1. Introduction
Colorectal cancer is considered the third most common cancer and the second leading source of death globally [
1]. The occurrence of colorectal cancer is increasing in developed countries, as well as in middle- and low-income countries due to westernization. Unhealthy living habits, especially adaptation to fast food, packed food items, and lack of exercise, are the leading causes of the increase in the incidence of colorectal cancer [
2].
Titanium dioxide nanoparticles (TiO
2-NPs), a white-colored food additive (E171), are proven to pose a major risk to health. TiO
2-NPs can induce inflammation due to oxidative stress and can significantly cause colorectal cancer in human beings and animals [
3]. TiO
2-NPs have a broad range of applications in the food industry, pharmaceuticals, cosmetic industry, and toothpaste.
This idea is also used to induce colorectal cancer in the animal model. The use of TiO
2-NPs has already been shown to cause cancer and persistent intestinal irritation [
4]. Food-grade TiO
2-NPs are frequently utilized as a white pigment in the food sector, particularly in confectionery. In carcinogenic-chemical-induced models, 100-day treatment with TiO
2-NPs causes colon microinflammation, pre-neoplastic lesions, and the formation of aberrant crypt foci, according to research. These findings yield a risk assessment of colon cancer susceptibility in human candidates exposed to TiO
2 through food [
5]. Previous research indicates that oral treatment of TiO
2-NPs in mice raises plasma glucose levels, and, as a result, the body weight of the animals eventually increases. As a result, oral TiO
2-NPs and peritoneal DMH administration were chosen for colorectal cancer induction [
6].
Curcumin (CUR), also known as diferuloylmethane, is a yellow pigment found in turmeric, a spice and culinary coloring. According to toxicological research, CUR is non-toxic even at large doses [
7]. This drug was recently discovered to have a considerable inhibitory impact on the growth of several mouse tumors, including skin, stomach, colon, and liver malignancies. In humans, CUR is thought to be a potentially safe and non-toxic colorectal cancer chemopreventive medication [
8]. As a result, CUR has already been studied as a chemopreventive drug in several preclinical investigations, and its effectiveness implies that CUR’s usage will certainly rise in the future. Because of its extensive use and multiple advantages as a chemopreventive agent in colon cancer, we believe that CUR-induced inhibition of COX-2 expression may improve the efficacy of standard anti-colon-cancer medications such as 5-fluorouracil (5-FU) [
9].
5-FU is the first line chemotherapeutic agent used for the treatment of CRC in both palliative and adjuvant situations. Over the last four decades, various techniques have been developed and used to increase the anti-tumor [
10] effectiveness of 5-FU and overcome clinical resistance, including the use of 5-FU-based combination regimens and 5-FU pro-drugs [
11]. More than 80% of 5-FU is catabolized by hepatic dihydropyrimidine dehydrogenase (DPD), while the remaining causes cell death by blocking RNA and DNA synthesis via fluorodeoxyuridine monophosphate (FdUMP) and fluouridine triphosphate (FUTP) [
12]. The most serious side effects of 5-FU include cardiotoxicity, diarrhea, dermatitis, myelosuppression, and mucositis, and it also possesses multidrug resistance (MDR) [
13]. The combination of 5-FU and CUR may have a synergistic effect in the treatment of colorectal cancer. Despite recent encouraging developments in colorectal cancer therapy, patient response rates continue to be modest, and the benefit of 5-FU-based therapy is usually jeopardized by chemoresistance. Each person’s genetic and epigenetic composition may have a role in inter-individual variation in therapy response in colorectal cancer patients [
14]. Dietary pectin supplementation has been demonstrated to significantly prevent colon cancer and have an anti-proliferative effect in mice’s distal colon [
15]. Pectin may help prevent colon cancer by inhibiting galectin-3’s biological effects. In turn, it helps in the site-specific delivery of the conjugate to the colorectal region [
16].
Here in this paper, we induce colorectal cancer in rats using TiO2-NPs as well as dimethylhydrazine (DMH). Then, we treat the animals using 5-fluorouracil (5-FU) and curcumin (CUR) in the ratio of 1:4. The effect of treatment is compared to that of the normal group (positive control-healthy animals) as well as colorectal cancer-induced group (negative control). Further blood parameters are analyzed, and we report on their effects.
2. Materials and Methods
2.1. Chemicals
Pectin was obtained from Sigma-Aldrich Pvt. Ltd. (Steinheim, Germany), and 5-FU and DMH from Avra Synthesis Pvt. Ltd. (Hyderabad, India). TiO2 was obtained as a gift sample from Bimal Pharma Pvt. Ltd. (Mumbai, India), and CUR was obtained as a gift sample from Himalaya Pvt. Ltd. (Bangalore, India). Before the start of the trial, all samples were tested for purity and confirmation.
2.2. Cell Culture
Human colorectal carcinoma cell line (HCT 116) cells were obtained from the American Type Culture Collection and was cultured at 37 °C in a humidified atmosphere containing 5% CO2 in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS and a 1% antibiotic-antimycotic cocktail. In tissue culture flasks, the cells were trypsinized using a PBS solution containing 0.25% trypsin and 0.03% EDTA until they achieved 70% confluence.
