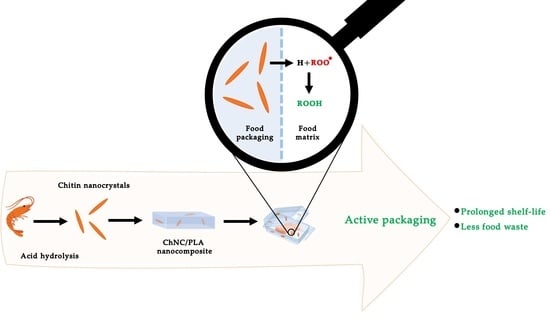
Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Chitin Nanocrystals Preparation
2.2.2. Nanocomposite Preparation
2.2.3. Deacetylation of ChNC
2.3. Sample Characterization
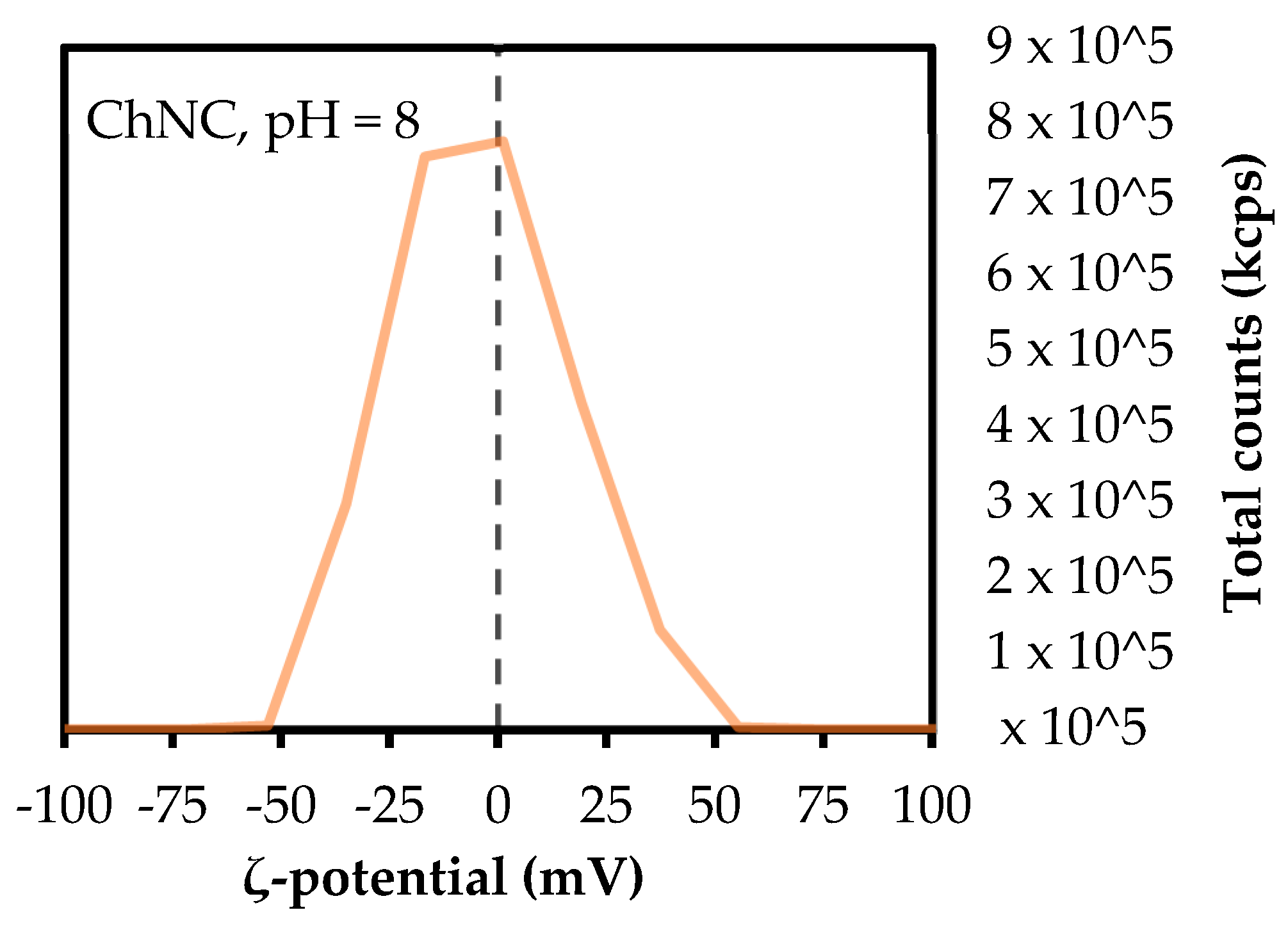
2.3.1. Particle Size and Zeta (ζ)-Potential
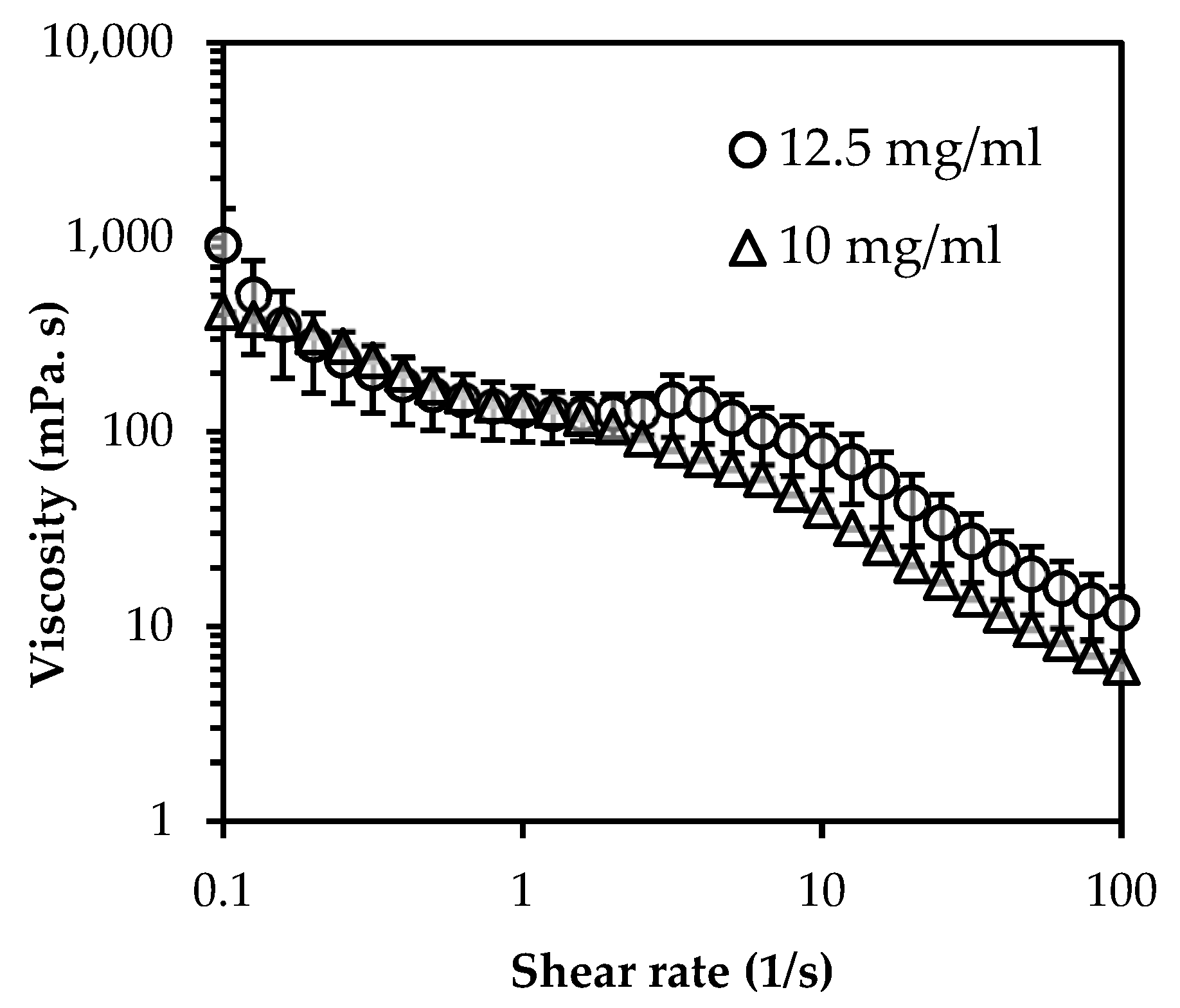
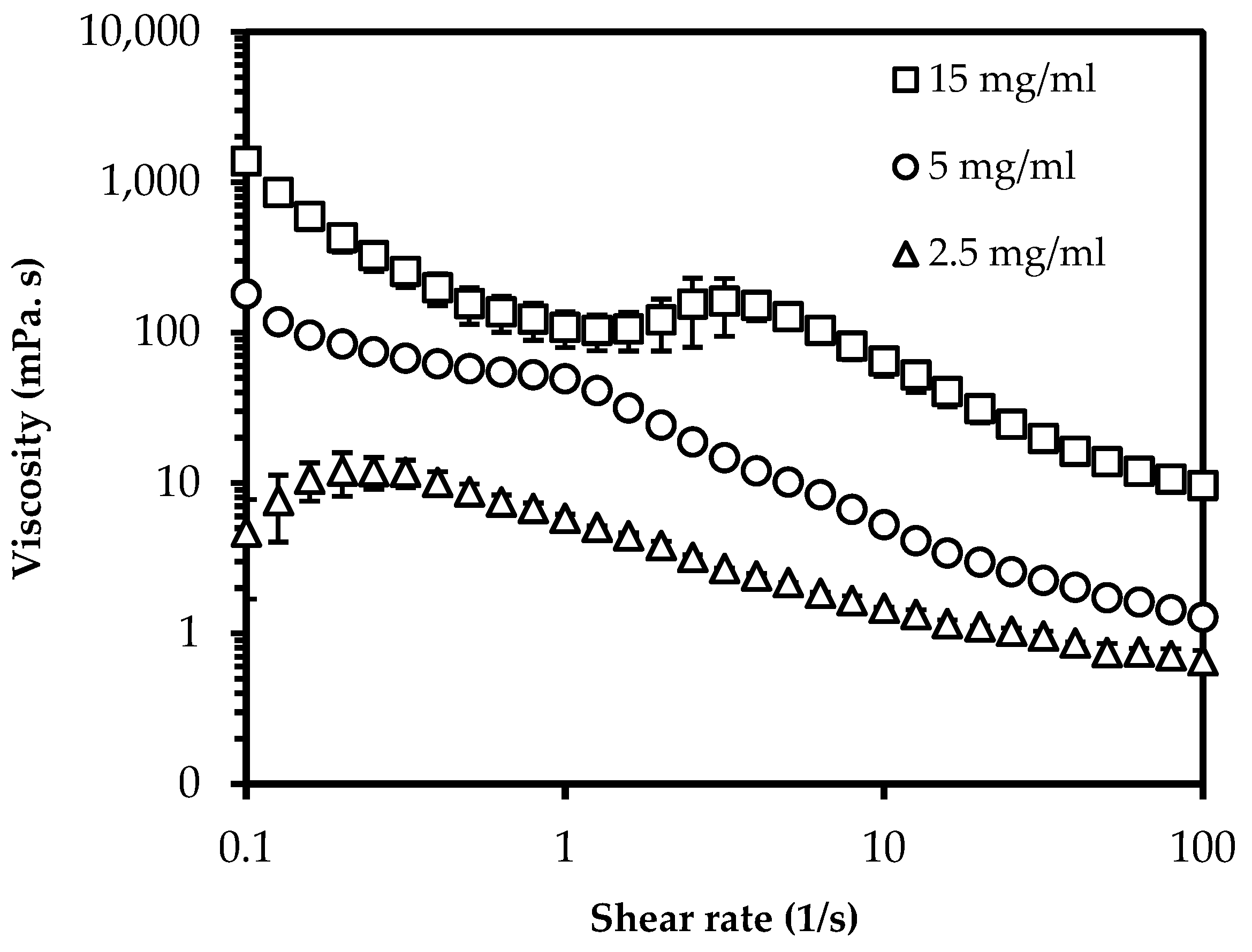
2.3.2. Viscosity Measurement
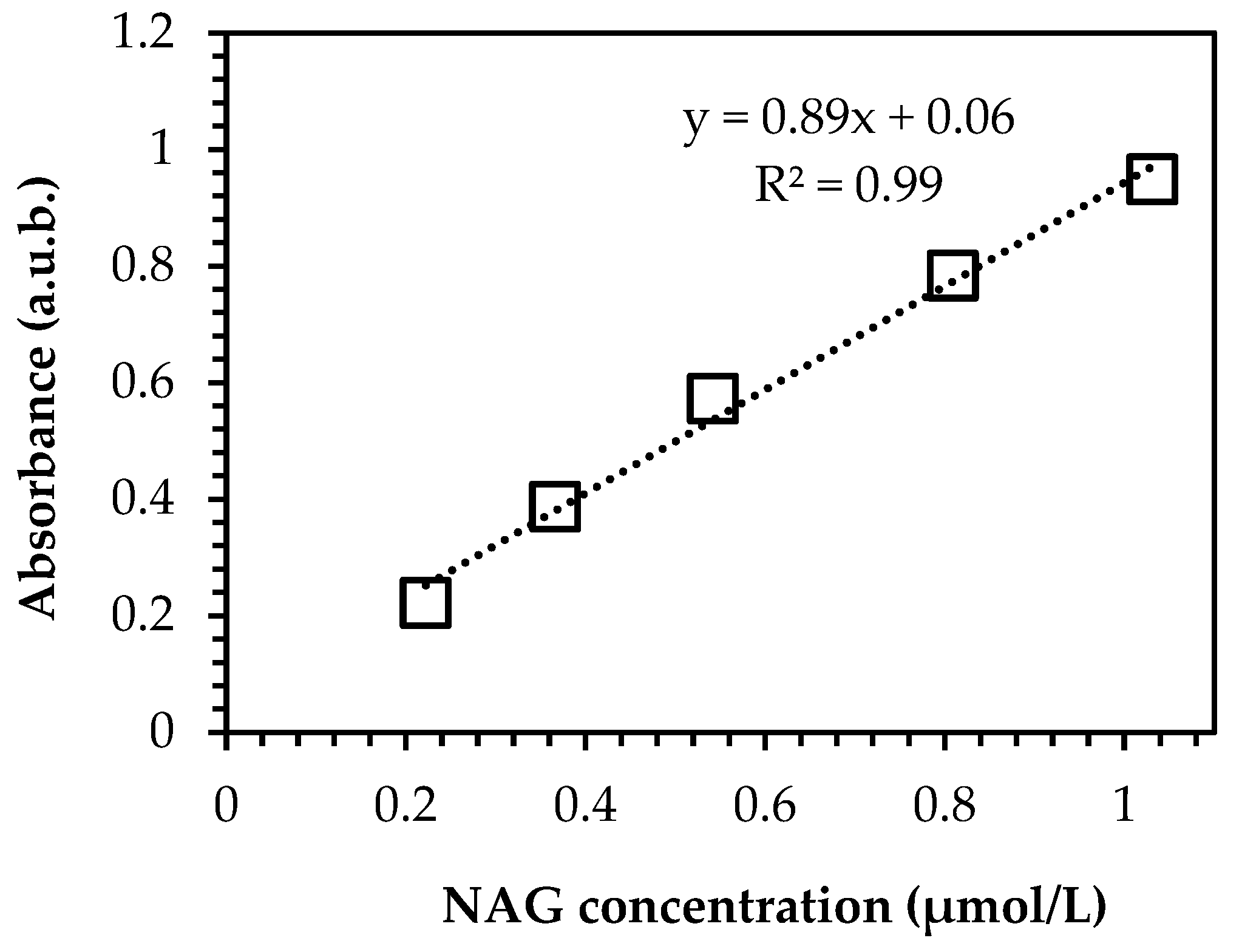
2.3.3. Degree of Deacetylation
2.3.4. Nanocomposite Morphology
2.4. Antioxidant Activity
3. Results
3.1. Particle Characterization
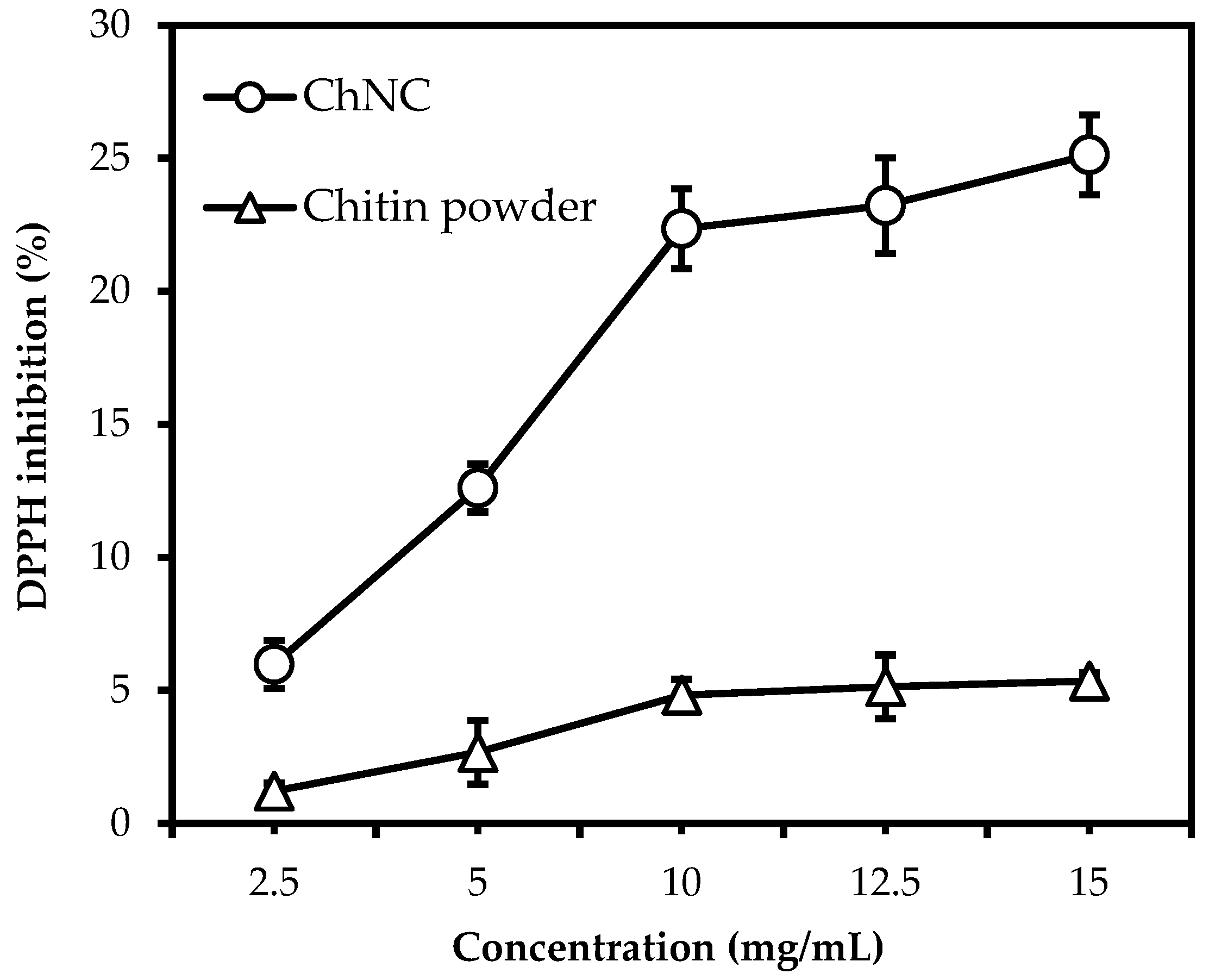
3.2. The Effect of Particle Size and Concentration on Radical Scavenging Activity
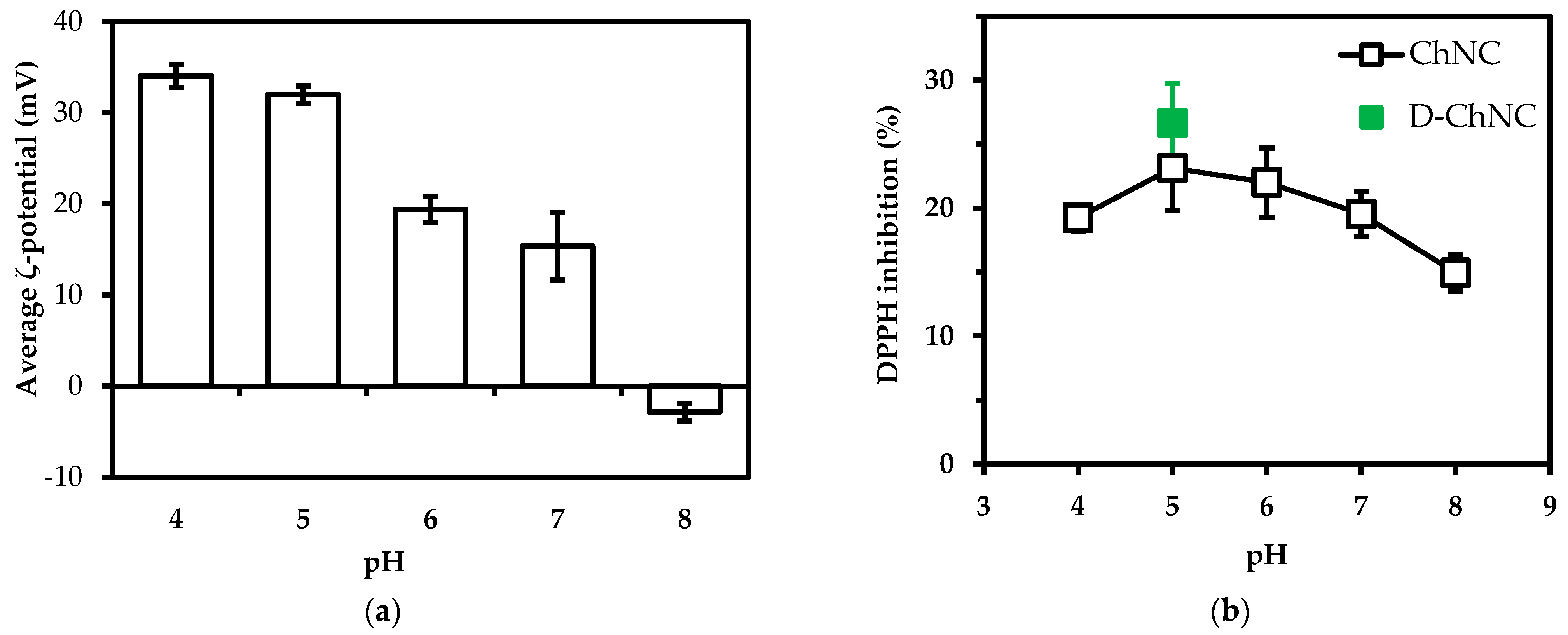
3.3. The Effect of pH and Degree of Deacetylation on Radical Scavenging Activity
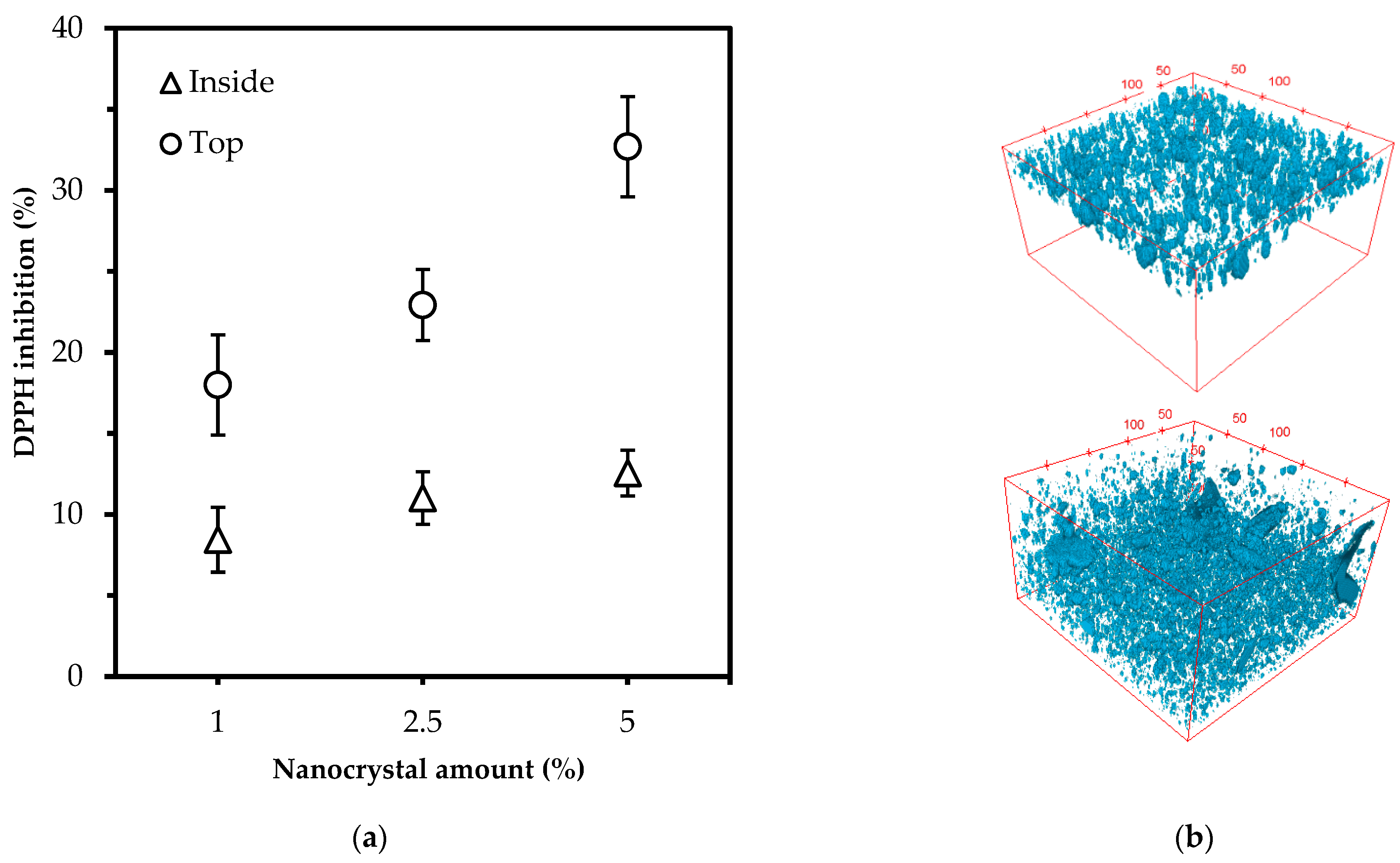
3.4. The Antioxidant Activity in Polylactic Acid Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Degree of Deacetylation (%) | DPPH Inhibition (%) |
|---|---|---|
| D-ChNC | 69.2 ± 3.8 | 26.63 ± 3.10 |
| D2-ChNC | 53.1 ± 5.2 | 22.7 ± 1.91 |
| D3-ChNC | 33.4 ± 2.8 | 20.8 ± 2.89 |
References
- Jacobsen, C.; Meyer, A.S.; Adler-Nissen, J. Oxidation Mechanisms in Real Food Emulsions: Oil-Water Partition Coefficients of Selected Volatile off-Flavor Compounds in Mayonnaise. Eur. Food Res. Technol. 1999, 208, 317–327. [Google Scholar] [CrossRef]
- Farhoosh, R. A Reconsidered Approach Providing Kinetic Parameters and Rate Constants to Analyze the Oxidative Stability of Bulk Lipid Systems. Food Chem. 2020, 327, 127088. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Schröder, A.; Rovalino-Cordova, A.; Schroën, K.; Sagis, L. Protein and Lipid Oxidation Affect the Viscoelasticity of Whey Protein Layers at the Oil–Water Interface. Eur. J. Lipid Sci. Technol. 2016, 118, 1630–1643. