter, R.M.; Huckle, W.R.; Goldstein, A.S. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J. Cell. Biochem. 2003, 90, 13–22. [Google Scholar] [CrossRef]
- Kim, H.; Suh, H.; Jo, S.A.; Kim, H.W.; Lee, J.M.; Kim, E.H.; Reinwald, Y.; Park, S.-H.; Min, B.-H.; Jo, I. In vivo bone formation by human marrow stromal cells in biodegradable scaffolds that release dexamethasone and ascorbate-2-phosphate. Biochem. Biophys. Res. Commun. 2005, 332, 1053–1060. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Chu, T.-M.G.; Chang, C.; Kang, H.-Y.; Huang, K.-E. Testosterone Delivered with a Scaffold Is as Effective as Bone Morphologic Protein-2 in Promoting the Repair of Critical-Size Segmental Defect of Femoral Bone in Mice. PLoS ONE 2013, 8, e70234. [Google Scholar] [CrossRef]
- Teitelbaum, S. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Capriani, C.; Irani, D.; Bilezikian, J.P.; Research, M. Safety of osteoanabolic therapy: A decade of experience. JBMR 2012, 27, 2419–2428. [Google Scholar] [CrossRef]
- Aspenberg, P.; Genant, H.K.; Johansson, T.; Nino, A.J.; See, K.; Krohn, K.; García-Hernández, P.A.; Recknor, C.P.; Einhorn, T.A.; Dalsky, G.P.; et al. Teriparatide for acceleration of fracture repair in humans: A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010, 25, 404–414. [Google Scholar] [CrossRef]
- Holzer, G.; Majeska, R.J.; Lundy, M.W.; Hartke, J.R.; Einhorn, T.A. Parathyroid hormone enhances fracture healing: A preliminary report. Clin. Orthop. Relat. Res. 1999, 366, 258–263. [Google Scholar] [CrossRef]
- Wojda, S.J.; Donahue, S.W. Parathyroid hormone for bone regeneration. J. Orthop. Res. 2018, 36, 2586–2594. [Google Scholar] [CrossRef]
- Huang, J.; Lin, D.; Wei, Z.; Li, Q.; Zheng, J.; Zheng, Q.; Cai, L.; Li, X.; Yuan, Y.; Li, J. Parathyroid Hormone Derivative with Reduced Osteoclastic Activity Promoted Bone Regeneration via Synergistic Bone Remodeling and Angiogenesis. Small 2020, 16, 1905876. [Google Scholar] [CrossRef]
- Dang, M.; Koh, A.J.; Jin, X.; McCauley, L.K.; Ma, P.X. Local pulsatile PTH delivery regenerates bone defects via enhanced bone remodeling in a cell-free scaffold. Biomaterials 2017, 114, 1–9. [Google Scholar] [CrossRef]
- Vanea, E.; Moraru, C.; Vulpoi, A.; Cavalu, S.; Simon, V. Freeze-dried and spray-dried zinc-containing silica microparticles entrapping insulin. J. Biomater. Appl. 2014, 28, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Maratova, K.; Soucek, O.; Matyskova, J.; Hlavka, Z.; Petruzelkova, L.; Obermannova, B.; Pruhova, S.; Kolouskova, S.; Sumnik, Z. Muscle functions and bone strength are impaired in adolescents with type 1 diabetes. Bone 2018, 106, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Nordklint, A.K.; Almdal, T.P.; Vestergaard, P.; Lundby-Christensen, L.; Jørgensen, N.R.; Boesgaard, T.W.; Breum, L.; Gade-Rasmussen, B.; Sneppen, S.B.; Gluud, C.; et al. Effect of Metformin vs. Placebo in Combination with Insulin Analogues on Bone Markers P1NP and CTX in Patients with Type 2 Diabetes Mellitus. Calcif. Tissue Res. 2020, 107, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 2005, 48, 1292–1299. [Google Scholar] [CrossRef]
- Hynes, B.; Kumar, A.; O’Sullivan, J.; Buneker, C.K.; Leblond, A.-L.; Weiss, S.; Schmeckpeper, J.; Martin, K.; Caplice, N.M. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur. Hear. J. 2011, 34, 782–789. [Google Scholar] [CrossRef]