Cell Cytotoxicity Studies
To measure cell cytotoxicity, an MTT reduction assay was used. HCT-116 cells were seeded into a 96-well plate (5 × 10
3 cells per well) and incubated for 24 h at 60–70% confluence under conventional growth conditions. Cells were subsequently treated for 48 and 72 h with formulations of 5-FU, CUR, 5-FU+CUR (1:4 ratio) coated with pectin, and 5-FU+CUR (1:4 ratio). Following the drug treatment, the cells were incubated for 4 h with 10%
v/
v MTT (5 mg mL
−1 dissolved in PBS pH 7.4). The medium was then entirely removed, and the resulting formazan crystals were dissolved in 100 µL isopropyl alcohol. A Biorad plate reader Model 680 was used to detect absorbance at 570 nm [
6].
2.3. Pectin Coating
To achieve total solubility, pectin powder (6%
w/
v) was dissolved in distilled water at 20–22 °C and agitated at 500 rpm for 30 min. To coat, 10 mL of pectin solution was combined with 1 g of conjugated powder and spun for 15 min at 500 rpm. A physical conjugate of 5-FU:CUR (1:4 ratio; 60 mg of 5-FU and 240 mg CUR) was coated with pectin. The pH of the conjugates was also noted [
17].
2.4. Colorectal Cancer Induction in Animals
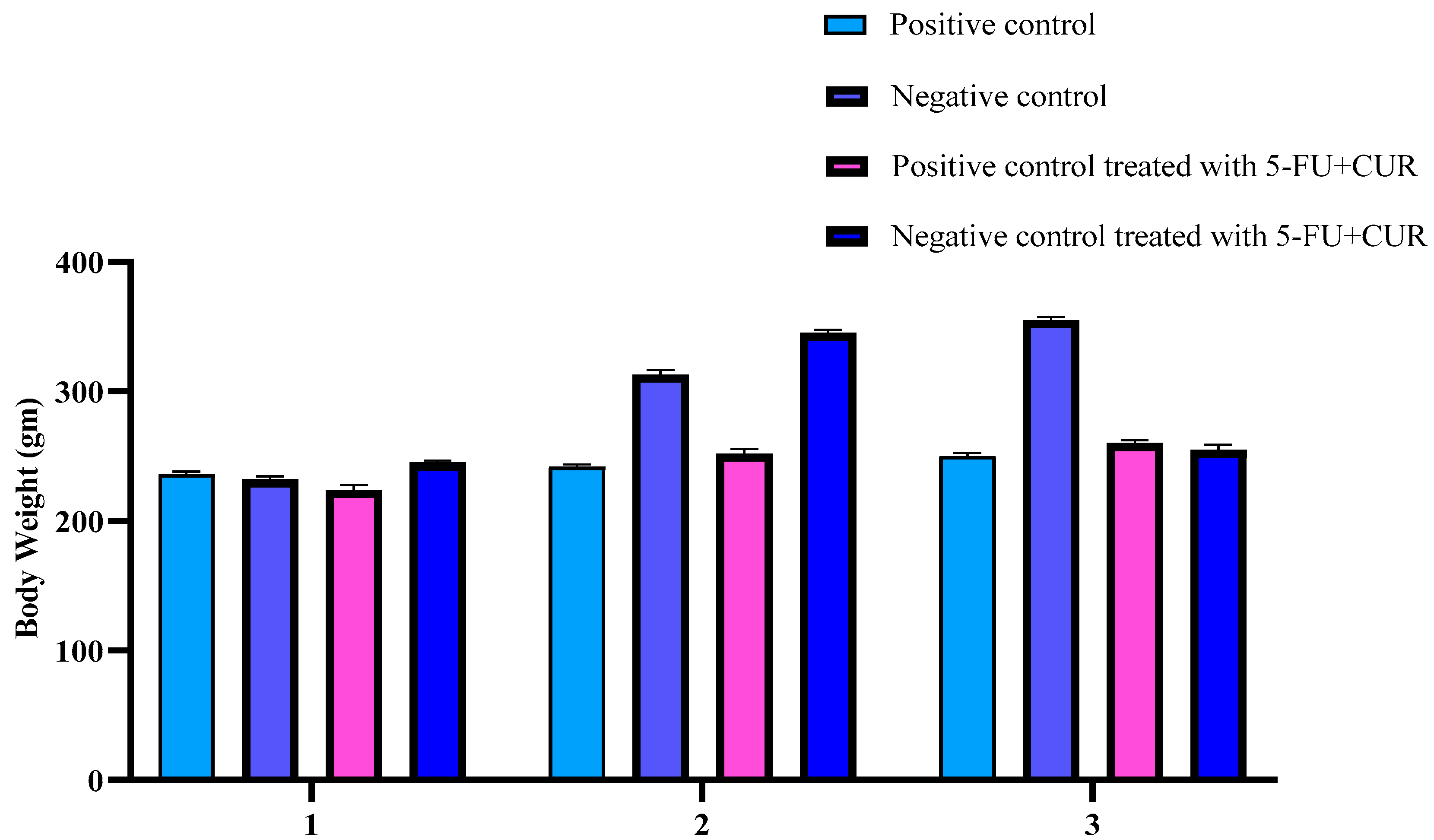
For a total of 70 days, the Sprague–Dawley male rats were given 5 mg/kg body weight TiO2-NPs (oral route) 5 days per week and 1 mg/kg body weight DMH (peritoneal route) 1 day per week. Body mass index and hematological findings were obtained. The animal’s weights were recorded every week.
