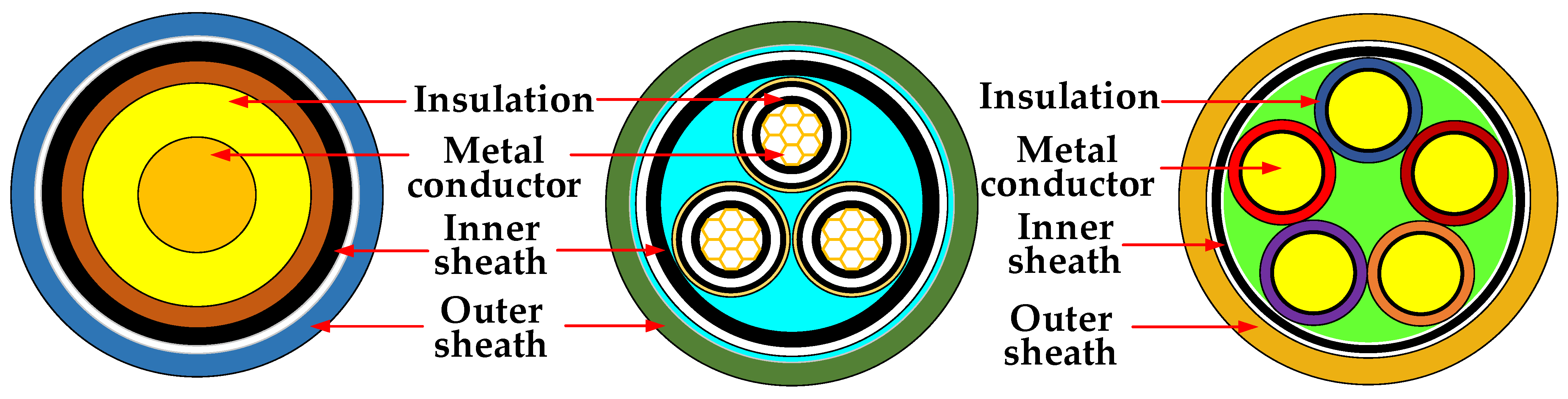
Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials
Abstract
1. Introduction
| Performance | Unit | Index |
|---|---|---|
| LOI | % | ≥30 |
| Tensile strength | MPa | ≥10 |
| Elongation at break | % | ≥150 |
2. Flame Retardant Mode of Action
- (i)
- Covering effect: When heated, some FRs can form a non-flammable protective layer, thereby blocking the two elements, i.e., oxygen and heat, necessary for combustion.
- (ii)
- Dilution effect: Some FRs can release incombustible gases, such as CO2 and water vapor, when heated, and this can reduce the oxygen concentration around the polymers, thereby inhibiting the combustion process.
- (iii)
- Endothermic effect: Some FRs can undergo a decomposition reaction to absorb a large amount of heat, leading to a cooling effect on the polymer.
- (iv)
- Inhibition effect: The thermal decomposition of some FRs will generate a large number of radicals, which can combine with the reactive radicals released by the polymer matrix to interrupt the process of the chain reaction.
3. Inorganic Flame Retardants
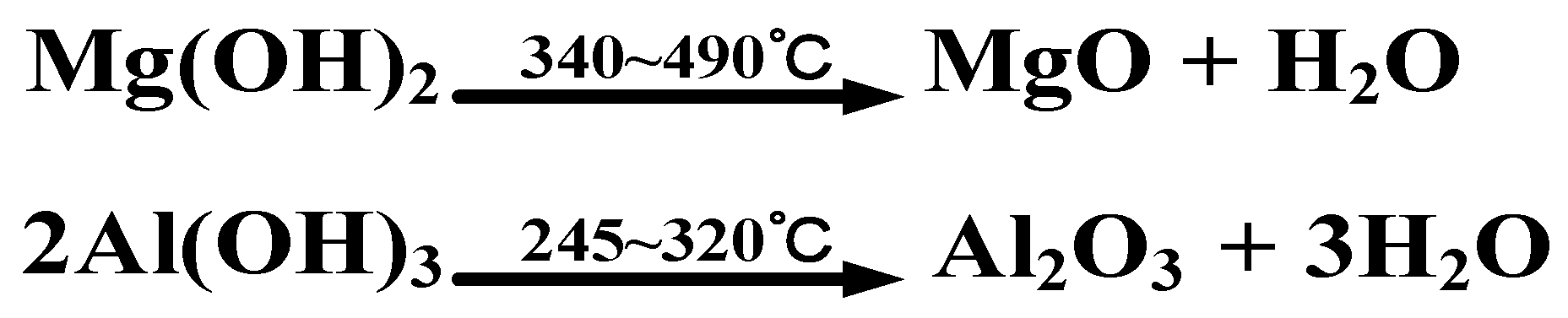
3.1. Metal Hydroxide
3.2. Inorganic Phosphorus

3.3. Inorganic Silicon
3.3.1. SiO2
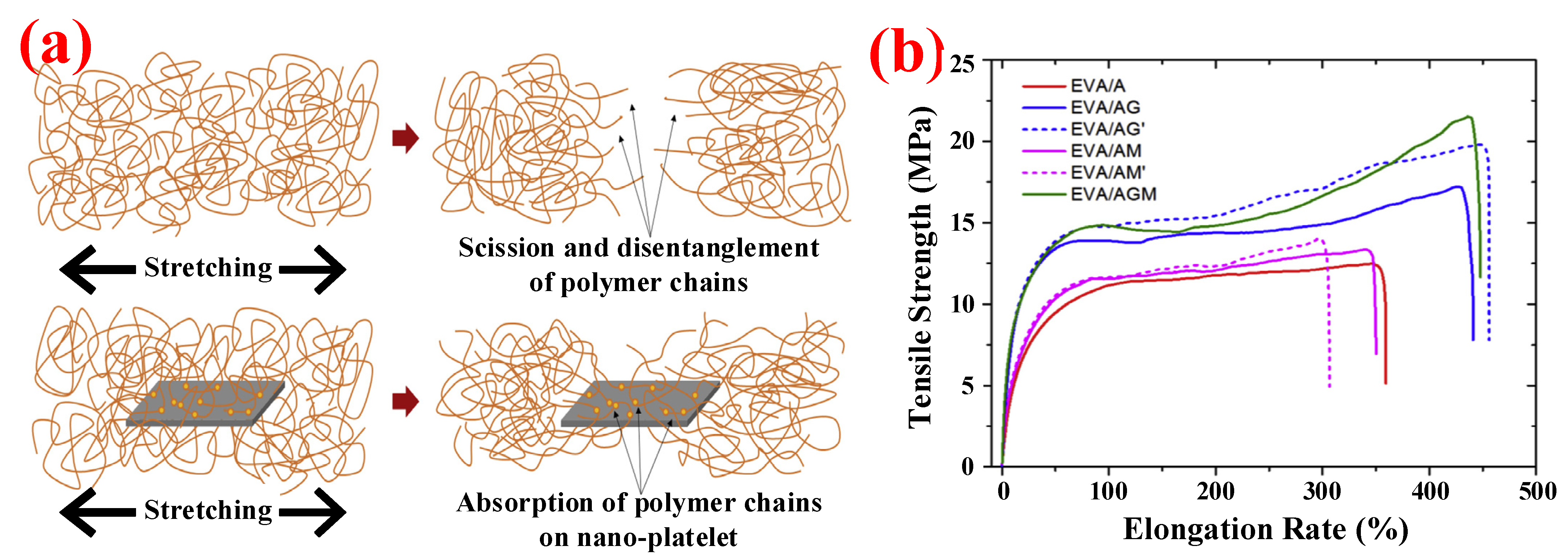
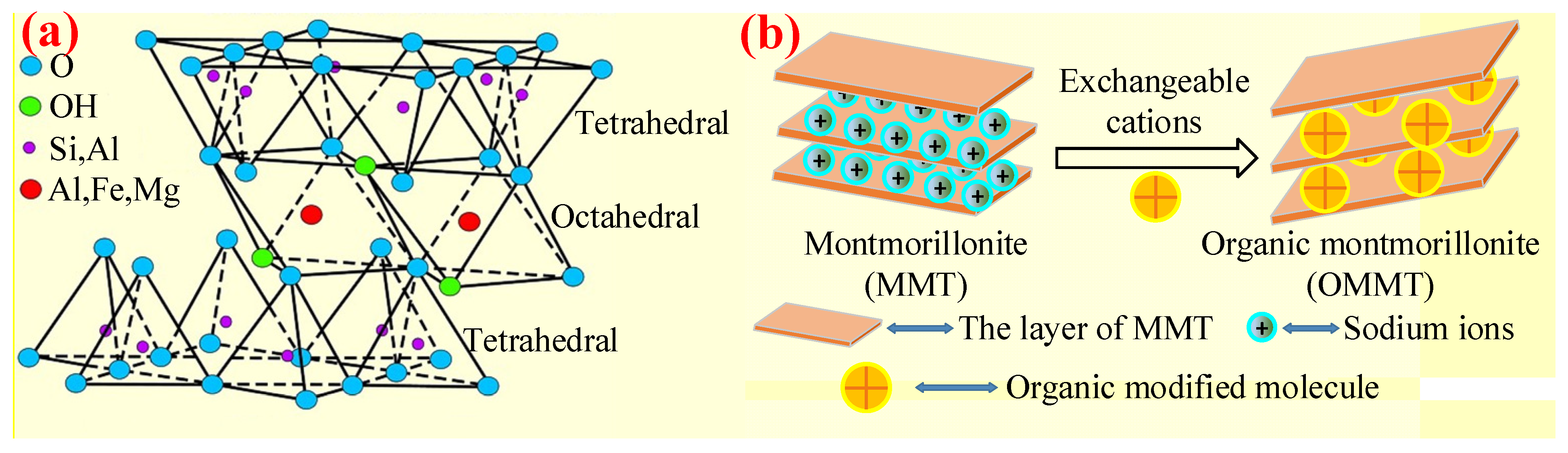
3.3.2. MMT/OMMT
4. Organic Flame Retardants
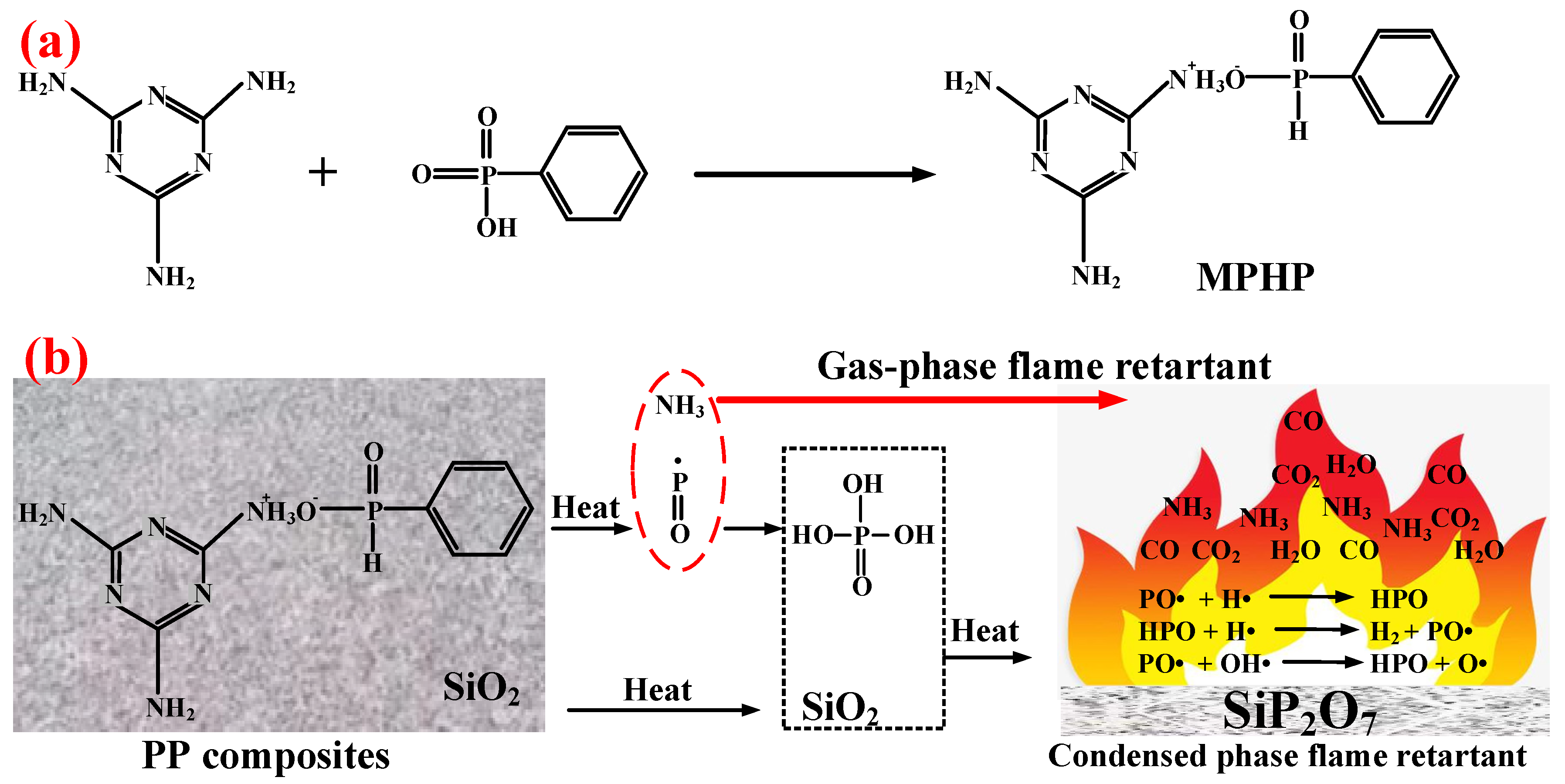
4.1. Organic Phosphorus
4.2. Organic Silicon