- Paglia, D.N.; Wey, A.; Breitbart, E.A.; Faiwiszewski, J.; Mehta, S.K.; Al-Zube, L.; Vaidya, S.; Cottrell, J.A.; Graves, D.; Benevenia, J.; et al. Effects of local insulin delivery on subperiosteal angiogenesis and mineralized tissue formation during fracture healing. J. Orthop. Res. 2012, 31, 783–791. [Google Scholar] [CrossRef]
- Rabinovsky, E.D.; Draghia-Akli, R. Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol. Ther. 2004, 9, 46–55. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Reid, I.R. Insulin increases histomorphometric indices of bone formation in vivo. Calcif. Tissue Int. 1996, 59, 492–495. [Google Scholar] [CrossRef]
- Thomas, D.; Udagawa, N.; Hards, D.; Quinn, J.; Moseley, J.; Findlay, D.; Best, J.D. Insulin receptor expression in primary and cultured osteoclast-like cells. Bone 1998, 23, 181–186. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Mat. 2012, 18, 1095–1101. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local delivery of insulin/IGF-1 for bone regeneration: Carriers, strategies, and effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef]
- Wang, X.; Qi, F.; Xing, H.; Zhang, X.; Lu, C.; Zheng, J.; Ren, X. Uniform-sized insulin-loaded PLGA microspheres for improved early-stage peri-implant bone regeneration. Drug Deliv. 2019, 26, 1178–1190. [Google Scholar] [CrossRef]
- Krajcer, A.; Klara, J.; Horak, W.; Lewandowska-Łańcucka, J. Bioactive injectable composites based on insulin-functionalized silica particles reinforced polymeric hydrogels for potential applications in bone tissue engineering. J. Mater. Sci. Technol. 2022, 105, 153–163. [Google Scholar] [CrossRef]
- Wang, B.; Song, Y.; Wang, F.; Li, D.; Zhang, H.; Ma, A.; Huang, N. Effects of local infiltration of insulin around titanium implants in diabetic rats. Br. J. Oral Maxillofac. Surg. 2011, 49, 225–229. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Heufelder, A.E. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 2001, 79, 243–253. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Irmak, G.; Demirtaş, T.T.; Altındal, D.Ç.; Çalış, M.; Gümüşderelioğlu, M. Sustained release of 17β-estradiol stimulates osteogenic differentiation of adipose tissue-derived mesenchymal stem cells on chitosan-hydroxyapatite scaffolds. Cells Tissues Organs 2014, 199, 37–50. [Google Scholar] [CrossRef]
- Ettinger, B.; Pressman, A.; Sklarin, P.; Bauer, D.C.; Cauley, J.A.; Cummings, S.R. Associations between Low Levels of Serum Estradiol, Bone Density, and Fractures among Elderly Women: The Study of Osteoporotic Fractures1. J. Clin. Endocrinol. Metab. 1998, 83, 2239–2243. [Google Scholar] [CrossRef]
- Bowring, C.; Francis, R.M. National Osteoporosis Society’s Position statement on hormone replacement therapy in the prevention and treatment of osteoporosis. Menopause Int. 2011, 17, 63–65. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Hu, Y.; Yuan, W.; Qiu, X.; Jiang, T.; Xia, C.; Xiong, L.; Li, F.; Gao, Y. EDTA-Modified 17β-Estradiol-Laden Upconversion Nanocomposite for Bone-Targeted Hormone Replacement Therapy for Osteoporosis. Theranostics 2020, 10, 3281–3292. [Google Scholar] [CrossRef]
- Segredo-Morales, E.; Reyes, R.; Arnau, M.R.; Delgado, A.; Évora, C. In situ gel-forming system for dual BMP-2 and 17β-estradiol controlled release for bone regeneration in osteoporotic rats. Drug Deliv. Transl. Res. 2018, 8, 1103–1113. [Google Scholar] [CrossRef]
- Ott, S.M.; Oleksik, A.; Lu, Y.; Harper, K.; Lips, P. Bone Histomorphometric and Biochemical Marker Results of a 2-Year Placebo-Controlled Trial of Raloxifene in Postmenopausal Women. J. Bone Miner. Res. 2002, 17, 341–348. [Google Scholar] [CrossRef]