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling Lipid Oxidation of Food by Active Packaging Technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Laskar, A.; Younus, H. Aldehyde Toxicity and Metabolism: The Role of Aldehyde Dehydrogenases in Detoxification, Drug Resistance and Carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Li, Z.; Soladoye, O.P.; Van-Hecke, T. Health Risks of Food Oxidation. Adv. Food Nutr. Res. 2017, 82, 45–81. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A Comprehensive Review on Natural Bioactive Films with Controlled Release Characteristics and Their Applications in Foods and Pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The Potential of Anthocyanins in Smart, Active, and Bioactive Eco-Friendly Polymer-Based Films: A Review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and Evaluation of a Novel High Internal Phase Pickering Emulsion Based on Whey Protein Isolate Nanofibrils Derived by Hydrothermal Method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Chan-Matú, D.I.; Toledo-López, V.M.; Vargas, M.d.L.V.y.; Rincón-Arriaga, S.; Rodríguez-Félix, A.; Madera-Santana, T.J. Preparation and Characterization of Chitosan-Based Bioactive Films Incorporating Moringa Oleifera Leaves Extract. J. Food Meas. Charact. 2021, 15, 4813–4824. [Google Scholar] [CrossRef]
- Ribeiro, A.C.B.; Cunha, A.P.; da Silva, L.M.R.; Mattos, A.L.A.; de Brito, E.S.; de Souza Filho, M.d.S.M.; de Azeredo, H.M.C.; Ricardo, N.M.P.S. From Mango By-Product to Food Packaging: Pectin-Phenolic Antioxidant Films from Mango Peels. Int. J. Biol. Macromol. 2021, 193, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Fabrication of Chitosan-Based Functional Nanocomposite Films: Effect of Quercetin-Loaded Chitosan Nanoparticles. Food Hydrocoll. 2021, 121, 107065. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Taha, A.; Khalifa, I.; Khan, S.; Rostamabadi, H.; Jafari, S.M. Recent Advances in Food Applications of Phenolic-Loaded Micro/Nanodelivery Systems. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, M.; Cheng, M.; Yan, X.; Zhang, R.; Kong, R.; Wang, J.; Wang, X. Development of Antioxidant and Antimicrobial Bioactive Films Based on Oregano Essential Oil/Mesoporous Nano-Silica/Sodium Alginate. Food Packag. Shelf Life 2021, 29, 100691. [Google Scholar] [CrossRef]