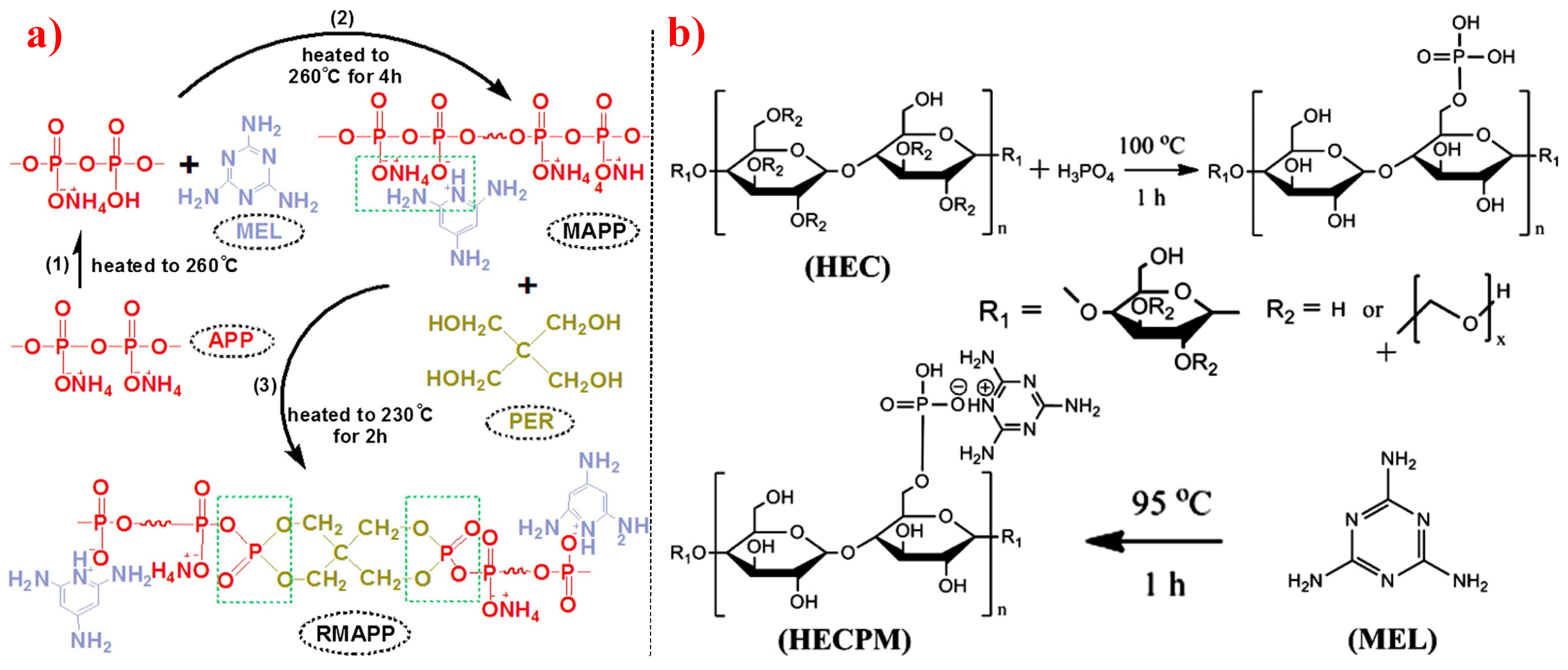
5. Intumescent Flame Retardants
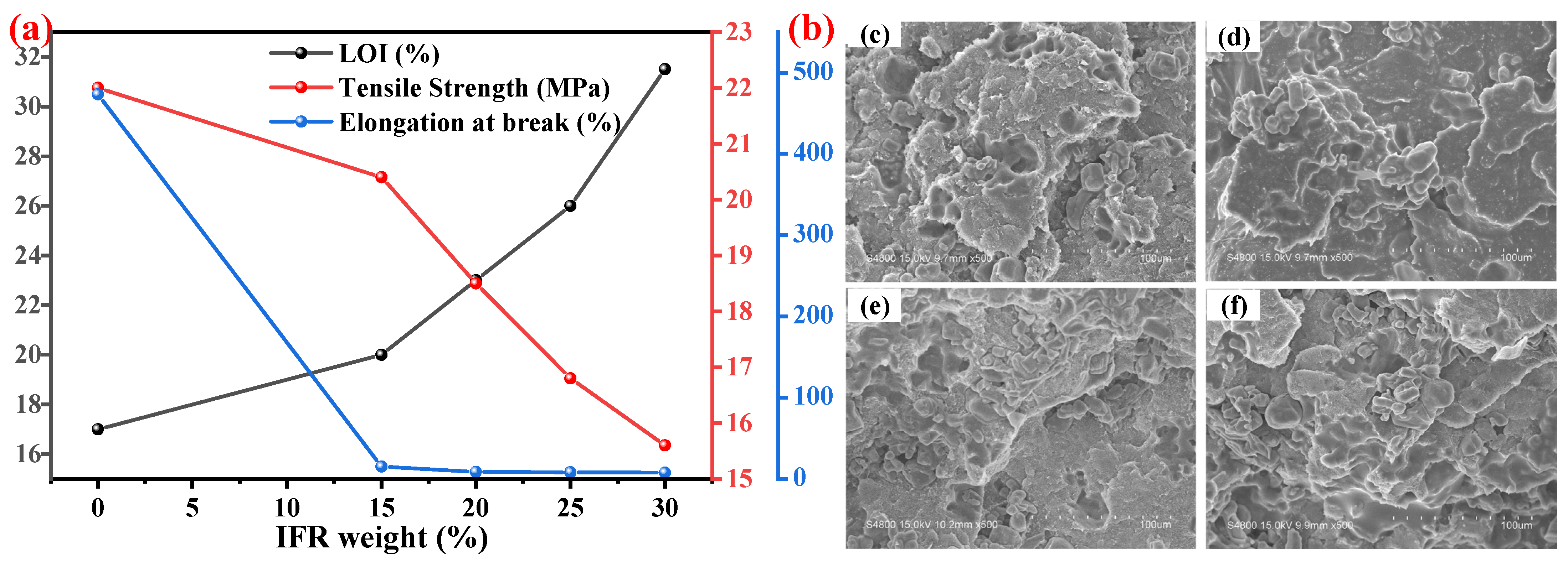
5.1. P-N-Based IFR
5.1.1. Surface Treatment of IFR
5.1.2. Adjuvants for IFR
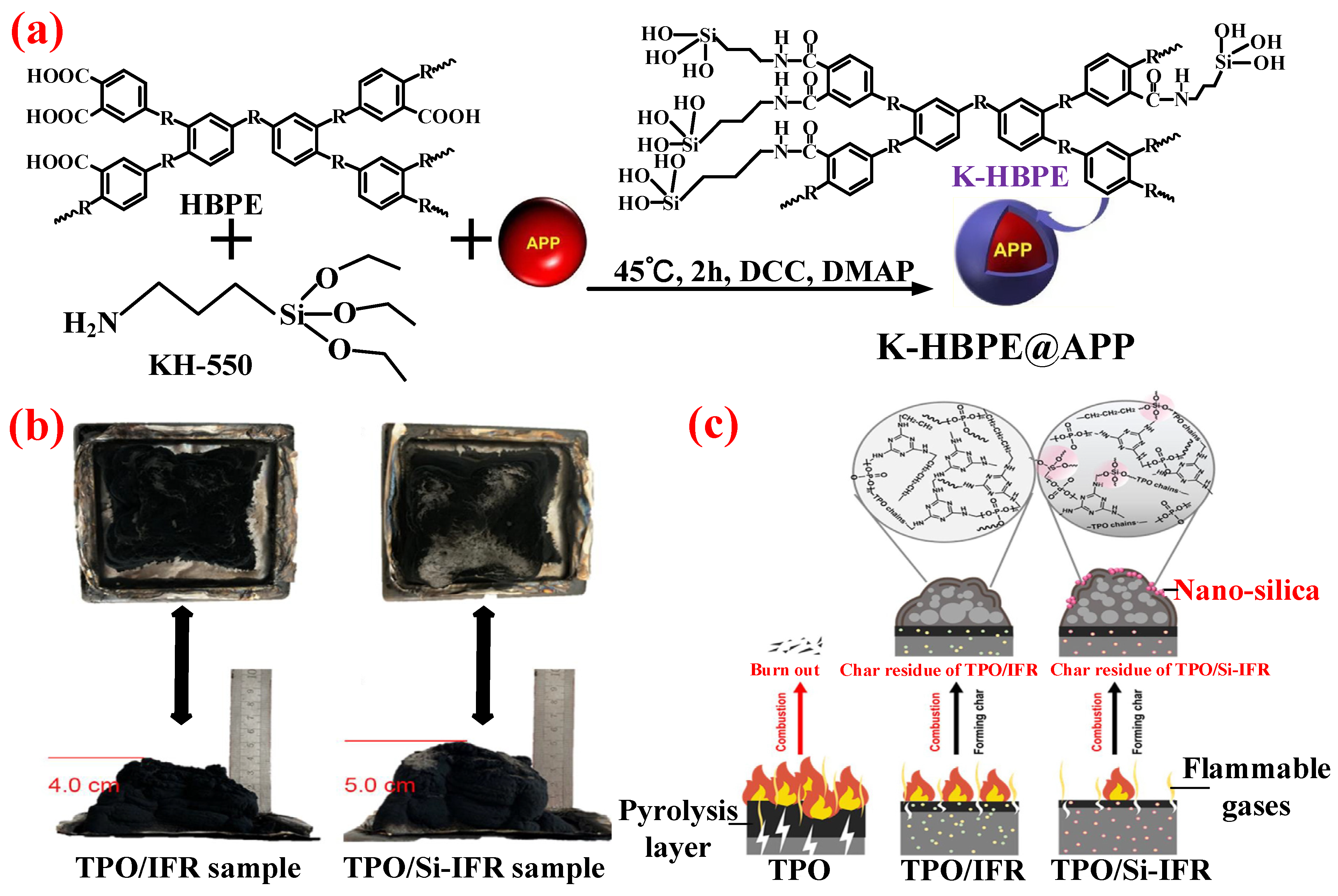
5.1.3. New IFRs
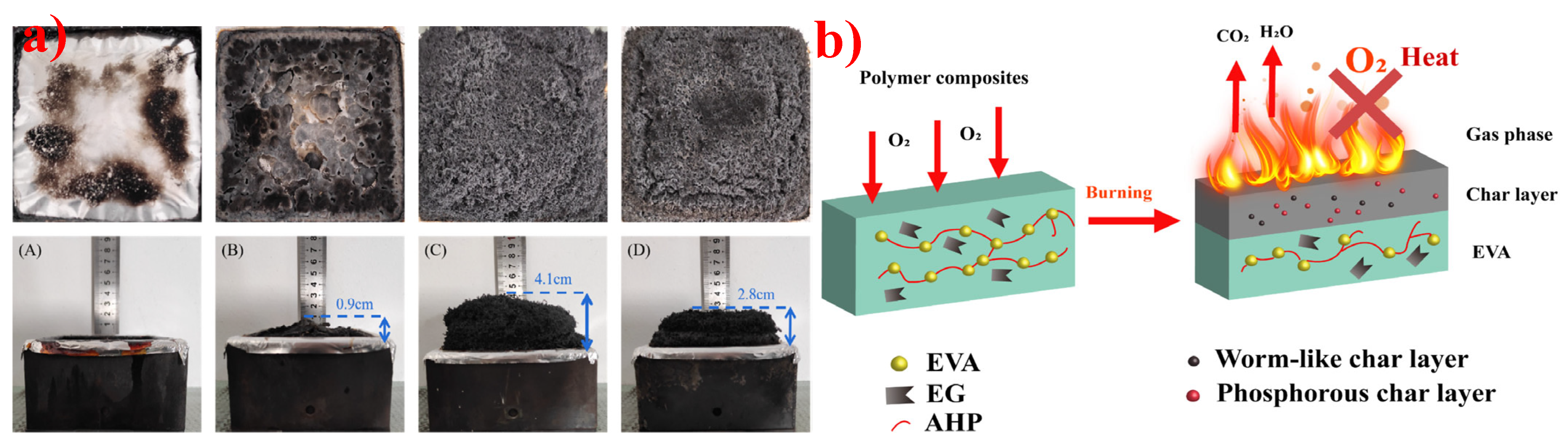
5.2. Expandable Graphite
6. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, S.J.; Sun, Q.H. The Law of Insulation Failure of ZR-VV Cables under the Circumstances of Different Thermal Environments. Procedia Eng. 2016, 135, 362–367. [Google Scholar] [CrossRef][Green Version]
- Courty, L.; Garo, J.P. External heating of electrical cables and auto-ignition investigation. J. Hazard. Mater. 2017, 321, 528–536. [Google Scholar] [CrossRef]
- Witkowski, A.; Girardin, B.; Foersth, M.; Hewitt, F.; Fontaine, G.; Duquesne, S.; Bourbigot, S.; Hull, T.R. Development of an anaerobic pyrolysis model for fire retardant cable sheathing materials. Polym. Degrad. Stab. 2015, 113, 208–217. [Google Scholar] [CrossRef]
- Meinier, R.; Sonnier, R.; Zavaleta, P.; Suard, S.; Ferry, L. Fire behavior of halogen-free flame retardant electrical cables with the cone calorimeter. J. Hazard. Mater. 2018, 342, 306–316. [Google Scholar] [CrossRef]
- Wang, B.; Qian, X.; Shi, Y.; Yu, B.; Hong, N.; Song, L.; Hu, Y. Cyclodextrin microencapsulated ammonium polyphosphate: Preparation and its performance on the thermal, flame retardancy and mechanical properties of ethylene vinyl acetate copolymer. Compos. B Eng. 2015, 69, 22–30. [Google Scholar] [CrossRef]