- Urano, T.; Shiraki, M.; Kuroda, T.; Tanaka, S.; Uenishi, K.; Inoue, S. Preventive effects of raloxifene treatment on agerelated weight loss in postmenopausal women. J. Bone Miner. Metab. 2017, 35, 108–113. [Google Scholar] [CrossRef]
- Spiro, A.S.; Khadem, S.; Jeschke, A.; Marshall, R.P.; Pogoda, P.; Ignatius, A.; Amling, M.; Beil, F.T. The SERM raloxifene improves diaphyseal fracture healing in mice. J. Bone Miner. Metab. 2013, 31, 629–636. [Google Scholar] [CrossRef]
- Stringhetta-Garcia, C.T.; Singulani, M.P.; Santos, L.F.; Louzada, M.J.Q.; Nakamune, A.C.S.; Chaves-Neto, A.H.; Rossi, A.C.; Ervolino, E.; Dornelles, R.C.M. The effects of strength training and raloxifene on bone health in aging ovariectomized rats. Bone 2016, 85, 45–54. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Mirkin, S.; Pickar, J.H. Selective estrogen receptor modulators (SERMs): A review of clinical data. Maturitas 2015, 80, 52–57. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Thomas, S. Use of SERMs for treatment in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2014, 142, 142–154. [Google Scholar] [CrossRef]
- Powles, T.J.; Hickish, T.; Kanis, J.A.; Tidy, A.; Ashley, S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J. Clin. Oncol. 1996, 14, 78–84. [Google Scholar] [CrossRef]
- Love, R.; Mazess, R.; Barden, H.; Epstein, S.; Newcomb, P.; Jordan, V.; Carbone, P.; DeMets, D.L. Effects of tamoxifen on bone material density and metabolism in postmenopausal women with breast cancer. N. Engl. J. Med. 1992, 326, 852–856. [Google Scholar] [CrossRef]
- Blair, J.M.; Hanson, D.L.; Jones, J.L.; Dworkin, M.S. Trends in Pregnancy Rates Among Women With Human Immunodeficiency Virus. Obstet. Gynecol. 2004, 103, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.L.; Christiansen, C.; Genant, H.K.; Vukicevic, S.; Zanchetta, J.R.; de Villiers, T.J.; Constantine, G.D.; Chines, A.A. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: Results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J. Bone Miner. Res. 2008, 23, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jeyakumar, M.; Petukhov, S.; Bagchi, M.K. A nuclear receptor corepressor modulates transcriptional activity of antagonist-occupied steroid hormone receptor. Mol. Endocrinol. 1998, 12, 513–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Couse, J.F.; Korach, K.S. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef]
- Riggs, B.L.; Hartmann, L.C. Selective estrogen-receptor modulators—Mechanisms of action and application to clinical practice. N. Engl. J. Med. 2003, 348, 618–629. [Google Scholar] [CrossRef]
- Arnott, J.; Martinkovich, S.; Planey, S.L.; Shah, D. Selective estrogen receptor modulators: Tissue specificity and clinical utility. Clin. Interv. Aging 2014, 9, 1437–1452. [Google Scholar] [CrossRef]
- Maximov, P.Y.; Lee, M.T.; Jordan, V.C. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef]
- Elkasabgy, N.; Abdel-Salam, F.S.; Mahmoud, A.A.; Basalious, E.B.; Amer, M.S.; Mostafa, A.A.; Elkheshen, S.A. Long lasting in-situ forming implant loaded with raloxifene HCl: An injectable delivery system for treatment of bone injuries. Int. J. Pharm. 2019, 571, 118703. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Cheng, J.; Xiao, Y.-C.; Yin, R.-F.; Feng, X. Raloxifene microsphere-embedded collagen/chitosan/β-tricalcium phosphate scaffold for effective bone tissue engineering. Int. J. Pharm. 2017, 518, 80–85. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Nanofibrillated cellulose/cyclodextrin based 3D scaffolds loaded with raloxifene hydrochloride for bone regeneration. Int. J. Biol. Macromol. 2020, 156, 704–716. [Google Scholar] [CrossRef]
