Freestanding Activated Carbon Nanocomposite Electrodes for Capacitive Deionization of Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
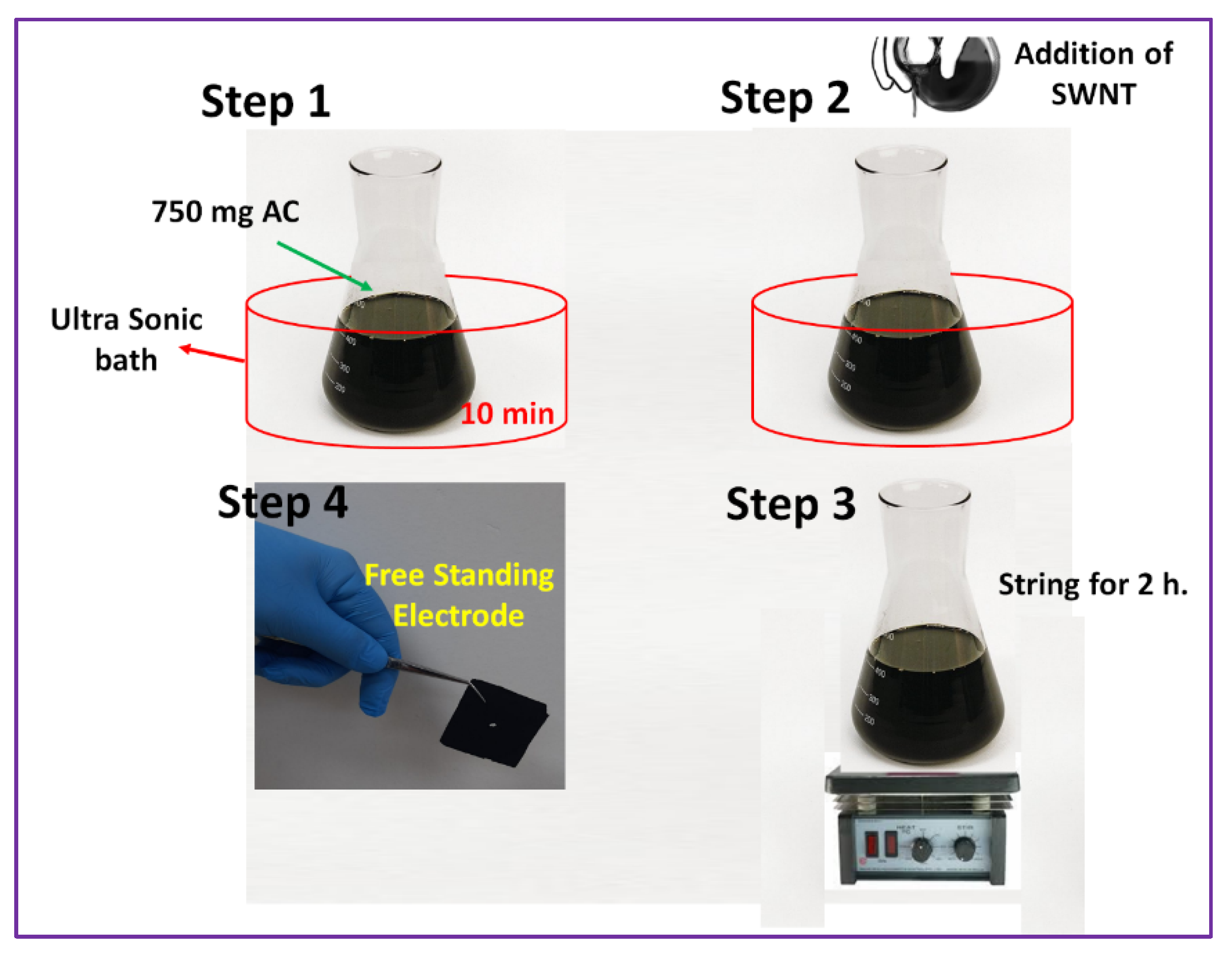
2.2. Synthesis of Freestanding Electrodes
2.3. Characterization
3. Results
3.1. Surface Morphology
3.2. Mechanical Property
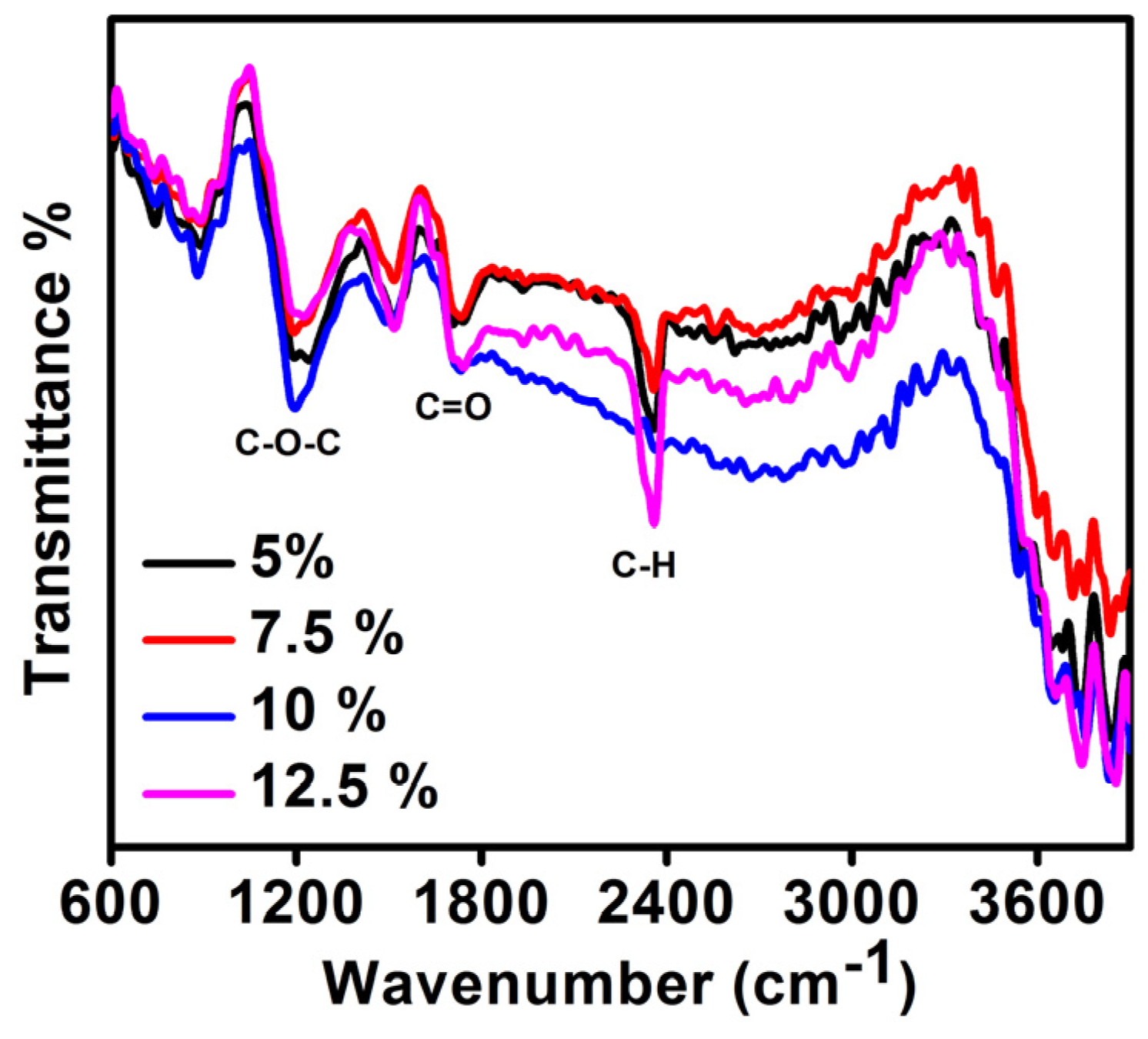
3.3. FTIR Results
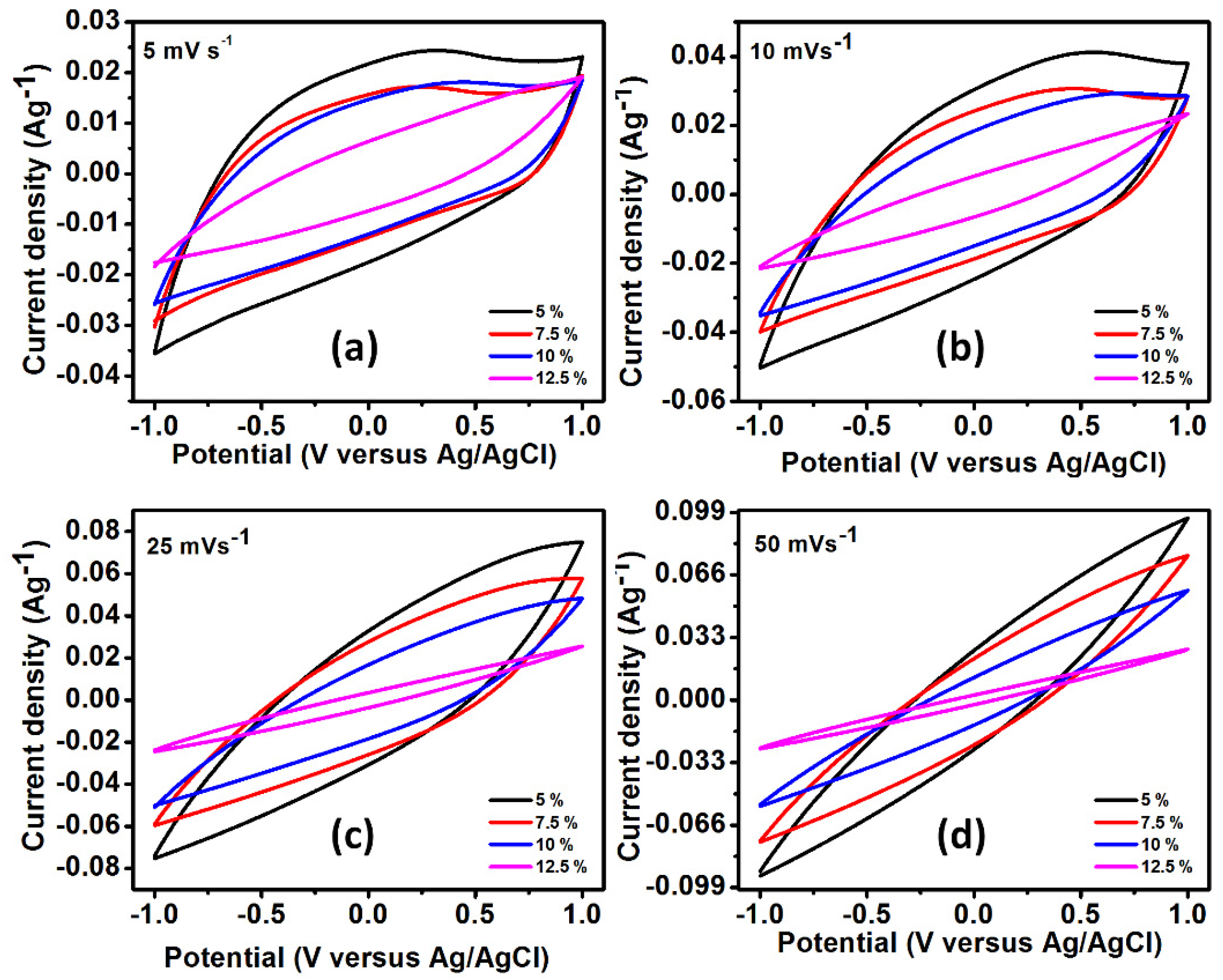
3.4. Electrochemical Characteristics of the Electrodes
3.5. Surface Area Analysis
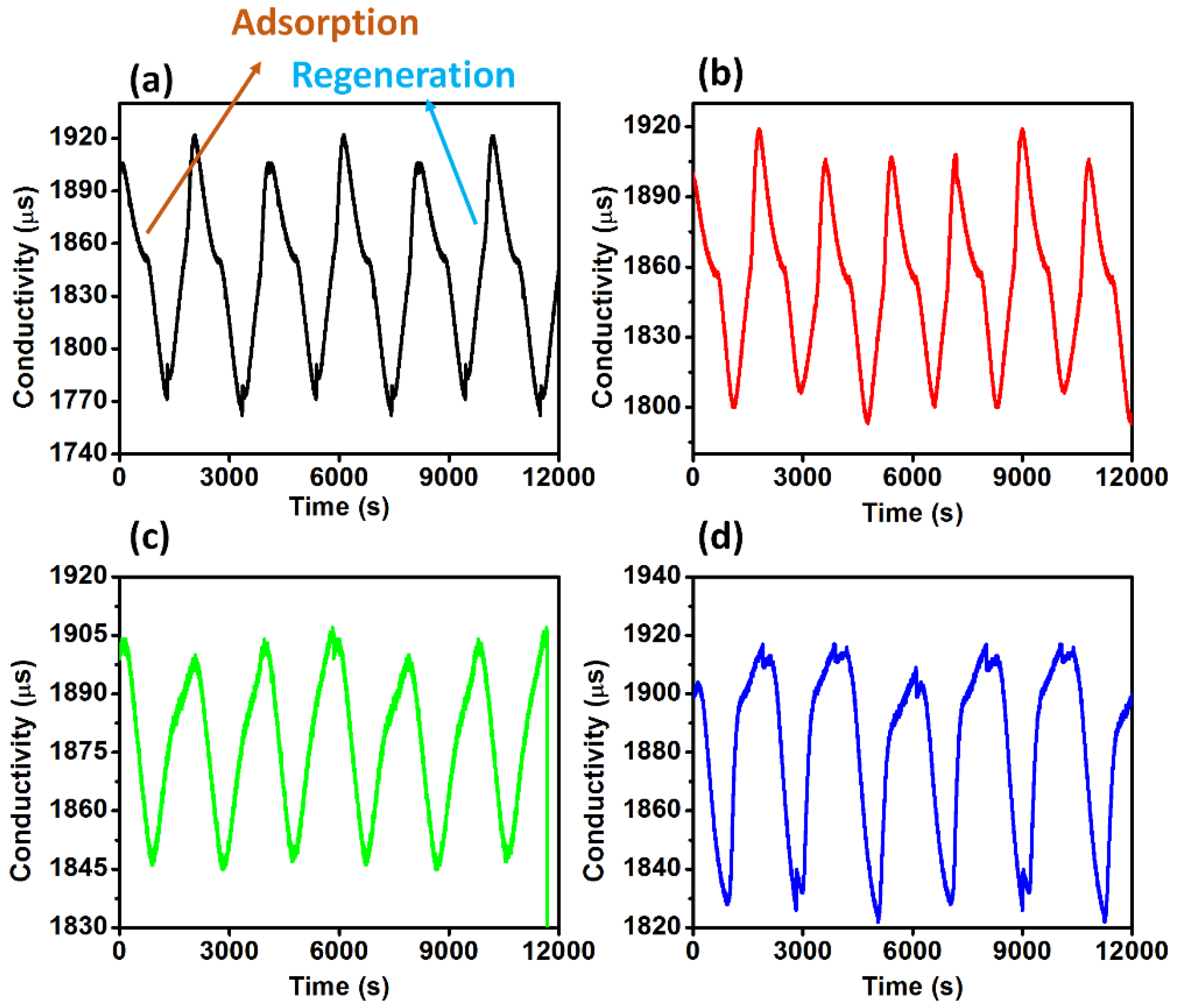
3.6. Deionization of Saline Water
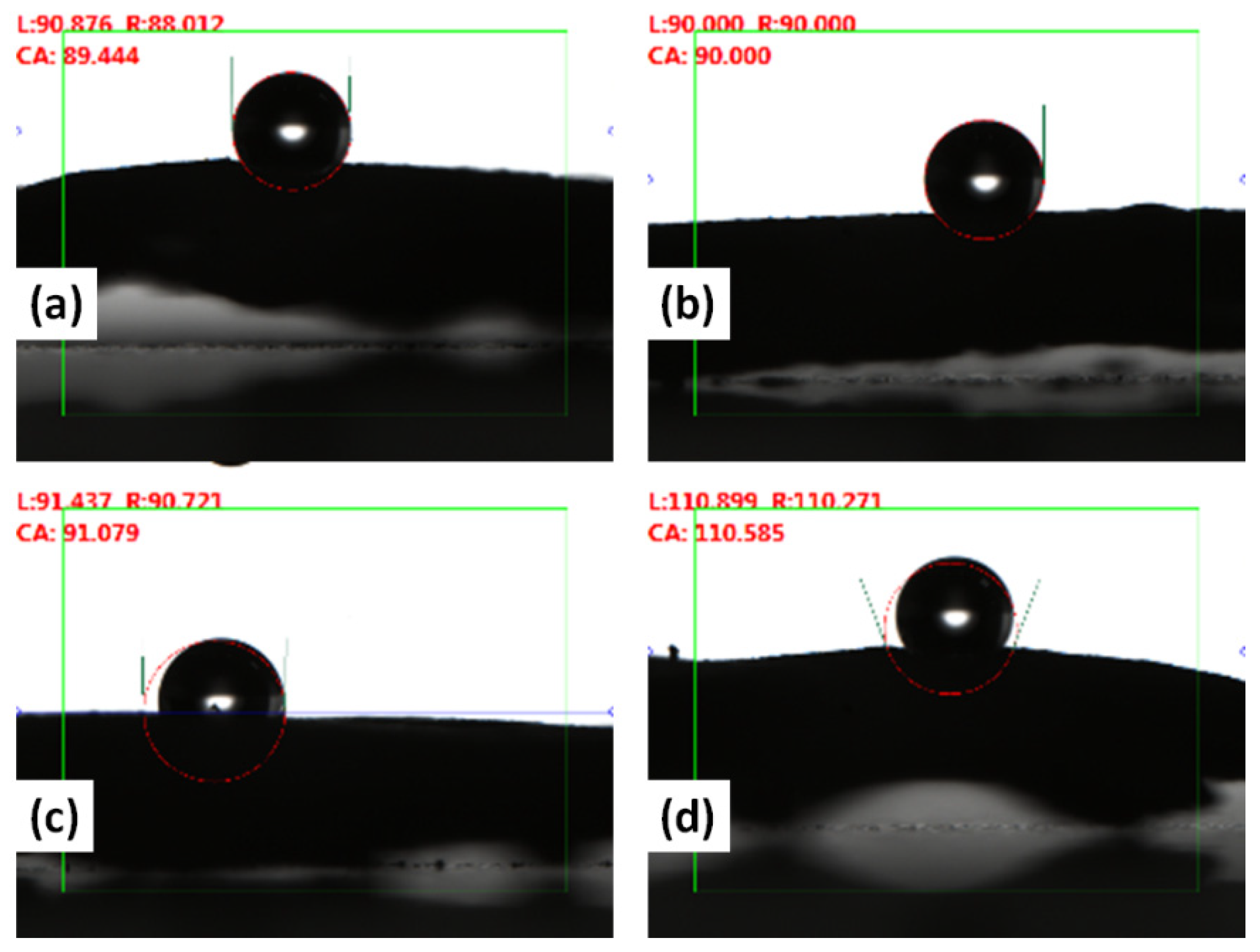
3.7. Contact Angle Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.; Cai, Z.; Sun, X.; Chong, C.; Yan, X.; Li, M.; Xu, J. Electrospun Nanofiber-Based Membranes for Water Treatment. Polymers 2022, 14, 2004. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Orlov, M.; Grokhovsky, V.; AlGhunaimi, F.I.; Ayirala, S. Functionalized Nanomembranes and Plasma Technologies for Produced Water Treatment: A Review. Polymers 2022, 14, 1785. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Kasongo, G. The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study. Membranes 2021, 11, 117. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Jain, H.; Garg, M.C. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination—A review. Environ. Technol. Innov. 2021, 23, 101561. [Google Scholar] [CrossRef]
- Ahualli, S.; Orozco-Barrera, S.; Fernández, M.d.M.; Delgado, Á.V.; Iglesias, G.R. Assembly of Soft Electrodes and Ion Exchange Membranes for Capacitive Deionization. Polymers 2019, 11, 1556. [Google Scholar] [CrossRef] [Green Version]
- Lado, J.J.; Cartolano, V.; García-Quismondo, E.; García, G.; Almonacid, I.; Senatore, V.; Naddeo, V.; Palma, J.; Anderson, M.A. Performance analysis of a capacitive deionization stack for brackish water desalination. Desalination 2021, 501, 114912. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, H.; Zhang, H.; Zhang, Y.; Zhao, H.; Wang, G. Synchronous removal of tetracycline and water hardness ions by capacitive deionization. J. Clean. Prod. 2021, 316, 128251. [Google Scholar] [CrossRef]
- Wimalasiri, Y.; Zou, L. Carbon nanotube/graphene composite for enhanced capacitive deionization performance. Carbon 2013, 59, 464–471. [Google Scholar] [CrossRef]
- Oladunni, J.; Zain, J.H.; Hai, A.; Banat, F.; Bharath, G.; Alhseinat, E. A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: From theory to practice. Sep. Purif. Technol. 2018, 207, 291–320. [Google Scholar] [CrossRef]
- Hou, C.-H.; Liu, N.-L.; Hsu, H.-L.; Den, W. Development of multi-walled carbon nanotube/poly(vinyl alcohol) composite as electrode for capacitive deionization. Sep. Purif. Technol. 2014, 130, 7–14. [Google Scholar] [CrossRef]
- Sandoval-Olvera, I.G.; González-Muñoz, P.; Díaz, D.R.; Maroto-Valiente, Á.; Ochoa, N.A.; Carmona, F.J.; Palacio, L.; Calvo, J.I.; Hernández, A.; Ávila-Rodríguez, M.; et al. Morphological, Electrical, and Chemical Characteristics of Poly(sodium 4-styrenesulfonate) Coated PVDF Ultrafiltration Membranes after Plasma Treatment. Polymers 2019, 11, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-Y.; Choi, J.-H. A carbon electrode fabricated using a poly(vinylidene fluoride) binder controlled the Faradaic reaction of carbon powder. J. Ind. Eng. Chem. 2010, 16, 401–405. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, J.; Xiong, S.; Wang, Y. N-Doping Carbon-Nanotube Membrane Electrodes Derived from Covalent Organic Frameworks for Efficient Capacitive Deionization. Langmuir 2020, 36, 12030–12037. [Google Scholar] [CrossRef] [PubMed]
- Alluri, N.R.; Chandrasekhar, A.; Jeong, J.H.; Kim, S.-J. Enhanced electroactive β-phase of the sonication-process-derived PVDF-activated carbon composite film for efficient energy conversion and a battery-free acceleration sensor. J. Mater. Chem. C 2017, 5, 4833–4844. [Google Scholar] [CrossRef]
- Mattsson, B.; Ericson, H.; Torell, L.M.; Sundholm, F. Micro-Raman investigations of PVDF-based proton-conducting membranes. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3317–3327. [Google Scholar] [CrossRef]
- Bharti, V.; Kaura, T.; Nath, R. Ferroelectric hysteresis in simultaneously stretched and corona-poled PVDF films. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 738–741. [Google Scholar] [CrossRef]
- Nagalakshmi, T.V.; Emmanuel, K.A.; Suresh Babu, C.; Chakrapani, C.; Divakar, P.P. Preparation of Mesoporous Activated Carbon from Jackfruit PPI-1 Waste and Development of Different Surface Functional Groups. Int. Lett. Chem. Phys. Astron. 2015, 54, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-E.; Li, X.-H.; Xu, J.-C.; Li, H.-L. Well-dispersed single-walled carbon nanotube/polyaniline composite films. Carbon 2003, 41, 2731–2736. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Briggs, M.E.; Hu, C.-C.; Cooper, A.I. Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers. Nano Energy 2018, 46, 277–289. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires. Energy Environ. Sci. 2011, 4, 1813–1819. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, F.; Meng, F.; Li, M.; Manivannan, A.; Wu, N. Effects of Pore Structure on Performance of An Activated-Carbon Supercapacitor Electrode Recycled from Scrap Waste Tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- Ahmad, A.; Ideris, N.; Ooi, B.; Low, S.; Ismail, A. Influence of polymer concentration on PVDF membrane fabrication for immunoassay analysis. J. Appl. Sci. 2014, 14, 1299–1303. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, C.; Pan, L.; Xu, X.; Sun, Z.; Chua, D.H.C. Carbon aerogels electrode with reduced graphene oxide additive for capacitive deionization with enhanced performance. Inorg. Chem. Front. 2014, 1, 249–255. [Google Scholar] [CrossRef]
- Quan, X.; Fu, Z.; Yuan, L.; Zhong, M.; Mi, R.; Yang, X.; Yi, Y.; Wang, C. Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes. RSC Adv. 2017, 7, 35875–35882. [Google Scholar] [CrossRef] [Green Version]
- Subasi, Y.; Cicek, B. Recent advances in hydrophilic modification of PVDF ultrafiltration membranes—A review: Part I. Membr. Technol. 2017, 2017, 7–12. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]






| PVDF Concentration (%) | Ck (Atomic %) | Fk (Atomic %) |
|---|---|---|
| 5 | 83.53 | 7.96 |
| 7.5 | 74.79 | 14.25 |
| 10 | 78.95 | 7.15 |
| 12.5 | 74.31 | 12.96 |
| Sample (PVDF) | SBET (m2g−1) | Vtotal (cm3g−1) | Vmicro (cm3g−1) | poreave (nm) | Vtotal (cm3g−1) |
|---|---|---|---|---|---|
| 5% | 721 | 0.51 | 0.70 | 3.26 | 0.92 |
| 7.5% | 521 | 0.50 | 0.64 | 3.24 | 0.85 |
| 10% | 233 | 0.46 | 0.62 | 3.05 | 0.81 |