- Pan, Y.T.; Yuan, Y.S.; Wang, D.Y.; Yang, R.J. An overview of the flame retardants for poly(vinyl chloride): Current states and perspective. Chin. J. Chem. 2020, 38, 1870–1896. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.Z.; Yang, B.; Liu, Y. A Novel Intumescent Flame-Retardant Polyethylene System. Macromol. Mater. Eng. 2010, 291, 247–253. [Google Scholar] [CrossRef]
- EN 50264; Railway Applications-Railway Rolling Stock Power and Control Cables Having Special Fire Performance. iTeh Standards: Etobicoke, ON, Canada, 2005.
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Watson, D.; Schiraldi, D.A. Biomolecules as Flame Retardant Additives for Polymers: A Review. Polymers 2020, 12, 849. [Google Scholar] [CrossRef]
- Zhao, W.J.; Kundu, C.K.; Li, Z.W.; Li, X.H.; Zhang, Z.J. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Feng, C.M.; Liang, M.Y.; Jiang, J.L.; Huang, J.G.; Liu, H.B. Flame retardancy and thermal degradation behavior of efficient intumescent flame retardant LDPE composite containing 4A zeotile. J. Anal. Appl. Pyrolysis 2015, 118, 9–19. [Google Scholar] [CrossRef]
- Cabrera-Alvarez, E.N.; Ramos-deValle, L.F.; Sanchez-Valdes, S.; Candia-Garcia, A.; Soriano-Corral, F.; Ramirez-Vargas, E.; Ibarra-Alonso, M.; Patino-Soto, P. Study of the silane modification of magnesium hydroxide and their effects on the flame retardant and tensile properties of high density polyethylene nanocomposites. Polym. Compos. 2013, 35, 1060–1069. [Google Scholar] [CrossRef]