- Sarker, A.; Grift, T.E. Bioactive Properties and Potential Applications of Aloe Vera Gel Edible Coating on Fresh and Minimally Processed Fruits and Vegetables: A Review. J. Food Meas. Charact. 2021, 15, 2119–2134. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan Nanoemulsions as Advanced Edible Coatings for Fruits and Vegetables: Composition, Fabrication and Developments in Last Decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and Characterisation of a Pectin-Based Edible Film That Contains Mulberry Leaf Extract and Its Bio-Active Components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Nerín, C. Antioxidant Active Food Packaging and Antioxidant Edible Films. In Oxidation in Foods and Beverages and Antioxidant Applications; Woodhead Publishing: Sawston, UK, 2010; pp. 496–515. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic Acid (PLA)/Silver-NP/VitaminE Bionanocomposite Electrospun Nanofibers with Antibacterial and Antioxidant Activity. J. Nanoparticle Res. 2014, 16, 2643. [Google Scholar] [CrossRef] [Green Version]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-Based Micro and Nanocomposites in Food Contact Materials and Active Food Packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal Oxide-Based Nanocomposites in Food Packaging: Applications, Migration, and Regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Ghozali, M.; Fahmiati, S.; Triwulandari, E.; Restu, W.K.; Farhan, D.; Wulansari, M.; Fatriasari, W. PLA/Metal Oxide Biocomposites for Antimicrobial Packaging Application. Polym. Technol. Mater. 2020, 59, 1332–1342. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Koppelmäki, K.; Helenius, J.; Schulte, R.P.O. Nested Circularity in Food Systems: A Nordic Case Study on Connecting Biomass, Nutrient and Energy Flows from Field Scale to Continent. Resour. Conserv. Recycl. 2021, 164, 105218. [Google Scholar] [CrossRef]