- Harmankaya, N.; Karlsson, J.; Palmquist, A.; Halvarsson, M.; Igawa, K.; Andersson, M.; Tengvall, P. Raloxifene and alendronate containing thin mesoporous titanium oxide films improve implant fixation to bone. Acta. Biomater. 2013, 9, 7064–7073. [Google Scholar] [CrossRef]
- Yang, D.; Anderson, P.H.; Wijenayaka, A.R.; Barratt, K.R.; Triliana, R.; Stapledon, C.J.; Zhou, H.; Findlay, D.M.; Morris, H.A.; Atkins, G.J.; et al. Both ligand and VDR expression levels critically determine the effect of 1α, 25-dihydroxyvitamin-D3 on osteoblast differentiation. J. Steroid. Biochem. Mol. Biol. 2018, 177, 83–90. [Google Scholar] [CrossRef]
- Griffin, A.C.; Kern, M.J.; Kirkwood, K. MKP-1 is essential for canonical vitamin D-induced signaling through nuclear import and regulates RANKL expression and function. Mol. Endocrinol. 2012, 26, 1682–1693. [Google Scholar] [CrossRef]
- Mucuk, G.; Sepet, E.; Erguven, M.; Ekmekcı, O.; Bılır, A. 1,25-Dihydroxyvitamin D3 stimulates odontoblastic differentiation of human dental pulp-stem cells in vitro. Connect. Tissue Res. 2017, 58, 531–541. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Cassiano, F.B.; Silva, I.S.P.; Usberti, F.R.; Anovazzi, G.; Pacheco, L.E.; Pansani, T.N.; Leite, M.L.; Hebling, J.; de Souza Costa, C.A. Synergistic potential of 1α, 25-dihydroxyvitamin D3 and calcium–aluminate–chitosan scaffolds with dental pulp cells. Clin. Oral. Investig. 2020, 24, 663–674. [Google Scholar] [CrossRef]
- Sumathra, M.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Osteoblast response to Vitamin D3 loaded cellulose enriched hydroxyapatite Mesoporous silica nanoparticles composite. Biomed. Pharmacother. 2018, 103, 858–868. [Google Scholar] [CrossRef]
- Leonida, A.; Favero, G.; Caccianiga, P.; Ceraulo, S.; Rodella, L.F.; Rezzani, R.; Caccianiga, G. Concentrated Growth Factors (CGF) Combined with Melatonin in Guided Bone Regeneration (GBR): A Case Report. Diagnostics 2022, 12, 1257. [Google Scholar] [CrossRef]
- Shino, H.; Hasuike, A.; Arai, Y.; Honda, M.; Isokawa, K.; Sato, S. Melatonin enhances vertical bone augmentation in rat calvaria secluded spaces. Oral Surg. 2016, 21, e122–e126. [Google Scholar] [CrossRef]
- Meenakshi, S.S.; Malaiappan, S. Role of melatonin in periodontal disease—A systematic review. Indian J. Dent. Res. 2020, 31, 593. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Rahim, I.; Acuña-Fernández, C.; Fernández-Ortiz, M.; Solera-Marín, J.; Sayed, R.K.A.; Díaz-Casado, M.E.; Rusanova, I.; Lopez, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef]
- Liu, J.; Huang, F.; He, H.-W. Melatonin Effects on Hard Tissues: Bone and Tooth. Int. J. Mol. Sci. 2013, 14, 10063–10074. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Fan, W.; Dong, W.; Fu, S.; He, H.; Huang, F. Melatonin influences proliferation and differentiation of rat dental papilla cells in vitro and dentine formation in vivo by altering mitochondrial activity. J. Pineal Res. 2012, 54, 170–178. [Google Scholar] [CrossRef]