- Ye, L.; Miao, Y.Y.; Yan, H.; Li, Z.; Zhou, Y.L.; Liu, J.X.; Liu, H. The synergistic effects of boroxo siloxanes with magnesium hydroxide in halogen-free flame retardant EVA/MH blends. Polym. Degrad. Stab. 2013, 98, 868–874. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, H.L.; Fang, Y.C. High-Efficiency and Durable Flame Retardant Cotton Fabric with Good Physical and Mechanical Properties by Layer-by-Layer Assembly Polyethylenimine/Phytic Acid Coating. J. Nat. Fibers 2022. [Google Scholar] [CrossRef]
- Liang, J.Z.; Feng, J.Q.; Tsui, C.P.; Tang, C.Y.; Liu, D.F.; Zhang, S.D.; Huang, W.F. Mechanical properties and flame-retardant of PP/MRP/Mg(OH)2/Al(OH)3 composites. Compos. B Eng. 2015, 71, 74–81. [Google Scholar] [CrossRef]
- Chen, X.L.; Yu, J.; Guo, S.Y. Structure and properties of polypropylene composites filled with magnesium hydroxide. J. Appl. Polym. Sci. 2006, 102, 4943–4951. [Google Scholar] [CrossRef]
- Ma, Z.L.; Wang, J.H.; Zhang, X.Y. Effect of silane KH-550 to polypropylene/brucite composite. J. Appl. Polym. Sci. 2010, 107, 1000–1005. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, J.; Zhou, C.G.; Zhou, D.; Chen, B.F.; Yang, H.; Cheng, R.S. Effects of the content of silane coupling agent KH-560 on the properties of LLDPE/magnesium hydroxide composites. J. Appl. Polym. Sci. 2010, 118, 2634–2641. [Google Scholar] [CrossRef]