Therapy
Following the conclusion of the 70-day period, the treatment was started. The first group serves as a positive control, while the second serves as a negative control (induction of CRC without therapy), with both groups receiving only ordinary saline. The third group received treatment with 60 mg of 5-FU and 240 mg of CUR (1:4) coated with pectin, whereas the fourth group received negative control therapy with 60 mg of 5-FU and 240 mg of CUR (1:4) coated with pectin. Intraperitoneally, the pectin-coated conjugate (a physical conjugation of 5-FU and CUR) was dissolved in phosphate buffer saline (PBS), whereas the control group, group 1, received phosphate buffer saline (PBS) solution. The Sprague–Dawley rats were kept in a room with a 24 h dark cycle, temperature of 24 ± 2 °C, and relative humidity of 60 ± 5%. All rats’ weight and diarrhea scores were recorded daily at the beginning the day after the treatment and induction [
6].
2.5. Analyze Your Body Weight
Bodyweight measurements were obtained once a week. The animals’ body weights were assessed before and after the cancer induction (on Sunday). Before and after induction, as well as during and after therapy, the animals were weighed. Following that, the animals were given unrestricted access to water, and their fasting blood glucose levels were tested [
18].
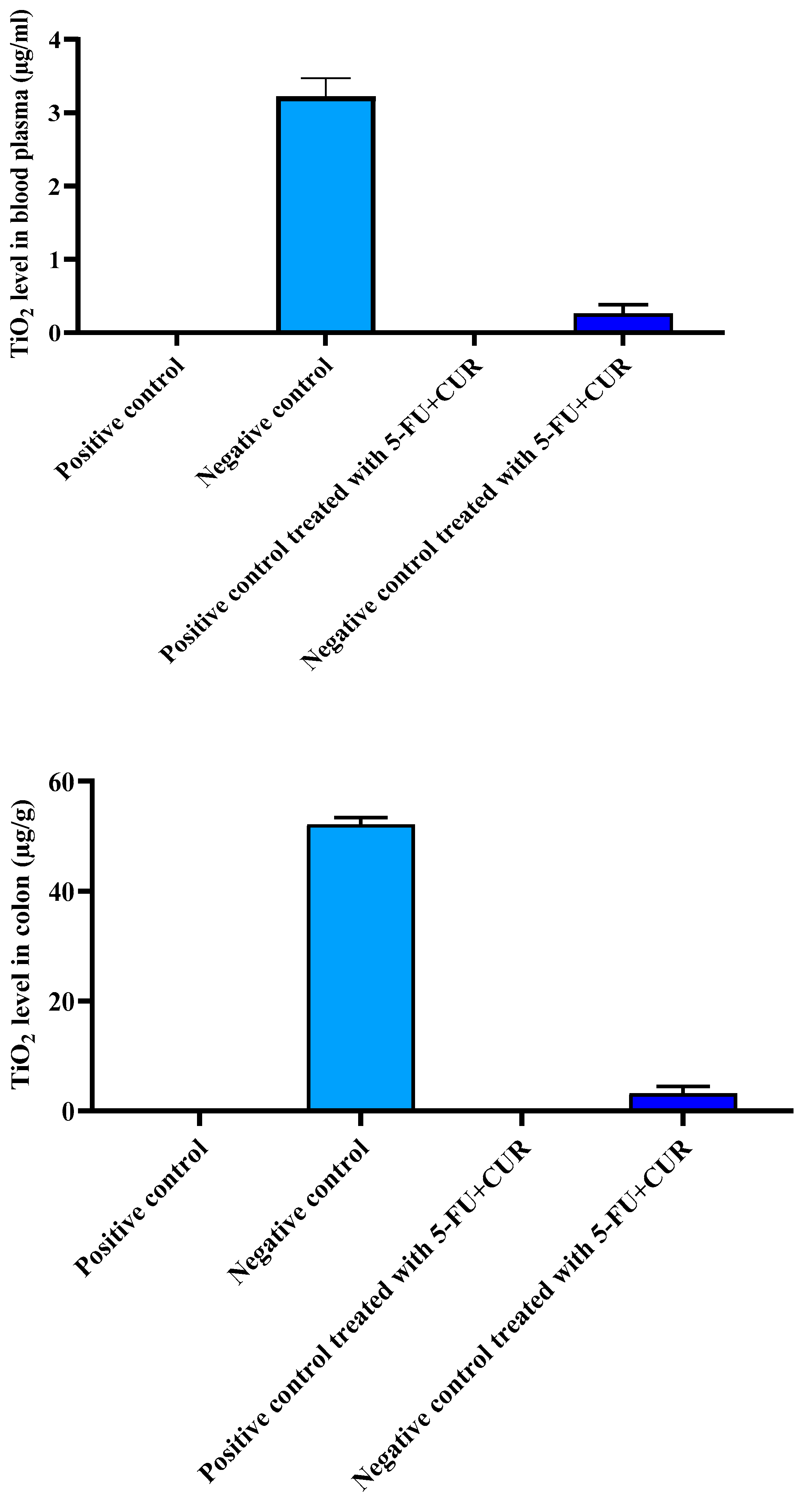
2.6. Elemental TiO2-NPs Concentrations in the Blood Plasma and Colon
0.1–0.5 g samples of blood plasma and colon were treated with 10 mL of nitric acid and 2 mL of perchloric acid in a 100 mL glass beaker, respectively. After that, the samples were digested for one hour at 230 °C. The digestion solution was heated to 280 °C until nearly completely evaporated, then cooled to room temperature and diluted with ultrapure water to a final volume of 25 mL. The concentrations of TiO
2-NPs were determined using an inductively coupled plasma-mass spectrometer (ICP-MS, Varian 820-MS, Palo Alto, CA, USA) [
4].