- Köse, O.; Arabaci, T.; Kizildag, A.; Erdemci, B.; Eminoğlu, D.; Gedikli, S.; Özkanlar, S.; Zihni, M.; Albayrak, M.; Kara, A.; et al. Melatonin prevents radiation-induced oxidative stress and periodontal tissue breakdown in irradiated rats with experimental periodontitis. J. Periodontal Res. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Fernández-Ortiz, M.; Sayed, R.K.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 connection in mouse heart mitochondria during aging. Antioxidants 2020, 9, 1187. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Marani, F.; Chiba, F.Y.; Mattera, M.S.D.L.C.; Tsosura, T.V.S.; Tessarin, G.W.L.; Pereira, R.F.; Belardi, B.E.; Pinheiro, B.C.E.S.; Sumida, D.H. Melatonin promotes reduction in TNF levels and improves the lipid profile and insulin sensitivity in pinealectomized rats with periodontal disease. Life Sci. 2018, 213, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gil, B.; Moneim, A.E.A.; Ortiz, F.; Shen, Y.-Q.; Soto-Mercado, V.; Mendivil-Perez, M.; Guerra-Librero, A.; Acuna-Castroviejo, D.; Molina-Navarro, M.M.; García-Verdugo, J.; et al. Melatonin protects rats from radiotherapy-induced small intestine toxicity. PLoS ONE 2017, 12, e0174474. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, F.P.; Vriend, J. The half-life of melatonin elimination from rat plasma. Endocrinology 1981, 109, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, D.; Afify, O.; El Soudany, K.; Ghoniem, S. The effect of local application of melatonin gel on the healing of periodontal osseous defects in experimentally induced diabetes in rabbits. Tanta Dent. J. 2013, 10, 48–57. [Google Scholar] [CrossRef]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Muñoz, F.; Lopez-Peña, M.; Stephenson, J.; Reiter, R.J. Melatonin stimulates osteointegration of dental implants. J. Pineal. Res. 2008, 45, 174–179. [Google Scholar] [CrossRef]
- Elgammal, M.Y.A.; Salem, A.S.; Anees, M.M.; Tawfik, M.A.-M. Clinical and Radiographic Evaluation of Immediate Loaded Dental Implants With Local Application of Melatonin: A Preliminary Randomized Controlled Clinical Trial. J. Oral Implant. 2016, 42, 119–125. [Google Scholar] [CrossRef]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2018, 66, e12534. [Google Scholar] [CrossRef]
- Rangarajan, V.; Juul, S.E. Erythropoietin: Emerging role of erythropoietin in neonatal neuroprotection. Pediatr. Neurol. 2014, 51, 481–488. [Google Scholar] [CrossRef]
- Bulmer, C.; Margaritis, A.; Xenocostas, A. Production and characterization of novel chitosan nanoparticles for controlled release of rHu-Erythropoietin. Biochem. Eng. J. 2012, 68, 61–69. [Google Scholar] [CrossRef]
- Fayed, B.E.; Tawfik, A.F.; Yassin, A.E.B. Novel erythropoietin-loaded nanoparticles with prolonged in vivo response. J. Microencapsul. 2012, 29, 650–656. [Google Scholar] [CrossRef]
- Jelkmann, W. Erythropoietin: Structure, control of production, and function. Physiol. Rev. 1992, 72, 449–489. [Google Scholar] [CrossRef]
- Anagnostou, A.; Lee, E.S.; Kessimian, N.; Levinson, R.; Steiner, M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5978–5982. [Google Scholar] [CrossRef]
- Anagnostou, A.; Liu, Z.; Steiner, M.; Chin, K.; Lee, E.S.; Kessimian, N.; Noguchi, C.T. Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3974–3978. [Google Scholar] [CrossRef]
- Haroon, Z.A.; Amin, K.; Jiang, X.; Arcasoy, M.O. A Novel Role for Erythropoietin During Fibrin-Induced Wound-Healing Response. Am. J. Pathol. 2003, 163, 993–1000. [Google Scholar] [CrossRef]
- Isogai, R.; Takahashi, M.; Aisu, K.; Horiuti, Y.; Aragane, Y.; Kawada, A.; Tezuka, T. The receptor for erythropoietin is present on cutaneous mast cells. Arch. Derm. Res. 2006, 297, 389–394. [Google Scholar] [CrossRef]
