Removal and Mechanism of Cadmium, Lead and Copper in Water by Functional Modification of Silkworm Excrement Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biochar
2.3. Characterization of Samples
2.4. Absorption Experiments
3. Results and Discussion
3.1. Characterization of Biochar
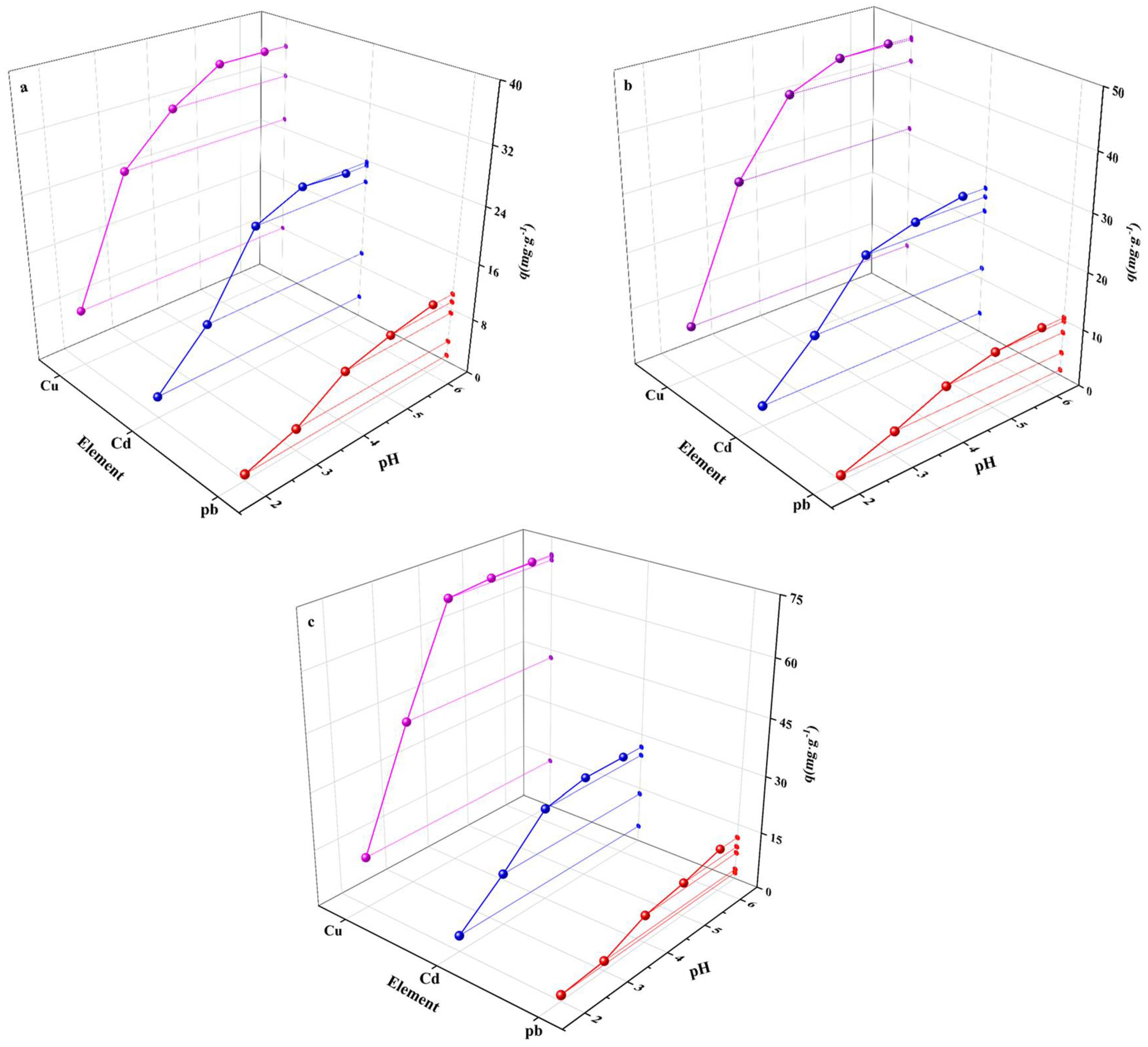
3.2. Influence of Initial pH Value
3.3. Competitive Adsorption
3.4. Adsorption Kinetics
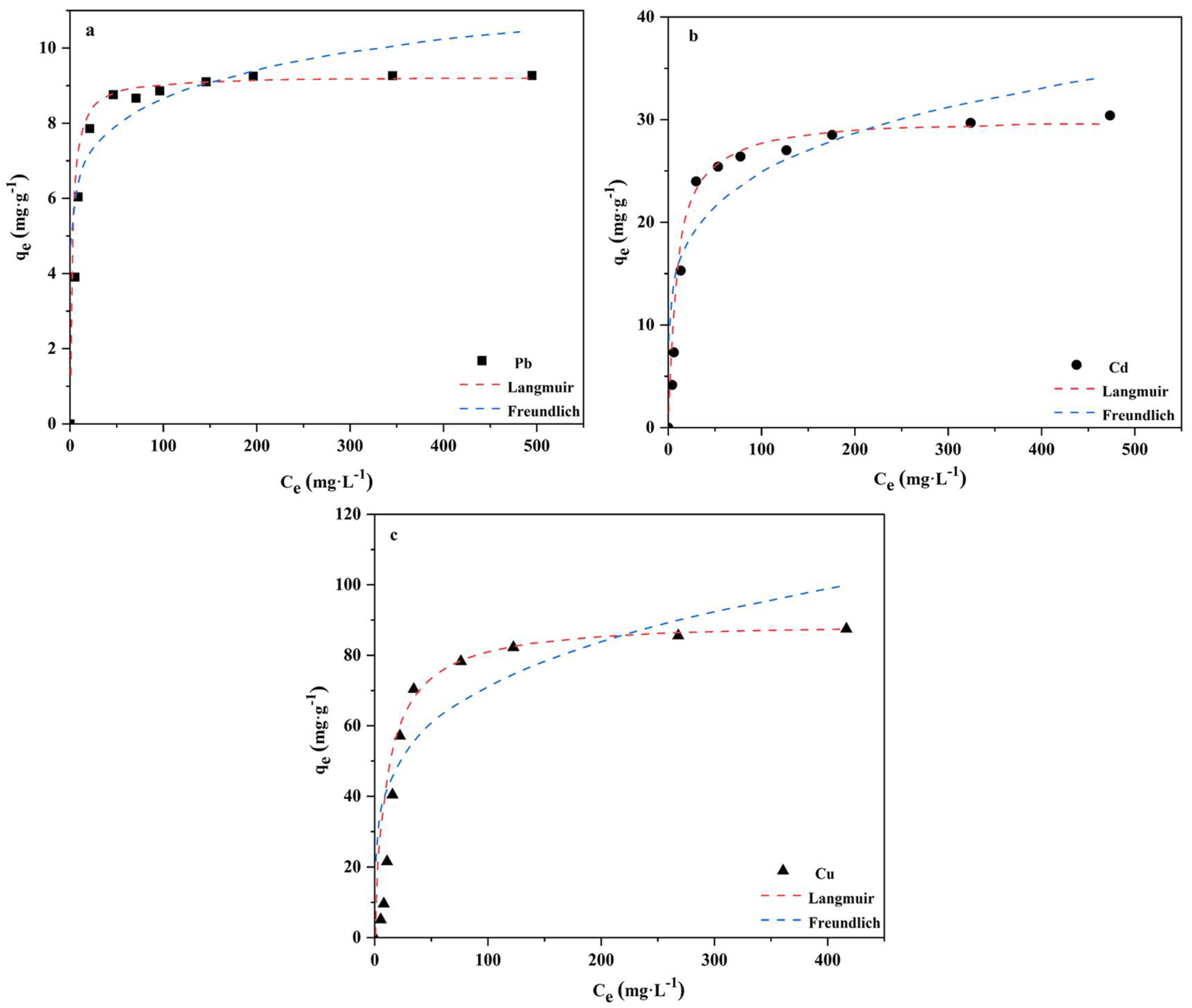
3.5. Adsorption Isotherms
3.6. Adsorption Mechanism
3.7. GBC Reproducibility and the Application of Actual Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Lashen, Z.M.; Shams, M.S.; El-Sheshtawy, H.S.; Slaný, M.; Antoniadis, V.; Yang, X.; Sharma, G.; Rinklebe, J.; Shaheen, S.M.; Elmahdy, S.M. Remediation of Cd and Cu contaminated water and soil using novel nanomaterials derived from sugar beet processing- and clay brick factory-solid wastes. J. Hazard. Mater. 2022, 428, 128205. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar]
- Liu, C.; Zhang, H.-X. Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: A critical review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Romero-Dondiz, E.M.; Almazán, J.E.; Rajal, V.B.; Castro-Vidaurre, E.F. Comparison of the performance of ultrafiltration and nanofiltration membranes for recovery and recycle of tannins in the leather industry. J. Clean. Prod. 2016, 135, 71–79. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wang, C.; Wang, H. Biochar/MnAl-LDH composites for Cu (ΙΙ) removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 443–450. [Google Scholar] [CrossRef]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Kearns, J.P.; Wellborn, L.S.; Summers, R.S.; Knappe, D.R.U. 2,4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res. 2014, 62, 20–28. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, Economical, and Climate-Related Aspects of Biochar Production Technologies: A Literature Review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-F.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Hu, B.; Chen, Z.; Yang, H.; Zhuang, L.; Wang, X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 2021, 3, 117–123. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: A review. Int. J. Biol. Macromol. 2016, 86, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, X.; Yuan, B.; Fu, M.-L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 2020, 239, 124745. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Han, L.; Hu, B.; Qiu, M. Reductive and adsorptive elimination of U(VI) ions in aqueous solution by SFeS@Biochar composites. Environ. Sci. Pollut. Res. 2021, 28, 55176–55185. [Google Scholar] [CrossRef]