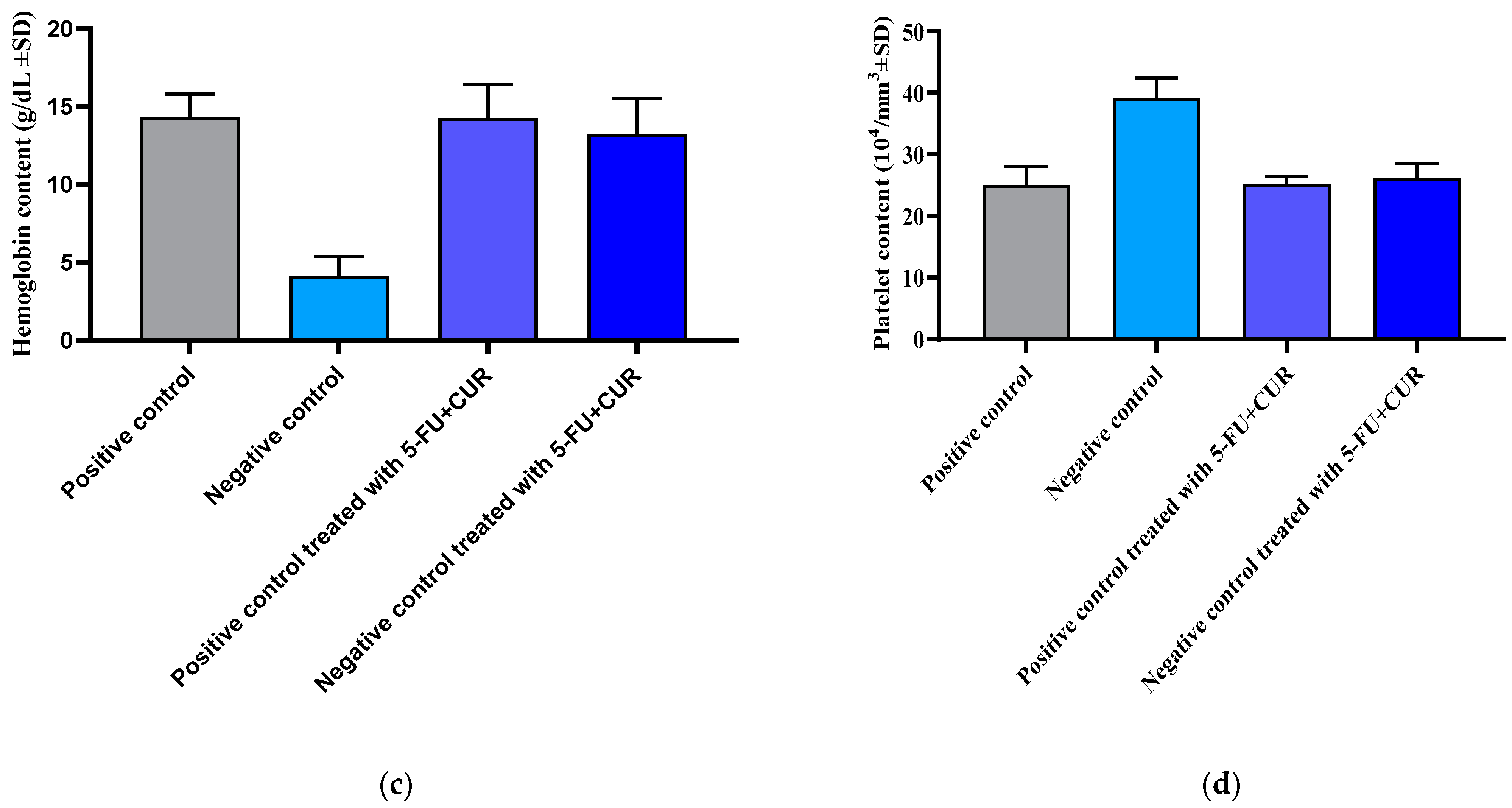
2.7. Hematological Parameters
At the time of sacrifice, all the animals were subjected to blood collection to measure hematological parameters. A retro-orbital puncture was used to collect blood. The above parameters were studied to learn more about the effects of CUR and 5-FU individually and in combination on the following specific blood components:
RBC;
WBC;
Hemoglobin-Hb content;
Platelet count.
Following the retro-orbital puncture, blood was collected into pre-coated (Heparin 25 IU/mL) blood collection tubes. Using a cell counter, the concentrations of these different components were then determined [
19].
2.8. Antioxidant Studies
For estimation of the molecular anticancer potential, antioxidant activities were evaluated [
20].