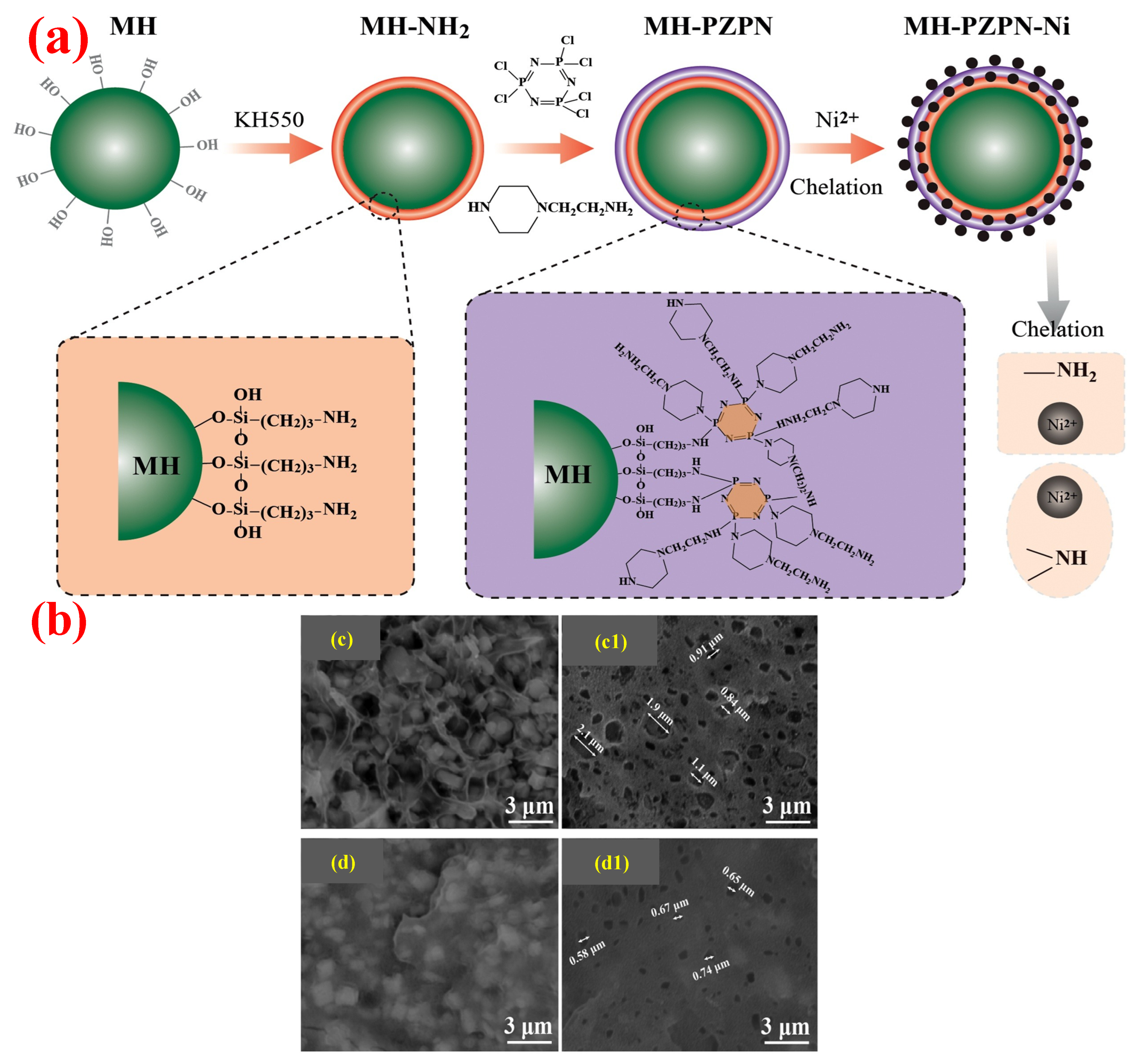
- Meng, W.H.; Wu, H.J.; Wu, R.F.; Wang, T.; Wang, A.Q.; Ma, J.; Xu, J.Z.; Qu, H.Q. Fabrication of surface-modified magnesium hydroxide using Ni2+ chelation method and layer-by-layer assembly strategy: Improving the flame retardancy and smoke suppression properties of ethylene-vinyl acetate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125712. [Google Scholar] [CrossRef]
- Meng, W.H.; Dong, Y.L.; Li, J.H.; Cheng, L.Y.; Zhang, H.J.; Wang, C.Z.; Jiao, Y.H.; Xu, J.Z.; Hao, J.W.; Qu, H.Q. Bio-based phytic acid and tannic acid chelate-mediated interfacial assembly of Mg(OH)2 for simultaneously improved flame retardancy, smoke suppression and mechanical properties of PVC. Compos. B Eng. 2020, 188, 107854. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, X.F.; Chen, J.Y.; Wang, J.X.; Zhang, L.L.; Chen, J.F. Magnesium hydroxide nanodispersion for polypropylene nanocomposites with high transparency and excellent fire-retardant properties. Polym. Degrad. Stab. 2018, 146, 327–333. [Google Scholar] [CrossRef]
- Liu, T.T.; Wang, F.; Li, G.; Liu, P.; Gao, C.; Ding, Y.F.; Zhang, S.M.; Kong, X.R.; Yang, M.S. Magnesium hydroxide nanoparticles grafted by DOPO and its flame retardancy in ethylene-vinyl acetate copolymers. J. Appl. Polym. Sci. 2020, 138, e49607. [Google Scholar] [CrossRef]