- Laus, R.; de Fávere, V.T. Competitive adsorption of Cu(II) and Cd(II) ions by chitosan crosslinked with epichlorohydrin–triphosphate. Bioresour. Technol. 2011, 102, 8769–8776. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Yoo, S.; Han, M.S.; Tae, G. Effective and prolonged targeting of a nanocarrier to the inflammation site by functionalization with ZnBPMP and chitosan. Mater. Sci. Eng. C 2021, 131, 112521. [Google Scholar] [CrossRef]
- Song, J.; Messele, S.A.; Meng, L.; Huang, Z.; Gamal El-Din, M. Adsorption of metals from oil sands process water (OSPW) under natural pH by sludge-based Biochar/Chitosan composite. Water Res. 2021, 194, 116930. [Google Scholar] [CrossRef]
- Kavianinia, I.; Plieger, P.G.; Cave, N.J.; Gopakumar, G.; Dunowska, M.; Kandile, N.G.; Harding, D.R.K. Design and evaluation of a novel chitosan-based system for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 85, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-X.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Chen, J.; Bian, P.; Huang, H.; Zhong, K.; Huang, D.; Nong, X.; Mao, J.; Zhang, C. The Mechanism of Silkworm Excrement Organic Fertilizer to Reduce the Cd Availability in Paddy Soil. Soil Sediment Contam. Int. J. 2022, 31, 1–14. [Google Scholar] [CrossRef]
- Arán, D.; Antelo, J.; Fiol, S.; Macías, F. Influence of feedstock on the copper removal capacity of waste-derived biochars. Bioresour. Technol. 2016, 212, 199–206. [Google Scholar] [CrossRef]
- Manakhov, A.; Michlíček, M.; Nečas, D.; Josef, P.; Makhneva, E.; Eliáb, M.; Zajíčková, L. Carboxyl-rich coatings deposited by atmospheric plasma co-polymerization of maleic anhydride and acetylene. Surf. Coat. Technol. 2016, 295, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Zieba-Palus, J.; Wesełucha-Birczyńska, A.; Trzcińska, B.; Kowalski, R.; Moskal, P. Analysis of degraded papers by infrared and Raman spectroscopy for forensic purposes. J. Mol. Struct. 2016, 1140, 154–162. [Google Scholar] [CrossRef]