2.8.1. Determination of Nitric Oxide (NO)
We started with 20 µL organ + 80 µL of PBS. Then, after 5 min, we added the 50 µL of salphanilamide solution (0.082 g + 25 mL distilled water). Then, we added 50 µL of NED solution (0.025 g + 25 mL distilled water) and waited for 10 min before measuring absorbance at 405 nm against a blank solution [
21].
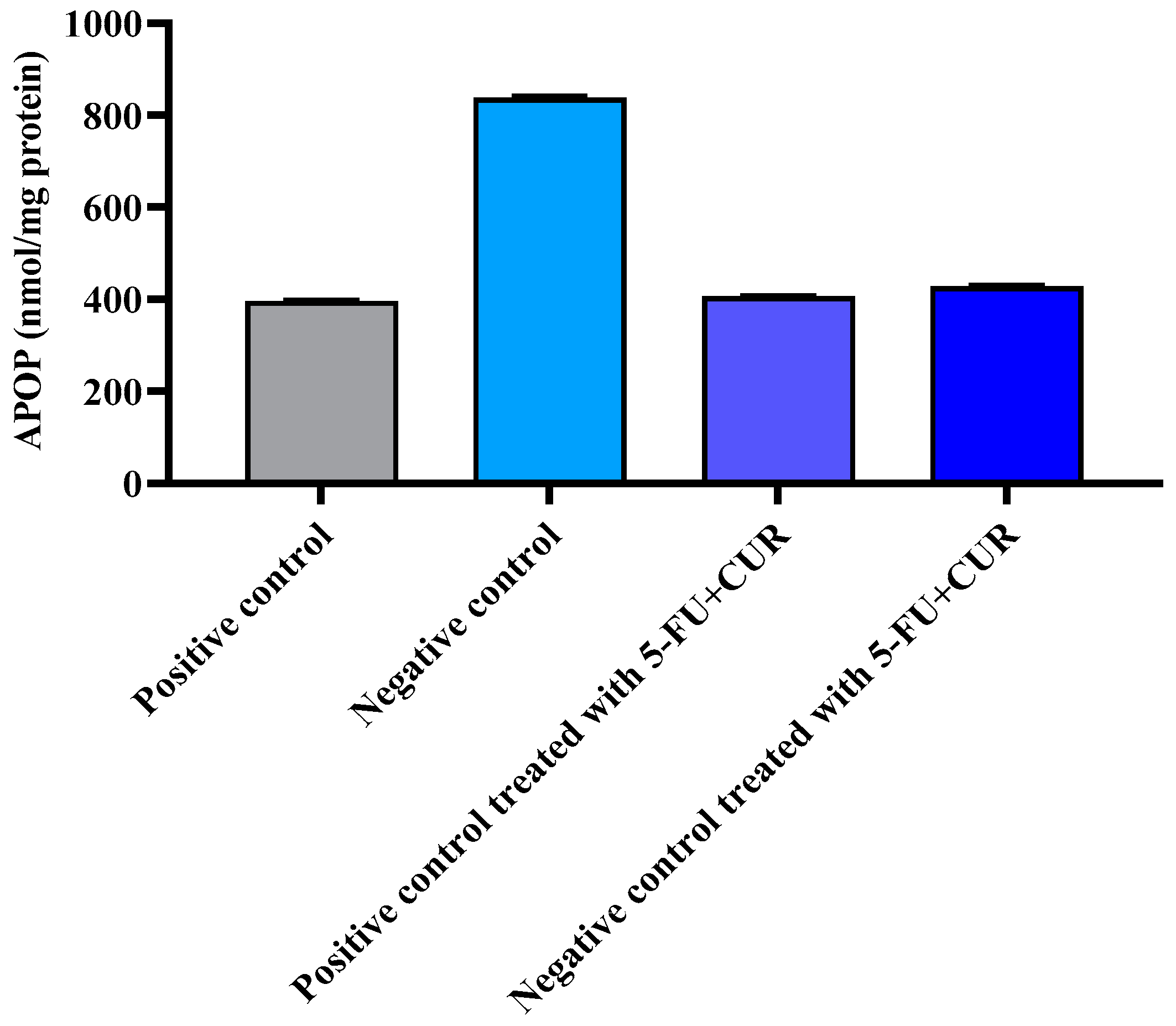
2.8.2. Determination of Advanced Protein Oxidation Products (APOP) Assay
We started with 10 µL organ + 190 µL of PBS. Then, we added 100 µL of acetic acid and 100 µL of KI (0.0134 g + 3 mL distilled water) and waited 2 min. Later, absorbance at 405 nm was measured in comparison to a blank solution [
22].
2.8.3. Detection of the Reactive Oxygen Species (ROS) Level
The levels of ROS were determined using dichloro-dihydro-fluorescein diacetate (DCFH-DA) reagent (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. In a 96-well black plate, 10 µL of serum and brain lysate samples were combined with 100 µL of 10 µM DCFH-DA and incubated for 30 min at 37 °C. The fluorescence was measured at 488 nm excitation or 525 nm emission using a DTX multi-mode microplate reader (Beckman Coulter Inc., Brea, CA, USA) [
23].