- Tseng, M.L.; Chiu, A.S.F.; Chien, C.F.; Tan, R.R. Pathways and Barriers to Circularity in Food Systems. Resour. Conserv. Recycl. 2019, 143, 236–237. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 73, ISBN 9780128002681. [Google Scholar]
- Hromis, N.; Lazic, V.; Popovic, S.; Suput, D.; Bulut, S. Antioxidative Activity of Chitosan and Chitosan Based Biopolymer Film. Food Feed Res. 2017, 44, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Herrera, N.; Salaberria, A.M.; Mathew, A.P.; Oksman, K. Plasticized Polylactic Acid Nanocomposite Films with Cellulose and Chitin Nanocrystals Prepared Using Extrusion and Compression Molding with Two Cooling Rates: Effects on Mechanical, Thermal and Optical Properties. Compos. Part A Appl. Sci. Manuf. 2016, 83, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized Blown Films of Plasticized Polylactic Acid/Chitin Nanocomposite: Preparation and Characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Broers, L.; van Dongen, S.; de Goederen, V.; Ton, M.; Spaen, J.; Boeriu, C.; Schroën, K. Addition of Chitin Nanoparticles Improves Polylactic Acid Film Properties. Nanotechnol. Adv. Mater. Sci. 2018, 1, 1–8. [Google Scholar]
- Coltelli, M.B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.Y.; Yuan, X.W.; Bhattacharyya, D.; Easteal, A.J. Characterisation of Solution Cast Cellulose Nanofibre—Reinforced Poly(Lactic Acid). Express Polym. Lett. 2010, 4, 26–31. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and Thermal Properties of Poly(Lactic Acid)/Cellulose Whiskers Nanocomposite Materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Hein, S.; Ng, C.H.; Stevens, W.F.; Wang, K. Selection of a Practical Assay for the Determination of the Entire Range of Acetyl Content in Chitin and Chitosan: UV Spectrophotometry with Phosphoric Acid as Solvent. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2008, 86, 558–568. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, W.H.; Ihm, D.; Hudson, S.M. Effect of the Degree of Deacetylation on the Thermal Decomposition of Chitin and Chitosan Nanofibers. Carbohydr. Polym. 