- Rich, P.; Maréchal, A. Carboxyl group functions in the heme-copper oxidases: Information from mid-IR vibrational spectroscopy. Biochim. Biophys. Acta 2008, 1777, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Chang, J.; Xu, L.; Liao, X.; Bai, L.; Lan, Y.; Li, M. Simultaneous analysis of uric acid, xanthine and hypoxanthine using voltammetric sensor based on nanocomposite of palygorskite and nitrogen doped graphene. J. Electroanal. Chem. 2017, 805, 159–170. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Y.; Xu, J.; Wen, Y.; Zhang, J.; Ding, W. Nanohybrid sensor based on carboxyl functionalized graphene dispersed palygorskite for voltammetric determination of niclosamide. Appl. Clay Sci. 2017, 143, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Zheng, T.; Wang, P.; Hao, L.; Wang, Y. Fast microwave-assisted preparation of a low-cost and recyclable carboxyl modified lignocellulose-biomass jute fiber for enhanced heavy metal removal from water. Bioresour. Technol. 2016, 201, 41–49. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lu, Y.; Luo, G. Ca(II) imprinted chitosan microspheres: An effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions. Chem. Eng. J. 2014, 244, 202–208. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, S.; Wang, L.; Liu, Y.; Qian, F.; Yao, W.; Zhang, S.; Chen, J.-M. Tracking the conversion of nitrogen during pyrolysis of antibiotic mycelial fermentation residues using XPS and TG-FTIR-MS technology. Environ. Pollut. 2016, 211, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Qi, J.H.; Wang, L.H. Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J. Hazard. Mater. 2010, 174, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Lai, C.; Zhao, M.H.; Wei, Z.; Li, N.J.; Huang, C.; Xie, G.X. Adsorption of Pb(II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: Equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem. Eng. J. 2012, 203, 423–431. [Google Scholar] [CrossRef]
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 2014, 105, 68–74. [Google Scholar] [CrossRef]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Hanif, M.U.; Capareda, S.; Chang, Z.; Huang, H.; Ai, Y. Copper(II) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of KOH activation. J. Environ. Chem. Eng. 2015, 4, 365–372. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chen, N. Anion Effects on the Adsorption of Acetylene by Nickel Halides. Langmuir 1999, 15, 7647–7652. [Google Scholar] [CrossRef]
- Narin, G.; Martins, V.; Campo, M.; Ribeiro, A.; Ferreira, A.; Santos, J.C.; Schumann, K.; Rodrigues, A.E. Light olefins/paraffins separation with 13X zeolite binderless beads. Sep. Purif. Technol. 2014, 133, 452–475. [Google Scholar] [CrossRef]