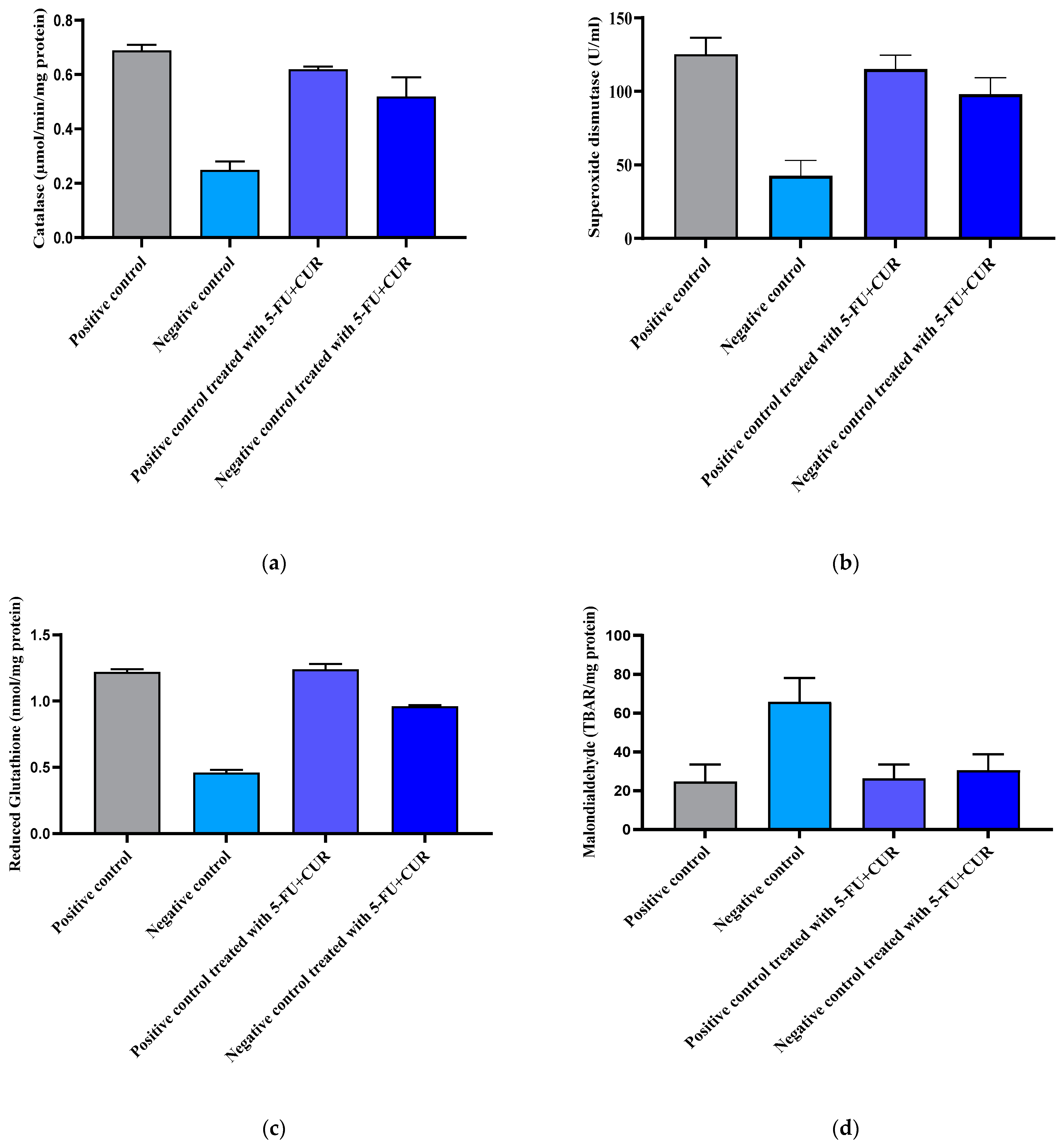
2.8.4. Catalase (CAT)
Hydrogen peroxide (H
2O
2) is said to be an oxidative stress inducer. In turn, it gets broken down into peroxidase free radicals (O
2−) and water (H
2O). Oxidative stress that aids in carcinogenesis is induced by O
2− free radicals. Hence, the potential of catalase to oxidase H
2O
2 was evaluated. Colonic mucosa homogenate (100 µL) was added into potassium phosphate buffer (2.25 mL) and was then incubated for 30 min at 25 °C. This was followed by the addition of 650 µL of 7.5 mM H
2O
2 into the mixture. The alteration in absorbance per minute for 2–3 min was analyzed using UV–visible spectrometry at 240 nm and was reported as µmol/min/mg of protein [
24].
2.8.5. Superoxide Dismutase (SOD)
For the assay, the method demonstrated by Winter Bourn et al. was adapted. Around 100 µL of the mucosa and homogenate supernatant was added into 8.3 pH 0.052 M sodium phosphate buffer (1.2 mL) with 186 µM phenazine methosulphate (100 µL) and nitro blue tetra tetrazolium (300 µL). In addition, 750 µM NADH (200 µL) was added and incubated for 90 s at 30 °C. To halt the reaction of the mixture, 100 µL of glacial acetic acid was added, followed by the addition of N-butanol (4 mL), and the mixture was then sonicated continuously. The chromogen intensity in butanol was evaluated by UV–visible spectrometry at 560 nm. The SOD content was expressed in U/mg of tissue [
25].