- Alam, A.; Zhang, Y.J.; Kuan, H.C.; Lee, S.H.; Ma, J. Polymer composite hydrogels containing carbon nanomaterials-Morphology and mechanical and functional performance. Prog. Polym. Sci. 2017, 77, 1–18. [Google Scholar] [CrossRef]
- Yen, Y.Y.; Wang, H.T.; Guo, W.J. Synergistic flame retardant effect of metal hydroxide and nanoclay in EVA composites. Polym. Degrad. Stab. 2012, 97, 863–869. [Google Scholar] [CrossRef]
- Guo, Y.C.; Xue, Y.; Zuo, X.H.; Zhang, L.X.; Yang, Z.H.; Zhou, Y.C.; Marmorat, C.; He, S.; Rafailovich, M. Capitalizing on the molybdenum disulfide/graphene synergy to produce mechanical enhanced flame retardant ethylene-vinyl acetate composites with low aluminum hydroxide loading. Polym. Degrad. Stab. 2017, 144, 155–166. [Google Scholar] [CrossRef]
- Vahidi, G.; Bajwa, D.S.; Shojaeiarani, J.; Stark, N.; Darabi, A. Advancements in traditional and nanosized flame retardants for polymers—A review. J. Appl. Polym. Sci. 2020, 138, e50050. [Google Scholar] [CrossRef]
- Chang, S.K.; Zeng, C.; Yuan, W.Z.; Ren, J. Preparation and characterization of double-layered microencapsulated red phosphorus and its flame retardance in poly(lactic acid). J. Appl. Polym. Sci. 2012, 125, 3014–3022. [Google Scholar] [CrossRef]
- Battig, A.; Sanchez-Olivares, G.; Rockel, D.; Maldonado-Santoyo, M.; Schartel, B. Waste not, want not: The use of leather waste in flame retarded EVA. Mater. Des. 2021, 210, 110100. [Google Scholar] [CrossRef]