- Bodó, E.; Kromminga, A.; Funk, W.; Laugsch, M.; Duske, U.; Jelkmann, W.; Paus, R. Human hair follicles are an extrarenal source and a nonhematopoietic target of erythropoietin. FASEB J. 2007, 21, 3346–3354. [Google Scholar] [CrossRef]
- Campana, W.M.; Myers, R.R. Erythropoietin and erythropoietin receptors in the peripheral nervous system: Changes after nerve injury. FASEB J. 2001, 15, 1804–1806. [Google Scholar] [CrossRef]
- Bianchi, R.; Buyukakilli, B.; Brines, M.; Savino, C.; Cavaletti, G.; Oggioni, N.; Lauria, G.; Borgna, M.; Lombardi, R.; Cimen, B.; et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc. Natl. Acad. Sci. USA 2004, 101, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Lykissas, M.G.; Korompilias, A.V.; Vekris, M.D.; Mitsionis, G.I.; Sakellariou, E.; Beris, A.E. The role of erythropoietin in central and peripheral nerve injury. Clin. Neurol. Neurosurg. 2007, 109, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E. Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013, 210, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Erythropoietin: Back to basics. Blood 2010, 115, 4151–4152. [Google Scholar] [CrossRef]
- Jelkmann, W.; Elliott, S. Erythropoietin and the vascular wall: The controversy continues. Nutr. Metab. Cardiovasc. Dis. 2013, 23, S37–S43. [Google Scholar] [CrossRef]
- Nairz, M.; Sonnweber, T.; Schroll, A.; Theurl, I.; Weiss, G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012, 14, 238–246. [Google Scholar] [CrossRef]
- Brines, M.; Cerami, A. Erythropoietin-mediated tissue protection: Reducing collateral damage from the primary injury response. J. Intern. Med. 2008, 264, 405–432. [Google Scholar] [CrossRef]
- Hamed, S.; Ullmann, Y.; Masoud, M.; Hellou, E.; Khamaysi, Z.; Teot, L. Topical Erythropoietin Promotes Wound Repair in Diabetic Rats. J. Investig. Dermatol. 2010, 130, 287–294. [Google Scholar] [CrossRef]
- Hamed, S.; Ullmann, Y.; Egozi, D.; Daod, E.; Hellou, E.; Ashkar, M.; Gilhar, A.; Teot, L. Fibronectin Potentiates Topical Erythropoietin-Induced Wound Repair in Diabetic Mice. J. Investig. Dermatol. 2011, 131, 1365–1374. [Google Scholar] [CrossRef]
- Holstein, J.; Orth, M.; Scheuer, C.; Tami, A.; Becker, S.; Garcia, P.; Histing, T.; Mörsdorf, P.; Klein, M.; Pohlemann, T.; et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 2011, 49, 1037–1045. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Wang, J.; Lee, C.H.; Sud, S.; et al. Erythropoietin Couples Hematopoiesis with Bone Formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Sun, H.; Joseph, J.; Mishra, A.; Shiozawa, Y.; Wang, J.; Krebsbach, P.H.; Taichman, R.S. Erythropoietin mediated bone formation is regulated by mTOR signaling. J. Cell. Biochem. 2011, 113, 220–228. [Google Scholar] [CrossRef]
- Li, C.; Shi, C.; Kim, J.; Chen, Y.; Ni, S.; Jiang, L.; Zheng, C.; Li, D.; Hou, J.; Taichman, R.S.; et al. Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J. Dent. Res. 2015, 94, 455–463. [Google Scholar] [CrossRef]
- Yaghobee, S.; Rouzmeh, N.; Aslroosta, H.; Mahmoodi, S.; Khorsand, A.; Kharrazifard, M.J. Effect of Topical Erythropoietin (EPO) on palatal wound healing subsequent to Free Gingival Grafting (FGG). Braz. Oral Res. 2018, 32, e55. [Google Scholar] [CrossRef]