The estimation of glutathione was also performed as per the method demonstrated earlier. The tissue and mucosa of the colon were homogenized along with 0.2 M ice-cold perchloric acid, and 0.01% EDTA. This was followed by its centrifugation at 4 °C and 10,000 rpm for 10 min. Then, 0.3 mM of reduced NADPH (500 µL), 6 mM DTNB, and 25 units/µL of glutathione reductase (10 µL) freshly prepared in pH 7.5 phosphate buffer were added. Initiation of the reaction was carried out with the addition of 200 µL homogenate supernatant and the reaction mixture into a cuvette. The absorbance was measured at a 412 nm wavelength at 30 °C, and the amount of glutathione was analyzed every 3 min. The change in the test solution absorbance was compared with that of the standard and was indicated as nmol/mg protein [
26].
2.8.6. Malondialdehyde
The thiobarbituric acid reactive substances (TBARS) level in colonic mucosa was estimated by using the standard method: lipid peroxidation marker with the addition of 10% mucosal homogenate (0.4 mL), 8.1% sodium dodecyl sulfate (1.5 mL, 3.5 pH), 20% acetate buffer (1.5 mL), and 0.8% solution of thiobarbituric acid (1.5 mL) were added, sonicated, and heated for 1 h at 95 °C. The mixture was then cooled, and a 15:1 ratio of n-butanol-pyridine (5.0 mL) was added. The n-butyl-pyridine layer absorbance was analyzed at 532 nm. The levels were evaluated as mM/100 g of tissue [
27].
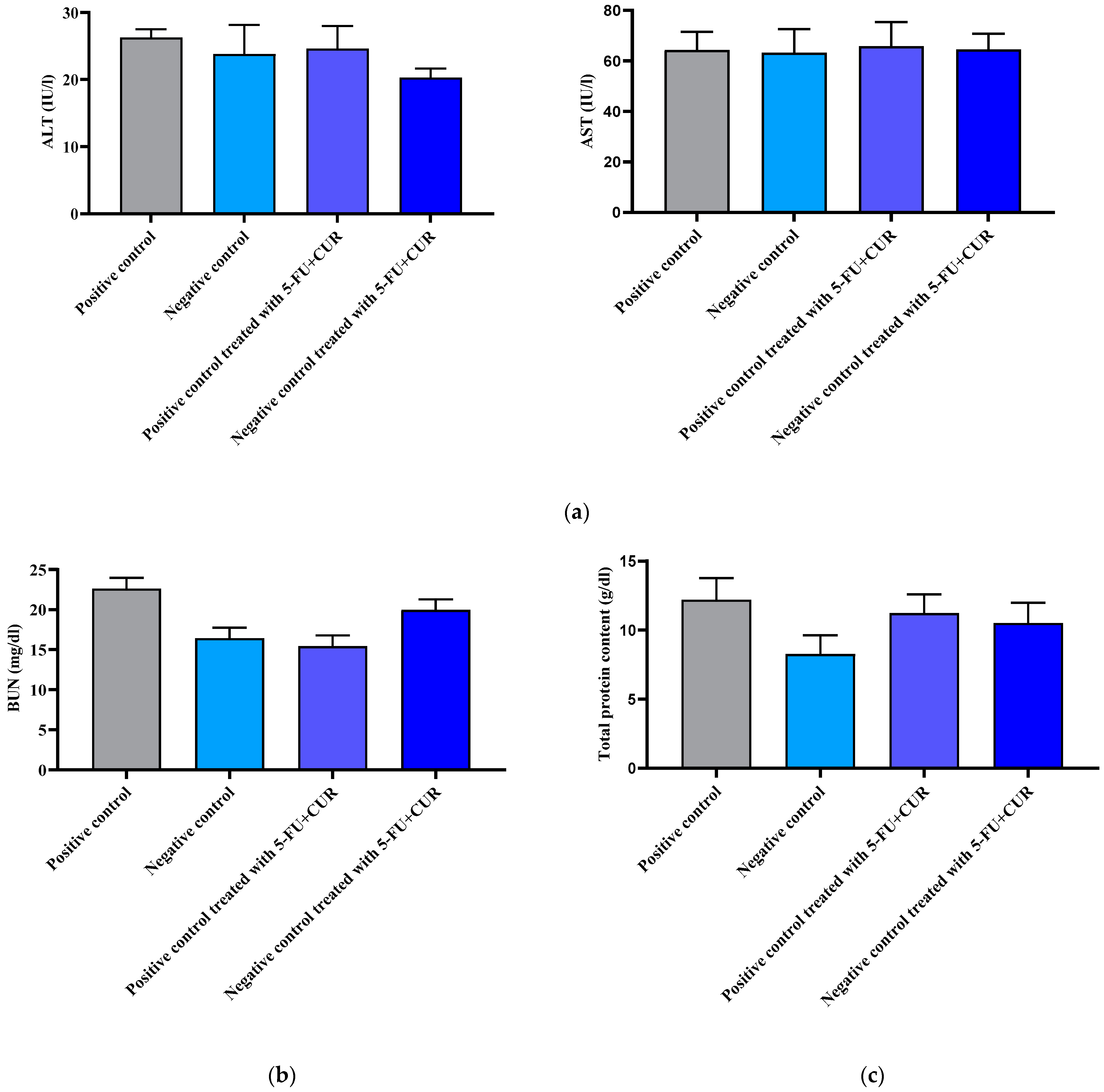
2.9. Biochemical Estimation
2.9.1. Liver Enzymes Analysis
To evaluate whether the drug conjugate has any effect on nearby tissues, liver enzyme analysis was performed and estimated. The two major enzymes involved are alanine transferase (ALT) as well as aspartate transferase (AST). An estimation kit procedure was performed for the analysis. The analysis was performed on mucosal homogenate, and liver tissue was centrifuged for 10 min at 400 rpm for separation of the supernatant. A multimode microplate reader was used for the estimation of the respective wavelengths of ALT and AST [
28].