- Mauerer, O. New reactive, halogen-free flame retardant system for epoxy resins. Polym. Degrad. Stab. 2005, 88, 70–73. [Google Scholar] [CrossRef]
- Huang, Y.W.; Song, M.L.; Ma, J.J.; Lu, Z.Y.; Yang, J.X.; Cao, K. Synthesis of a phosphorus/silicon hybrid and its synergistic effect with melamine polyphosphates on flame retardant polypropylene system. J. Appl. Polym. Sci. 2013, 129, 316–323. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R-Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wang, N.; Li, L.T.; Xu, Y.; Zhang, K.; Chen, X.L.; Wu, H. Synergistic effects of red phosphorus masterbatch with expandable graphite on the flammability and thermal stability of polypropylene/thermoplastic polyurethane blends. Polym. Polym. Compos. 2020, 28, 209–219. [Google Scholar] [CrossRef]
- Jian, R.K.; Chen, L.; Hu, Z.; Wang, Y.Z. Flame-retardant polycarbonate/acrylonitrile-butadiene-styrene based on red phosphorus encapsulated by polysiloxane: Flame retardance, thermal stability, and water resistance. J. Appl. Polym. Sci. 2011, 123, 2867–2874. [Google Scholar] [CrossRef]
- Qin, Z.L.; Li, D.H.; Yang, R.J. Preparation of Ammonium Polyphosphate Coated with Aluminium Hydroxide and Its Application in Polypropylene as Flame Retardant. J. Inorg. Mater. 2015, 30, 1267–1272. [Google Scholar] [CrossRef]