- Aslroosta, H.; Yaghobee, S.; Akbari, S.; Kanounisabet, N. The effects of topical erythropoietin on non-surgical treatment of periodontitis: A preliminary study. BMC Oral Health 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Wu, F.; Song, Y.; Duan, Y.; Jin, Z. Erythropoietin induces the osteogenesis of periodontal mesenchymal stem cells from healthy and periodontitis sources via activation of the p38 MAPK pathway. Int. J. Mol. Med. 2018, 41, 829–835. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Cong, M.; Liu, L.; Yan, G.; Li, Z.; Li, B.; Yu, W.; Sun, H.; Yang, B. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation in vivo. Dent. Mater. 2020, 36, e229–e240. [Google Scholar] [CrossRef]
- De Paula, F.J.A.; Rosen, C.J. Back to the Future: Revisiting Parathyroid Hormone and Calcitonin Control of Bone Remodeling. Horm. Metab. Res. 2010, 42, 299–306. [Google Scholar] [CrossRef]
- Wallach, S.; Carstens, J.; Avioli, L.V. Calcitonin, osteoclasts, and bone turnover. Calcif. Tissue Int. 1990, 47, 388–391. [Google Scholar] [CrossRef]
- Granholm, S.; Lundberg, P.; Lerner, U.H. Calcitonin inhibits osteoclast formation in mouse haematopoetic cells independently of transcriptional regulation by receptor activator of NF-kappa B and c-Fms. J. Endocrinol 2007, 195, 415–428. [Google Scholar] [CrossRef]
- Zaidi, M.; Moonga, B.S.; Abe, E. Calcitonin and bone formation: A knockout full of surprises. J. Clin. Investig. 2002, 110, 1769–1771. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Fahimipour, F.; Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Vargas, J.; Vashaee, D.; Lobner, D.C.; Kashi, T.S.J.; Ghasemzadeh, B.; Tayebi, L. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent. Mater. 2019, 35, 990–1006. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local drug delivery systems as therapeutic strategies against periodontitis: A systematic review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C 2018, 82, 363–371. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
| Biomaterial | Advantages | Disadvantages | Clinical Application |
|---|---|---|---|
| Ceramics |
|
|
|
| Natural Polymers |
|
|
|
| Synthetic polymers |
|
|
|
| Composites |
|
|
|
| Hydrogels |
|
|
| No. | Significance | Ref. | Challenges | Ref. |
|---|---|---|---|---|
| 1 | Ensures safety | [68] | Inadequate understanding of regulatory standards | [69] |
| 2 | It results in lowering tome and costs | [70] | Insufficient revenue motives | [69] |
| 3 | Opportunity for branding: increased worldwide income; drives market expansion | [65,68] | Clinical trial issues include the possibility of failed proof-of-concept studies for novel indications | [70,71] |
| 4 | Out licensing likelihood: new purposes while keeping rights to the old indication | [68] | Patent constraints obstruct the marketing of repurposed molecules | [70] |
| 5 | Satisfy unfulfilled medical needs through discovering new applications for existing medications to cure uncommon disorders and targeting tumors with non-cancer therapies | [69,72] | Economic needs assessment | [72,73] |
| Peptides | Glycoproteins | Amines | Eicosanoids | Steroid Hormones |
|---|---|---|---|---|
| Source: made up of amino acid residues | Source: conjugated protein bound to carbohydrate | Source: modification of amino acids | Source: small fatty acid derivatives with a variety of arachidonic acid | Source: derived from cholesterol |
| e.g., Thyrotropin (TSH) | e.g., thyroid hormones and catecholamines | e.g., Prostaglandins | Examples:
|
| Short peptides | e.g.,
| |||
| Intermediate peptides | e.g.,
| |||
| Glycoproteins |
| |||
| Peptide-based hormones | ||||
| Amino acid derivatives | ||||
| Iodothyronines |
| |||
| Amines | i.e., • Melatonin | |||