2010, 80, 291–295. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Yu, J.; Jiang, J.; Zheng, K.; Hu, L.; Wang, Z.; Fan, Y. Robust Self-Standing Chitin Nanofiber/Nanowhisker Hydrogels with Designed Surface Charges and Ultralow Mass Content via Gas Phase Coagulation. Biomacromolecules 2016, 17, 3773–3781. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitin Nanostructures in Living Organisms. In Chitin; Gupta, N.S., Ed.; Topics in Geobiology; Springer: Dordrecht, The Netherlands, 2011; Volume 34, ISBN 978-90-481-9683-8. [Google Scholar]
- Cai, J.; Kuga, S. Aerogels of Cellulose and Chitin Crystals. In Handbook of Green Materials; World Scientific: Singapore, 2014; pp. 139–161. [Google Scholar] [CrossRef]
- Colijn, I.; Fokkink, R.; Schroën, K. Quantification of Energy Input Required for Chitin Nanocrystal Aggregate Size Reduction through Ultrasound. Sci. Rep. 2021, 11, 17217. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Hu, X.; Zhang, Z.; Liang, L. Crystalline Reduction, Surface Area Enlargement and Pore Generation of Chitin by Instant Catapult Steam Explosion. Carbohydr. Polym. 2018, 200, 255–261. [Google Scholar] [CrossRef]
- Phongying, S.; Aiba, S.-i.; Chirachanchai, S. Direct Chitosan Nanoscaffold Formation via Chitin Whiskers. Polymer 2007, 48, 393–400. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Biliaderis, C.G. Metastability of Nematic Gels Made of Aqueous Chitin Nanocrystal Dispersions. Biomacromolecules 2010, 11, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-Water Emulsions Stabilized by Chitin Nanocrystal Particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Pignon, F.; Challamel, M.; De Geyer, A.; Elchamaa, M.; Semeraro, E.F.; Hengl, N.; Jean, B.; Putaux, J.L.; Gicquel, E.; Bras, J.; et al. Breakdown and Buildup Mechanisms of Cellulose Nanocrystal Suspensions under Shear and upon Relaxation Probed by SAXS and SALS. Carbohydr. Polym. 2021, 260, 117751. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of Chitosan Oligomers and Their Antioxidant Activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Saddam, N.S.; Hadi, A.G.; Mohammed, A.A. Enhanced Antioxidant Activity of Chitosan Extracted from Fish Shells Materials. Mater. Today Proc. 2021, 49, 2793–2796. [Google Scholar] [CrossRef]