- Xu, X.; Schierz, A.; Xu, N.; Cao, X. Comparison of the characteristics and mechanisms of Hg(II) sorption by biochars and activated carbon. J. Colloid Interface Sci. 2015, 463, 55–60. [Google Scholar] [CrossRef]
- Smolyakov, B.S.; Sagidullin, A.K.; Chikunov, A.S. Removal of Cd(II), Zn(II), and Cu(II) from aqueous solutions using humic-modified moss (Polytrichum Comm.). J. Environ. Chem. Eng. 2017, 5, 1015–1020. [Google Scholar] [CrossRef]
- Harish, S.; Navaneethan, M.; Archana, J.; Ponnusamy, S.; Muthamizhchelvan, C.; Hayakawa, Y. Controlled synthesis and morphological investigation of self-assembled CuO nanostructures. Mater. Lett. 2014, 121, 129–132. [Google Scholar] [CrossRef]


| pH | Kd(Pb) | Kd(Cd) | Kd(Cu) | ||
|---|---|---|---|---|---|
| 2 | 0.0071 | 0.0133 | 0.1026 | 7.6232 | 13.8061 |
| 3 | 0.0137 | 0.0456 | 0.6563 | 14.2818 | 48.0182 |
| 4 | 0.0326 | 0.0721 | 1.4246 | 21.8139 | 43.4567 |
| 5 | 0.0503 | 0.1028 | 1.7428 | 16.8621 | 34.4987 |
| 6 | 0.0536 | 0.0994 | 1.7616 | 17.9672 | 33.6263 |
| Element | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg·g−1) | R2 | K2 (g·(mg·min)−1) | qe (mg·g−1) | R2 | |
| Pb | 0.0631 | 8.2339 | 0.9593 | 0.0117 | 8.5933 | 0.9924 |
| Cd | 0.0558 | 24.6435 | 0.9516 | 0.0034 | 25.7601 | 0.9976 |
| Cu | 0.0269 | 66.7413 | 0.9788 | 0.0006 | 71.4227 | 0.9971 |
| Element | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| KF | 1/n | R2 | KL | qm | R2 | |
| Pb | 5.1107 | 0.1193 | 0.9142 | 0.5339 | 9.33 | 0.9348 |
| Cd | 9.8432 | 0.2108 | 0.8572 | 0.1197 | 30.14 | 0.9912 |
| Cu | 23.6736 | 0.2419 | 0.8599 | 0.0949 | 90.02 | 0.9951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, P.; Liu, Y.; Zheng, X.; Shen, W. Removal and Mechanism of Cadmium, Lead and Copper in Water by Functional Modification of Silkworm Excrement Biochar. Polymers 2022, 14, 2889. https://doi.org/10.3390/polym14142889
Bian P, Liu Y, Zheng X, Shen W. Removal and Mechanism of Cadmium, Lead and Copper in Water by Functional Modification of Silkworm Excrement Biochar. Polymers. 2022; 14(14):2889. https://doi.org/10.3390/polym14142889
Chicago/Turabian StyleBian, Pengyang, Yixuan Liu, Xiaoqin Zheng, and Weibo Shen. 2022. "Removal and Mechanism of Cadmium, Lead and Copper in Water by Functional Modification of Silkworm Excrement Biochar" Polymers 14, no. 14: 2889. https://doi.org/10.3390/polym14142889
APA StyleBian, P., Liu, Y., Zheng, X., & Shen, W. (2022). Removal and Mechanism of Cadmium, Lead and Copper in Water by Functional Modification of Silkworm Excrement Biochar. Polymers, 14(14), 2889. https://doi.org/10.3390/polym14142889