| Steroidal hormones | ||||
| ||||
| Hormone | Current Indication | Used Carrier | Repurposed Application | Reference |
|---|---|---|---|---|
| Thyroxin | Hypothyroidism and thyroid cancer | Chitosan/collagen hydrogel | Angiogenesis and neovascularization | [76] |
| Oxytocin | Postpartum hemorrhage, labor induction, and incomplete or inevitable abortion | Micro porous β-TCP | Osseo induction and enhanced osteogenesis | [77] |
| Dexamethasone | Arthritis, blood/hormone issues, allergic responses, skin illnesses, vision difficulties, respiratory problems, gastrointestinal problems, tumors, and hypersensitivity reactions are all examples of medical conditions | Chitosan-alginate-gelatin matrix | Increased proliferation and osteogenic-enhanced bone marrow | [78] |
| Androgens | Estradiol production, sex drive and muscular mass | PLGA-coated pericardial membranes | Enhanced implant Osseo-integration and repair of bone defects and fractures | [79] |
| Parathyroid Hormone | Calcium/Phosphorus homeostasis | Injectable Gelatin Methacrylate (GelMA) hydrogel | Increased ALP activity and mineralization | [80] |
| Insulin | Treatment of Diabetes | Poly lactic-co-glycolic-acid (PLGA) nano spheres were incorporated into nano hydroxyapatite/collagen (nHAC) scaffolds | Increased bone regeneration in rabbit mandible critical size defects | [81] |
| Raloxifene | Treatment and prevention of postmenopausal osteoporosis | Chitosan composite encapsulated with PLGA microspheres | Increased cell proliferation, greater mineralization capability, and ALP activity | [82] |
| Erythropoietin | Treatment of cancer induced anemia | Cs/β-GP/Gelatin hydrogel | Anti-inflammation and improved periodontal regeneration | [83] |
| Estrogen |
| β-cyclodextrin/silk fibroin (SF) | Improved cell proliferation and osteoblast differentiation markers | [84] |
| Vitamin D | Osteomalacia, Osteoporosis | Polycaprolactone/gelatin scaffold incorporating HA nanoparticles. | Increased hADSC osteogenic development and maturation | [85] |
| Melatonin | Insomnia | Chitosan micro particles | Accelerating osteogenic differentiation of preosteoblast cells in vitro | [86] |
| Calcitonin | Hypercalcemia, Paget’s disease of bone | Local injection | Reduced alveolar bone resorption by controlling the action of osteoclasts | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Nasser Atia, G.; Shalaby, H.K.; Zehravi, M.; Ghobashy, M.M.; Ahmad, Z.; Khan, F.S.; Dey, A.; Rahman, M.H.; Joo, S.W.; Barai, H.R.; et al. Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review. Polymers 2022, 14, 2964. https://doi.org/10.3390/polym14142964
Abdel Nasser Atia G, Shalaby HK, Zehravi M, Ghobashy MM, Ahmad Z, Khan FS, Dey A, Rahman MH, Joo SW, Barai HR, et al. Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review. Polymers. 2022; 14(14):2964. https://doi.org/10.3390/polym14142964
Chicago/Turabian StyleAbdel Nasser Atia, Gamal, Hany K. Shalaby, Mehrukh Zehravi, Mohamed Mohamady Ghobashy, Zubair Ahmad, Farhat S. Khan, Abhijit Dey, Md. Habibur Rahman, Sang Woo Joo, Hasi Rani Barai, and et al. 2022. "Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review" Polymers 14, no. 14: 2964. https://doi.org/10.3390/polym14142964
APA StyleAbdel Nasser Atia, G., Shalaby, H. K., Zehravi, M., Ghobashy, M. M., Ahmad, Z., Khan, F. S., Dey, A., Rahman, M. H., Joo, S. W., Barai, H. R., & Cavalu, S. (2022). Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review. Polymers, 14(14), 2964. https://doi.org/10.3390/polym14142964