- Tu, Y.; Xu, Y.; Ren, F.; Zhang, H. Characteristics and Antioxidant Activity of Maillard Reaction Products from α-Lactalbumin and 2′-Fucosyllactose. Food Chem. 2020, 316, 126341. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant Properties of Chitosan from Crab Shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho Dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Liu, C.; Gan, L.; Ma, X.; Huang, J. Polydopamine-Coated Cellulose Nanocrystals as an Active Ingredient in Poly(Vinyl Alcohol) Films towards Intensifying Packaging Application Potential. Cellulose 2019, 26, 9599–9612. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wang, W.; Yang, Y.; Shi, X.; Sun, M.; Hao, Y.; Li, Y. Comparison of Physicochemical and Rheology Properties of Shiitake Stipes-Derived Chitin Nanocrystals and Nanofibers. Carbohydr. Polym. 2020, 244, 116468. [Google Scholar] [CrossRef] [PubMed]
- Julianus Sohilait, H.; Kainama, H. Free Radical Scavenging Activity of Essential Oil of Eugenia caryophylata from Amboina Island and Derivatives of Eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Di Mambro, V.M.; Azzolini, A.E.C.S.; Valim, Y.M.L.; Fonseca, M.J.V. Comparison of Antioxidant Activities of Tocopherols Alone and in Pharmaceutical Formulations. Int. J. Pharm. 2003, 262, 93–99. [Google Scholar] [CrossRef]
- Oey, I.; Verlinde, P.; Hendrickx, M.; Van Loey, A. Temperature and Pressure Stability of L-Ascorbic Acid and/or [6s] 5-Methyltetrahydrofolic Acid: A Kinetic Study. Eur. Food Res. Technol. 2006, 223, 71–77. [Google Scholar] [CrossRef]