| 12.5% | 110 | 0.43 | 0.48 | 3.01 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, H.; Jilani, A.; Salah, N.; Alshahrie, A.; Memić, A.; Ansari, M.O.; Dutta, J. Freestanding Activated Carbon Nanocomposite Electrodes for Capacitive Deionization of Water. Polymers 2022, 14, 2891. https://doi.org/10.3390/polym14142891
Hussain H, Jilani A, Salah N, Alshahrie A, Memić A, Ansari MO, Dutta J. Freestanding Activated Carbon Nanocomposite Electrodes for Capacitive Deionization of Water. Polymers. 2022; 14(14):2891. https://doi.org/10.3390/polym14142891
Chicago/Turabian StyleHussain, Humair, Asim Jilani, Numan Salah, Ahmed Alshahrie, Adnan Memić, Mohammad Omaish Ansari, and Joydeep Dutta. 2022. "Freestanding Activated Carbon Nanocomposite Electrodes for Capacitive Deionization of Water" Polymers 14, no. 14: 2891. https://doi.org/10.3390/polym14142891
APA StyleHussain, H., Jilani, A., Salah, N., Alshahrie, A., Memić, A., Ansari, M. O., & Dutta, J. (2022). Freestanding Activated Carbon Nanocomposite Electrodes for Capacitive Deionization of Water. Polymers, 14(14), 2891. https://doi.org/10.3390/polym14142891