2.9.2. Kidney Function Analysis
Toxic activity with the adjoining kidney is analyzed using an estimation of blood urea nitrogen. The readings were analyzed using a multimode plate reader at their determined wavelength [
29].
2.9.3. Lowry’s Method for Protein Content Estimation
For the evaluation of total protein content, Lowry’s method was used. For obtaining the supernatant for this assay, the tissue samples were subjected to homogenization and centrifugation for 10 min at 400 rpm. Initially, 0.05–1 mg/mL of standard bovine serum albumin (BSA) was prepared. The protein sample (0.2 mL) with an analytical reagent, which was an alkaline copper sulfate solution (2 mL), was sonicated, followed by its incubation for 10 min. A Folin–Ciocalteu solution (0.2 mL) was pipetted and added to this mixture, and then incubated for around 30 min until a blue coloration was obtained. This was followed by measuring its absorbance at 660 nm [
30].
2.10. Histopathological Report
Each colon tissue sample was submerged in a 10% formaldehyde solution before being processed for histopathology. The tissues were immersed in molten liquid paraffin before being hardened into blocks, making slicing and staining simpler. Using a rotary microtome, tis-sue-paraffin blocks were cut into 6-µm-thick slices (Leica, UK; Model No. RM2135). After being placed on staining stands, tissue slices were stained with hematoxylin and eosin. A digital microscope was used to perform pathological investigations on tissue slices.
2.11. Statisticss
All datas are presented as the mean standard deviation of the mean (SEM). GraphPad Prism Software, version 9.3, was used to analyze the data, which included a one-way ANOVA followed by a Newman–Keuls post hoc test. In all situations, statistical significance was defined as
p < 0.05 [
31].
4. Discussion
Colorectal cancer is one of the most common kinds of cancer worldwide. As health-care systems have evolved, so has the number of cancer survivors [
18]. These cancer survivors have several difficulties, including a significant chance of recurrence. Furthermore, because of therapy, a considerable proportion of patients may develop comorbidities. Concerns are being raised about the global growth in colorectal cancer incidence, as well as the emergence of medication resistance. Cancer therapy methods now available, like those for other malignancies, are either invasive or have major negative effects. As a result, better therapeutic alternatives are needed. 5-FU, an effective chemotherapy drug, is commonly used to treat colorectal cancer [
32]. However, its toxicity to normal tissues and the development of cancer resistance are the primary impediments to successful cancer treatment, limiting its usage. CUR can enhance 5-FU-induced cytotoxicity, while decreasing negative side effects. CUR, derived from
Curcuma longa, has piqued the interest of experts as a viable chemotherapy drug alternative. In small dosages, it is non-toxic and safe against colorectal cancer. It is worth noting that it has a special effect on colorectal cancer apoptotic effects. CUR’s utility in aquatic environments is limited because of its weak solubility and bioavailability [
7].
A synergistic approach in the ratio of 1:4 works better, as has been proven in our previous research. 5-FU at 60 mg/kg body weight of the rat and curcumin at 240 mg/kg were given through an oral route. For the specific delivery to the colorectal region, pectin coating was done. Evidential support was also obtained using HCT-116 cell lines, as reported. The pH of the pectin-coated conjugate was in the range of 6–6.5. The bacteria and the microflora present in the colorectal region help in the specific delivery of the conjugate to the colorectal region by the degradation of the pectin-coated layer [
33]. Furthermore, animal studies are conducted where blood and plasma analyses are carried out. In vitro cell line studies in HCT-116 cell lines and histopathology reports are also done for supporting evidence.
It was reported that there is an increase in the bodyweight of the animals with the induction of colorectal cancer using TiO
2-NPS and DMH because of an increase in the blood sugar level. The level of TiO
2-NPs in the blood plasma and colon were also noted: the reports show that the TiO
2-NPs level was found to be high in the colon, which is the main reason for the inflammation in the colorectal region and the incidence of cancer [
34]. With the hematological parameters’ evaluation, the RBC count and hemoglobin count were found to be decreased with cancer incidence, and subsequently, with treatment, the level came back to be normal. The WBC and platelet count were found to be higher than that of the positive control with the incidence of cancer and returned to normal with treatment with 5-FU and CUR conjugate coated with pectin. This is seen along with significant changes in the level of nitric oxide, advanced protein oxidation products, ROS, catalase, and superoxide dismutase, and reduced glutathione and malondialdehyde activity. The biochemical analysis shows no significant change in the level of induction and treatment. From this, we can conclude that the effect is not shown in the liver, kidney, or other organs or that metastasis did not occur.