- Ali, A.; Chong, C.H.; Mah, S.H.; Abdullah, L.C.; Choong, T.S.Y.; Chua, B.L. Impact of Storage Conditions on the Stability of Predominant Phenolic Constituents and Antioxidant Activity of Dried Piper Betle Extracts. Molecules 2018, 23, 484. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.R.; Mount, E.M.; Giles, H.F. Processing Conditions. In Extrusion; William Andrew: Norwich, NY, USA, 2014; pp. 181–192. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrášek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-Million-Year Old Chitin in the Basal Demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef] [Green Version]
- Akopova, T.A.; Demina, T.S.; Khavpachev, M.A.; Popyrina, T.N.; Grachev, A.V.; Ivanov, P.L.; Zelenetskii, A.N. Hydrophobic Modification of Chitosan via Reactive Solvent-Free Extrusion. Polymers 2021, 13, 2807. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Lu, T.; Liu, S.; Jiang, M.; Xu, X.; Wang, Y.; Wang, Z.; Gou, J.; Hui, D.; Zhou, Z. Effects of Modifications of Bamboo Cellulose Fibers on the Improved Mechanical Properties of Cellulose Reinforced Poly(Lactic Acid) Composites. Compos. Part B Eng. 2014, 62, 191–197. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Abdelwahab, M.A.; Lopez-Rubio, A.; Lagaron, J.M.; Chiellini, E.; Williams, T.G.; Wood, D.F.; Orts, W.J.; Imam, S.H. Incorporation of Poly(Glycidylmethacrylate) Grafted Bacterial Cellulose Nanowhiskers in Poly(Lactic Acid) Nanocomposites: Improved Barrier and Mechanical Properties. Eur. Polym. J. 2013, 49, 2062–2072. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, S.; Huang, J.; Feng, J.; Chang, P.R. Effect of Surface Acetylated-Chitin Nanocrystals on Structure and Mechanical Properties of Poly(Lactic Acid). J. Appl. Polym. Sci. 2014, 131, 2–9. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface Acetylation of Cellulose Nanocrystal and Its Reinforcing Function in Poly(Lactic Acid). Carbohydr. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
- Shafiur Rahman, M.; Rahman, M.R.T. pH in Food Preservation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 287–298. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of Processing Conditions and Lignin Content on Thermal, Mechanical and Degradative Behavior of Lignin Nanoparticles/Polylactic (Acid) Bionanocomposites Prepared by Melt Extrusion and Solvent Casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Sarul, D.S.; Arslan, D.; Vatansever, E.; Kahraman, Y.; Durmus, A.; Salehiyan, R.; Nofar, M. Effect of Mixing Strategy on the Structure-Properties of the Pla/Pbat Blends Incorporated with Cnc. J. Renew. Mater. 2022, 10, 149–164. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and Characterization of α-Lipoic Acid Grafted Chitosan Derivatives with Antioxidant Activity. React. Funct. Polym. 2022, 172, 105205. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Preparation, Characterization and Antioxidant Property of Water-Soluble Ferulic Acid Grafted Chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef]
- Colijn, I.; Yanat, M.; Terhaerdt, G.; Molenveld, K.; Boeriu, C.G.; Schroën, K. Chitin Nanocrystal Hydrophobicity Adjustment by Fatty Acid Esterification for Improved Polylactic Acid Nanocomposites. Polymers 2022, 14, 2619. [Google Scholar] [CrossRef]
| Sample | Size | ζ-Potential | Degree of Deacetylation | Specific Surface Area |
|---|---|---|---|---|
| (μm) | (mV) | (%) | (m2/kg) | |
| ChNC | 0.4 ± 0.0 | +32.0 ± 1.0 | 16.3 ± 1.6 | 285 ± 2 |
| D-ChNC | 0.8 ± 0.0 | +35.9 ± 0.4 | 69.2 ± 3.8 | 184 ± 1 |
| Chitin powder | 122 ± 5 | +16.8 ± 2.7 | 6.8 ± 1.1 | 35.2 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanat, M.; Colijn, I.; Schroën, K. Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films. Polymers 2022, 14, 2965. https://doi.org/10.3390/polym14142965
Yanat M, Colijn I, Schroën K. Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films. Polymers. 2022; 14(14):2965. https://doi.org/10.3390/polym14142965
Chicago/Turabian StyleYanat, Murat, Ivanna Colijn, and Karin Schroën. 2022. "Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films" Polymers 14, no. 14: 2965. https://doi.org/10.3390/polym14142965
APA StyleYanat, M., Colijn, I., & Schroën, K. (2022). Chitin Nanocrystals Provide Antioxidant Activity to Polylactic Acid Films. Polymers, 14(14), 2965. https://doi.org/10.3390/polym14142965