- Shao, Z.B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.J.; Chen, L.; Wang, Y.Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Liang, J.Z.; Feng, J.Q.; Tsui, C.P.; Tang, C.Y.; Huang, W.F. Mechanical properties and morphology for polypropylene composites filled with microencapsulated red phosphorus. Polym. Adv. Technol. 2014, 25, 347–352. [Google Scholar] [CrossRef]
- Fang, S.L.; Hu, Y.; Song, L.; Zhan, J.; He, Q.L. Mechanical properties, fire performance and thermal stability of magnesium hydroxide sulfate hydrate whiskers flame retardant silicone rubber. J. Mater. Sci. 2008, 43, 1057–1062. [Google Scholar] [CrossRef]
- Jiang, W.J.; Li, Z.Z.; Zhang, C.X.; Fang, J.; Yang, X.J.; Lu, L.D.; Pu, L.J. Preparation of Microencapsulated Red Phosphorus and Its Flame-Retardant Application in PP Composites. Spectrosc. Spectr. Anal. 2010, 30, 1329–1335. [Google Scholar] [CrossRef]
- Chen, X.L.; Yu, J.; Qin, J.; Luo, Z.; Hu, S.C.; He, M. Combustion Behaviour and Synergistic Effect of Zinc Borate and Microencapsulated Red Phosphorus with Magnesium Hydroxide in Flame-Retarded Polypropylene Composites. Polym. Polym. Compos. 2011, 19, 491–496. [Google Scholar] [CrossRef]
- Wang, D.K.; He, H.; Yu, P. Flame-retardant and thermal degradation mechanism of low-density polyethylene modified with aluminum hypophosphite and microencapsulated red phosphorus. J. Appl. Polym. Sci. 2016, 133, 43225. [Google Scholar] [CrossRef]
- Huo, S.Q.; Song, P.A.; Yu, B.; Ran, S.Y.; Chevali, V.S.; Liu, L.; Fang, Z.P.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent Advances and Future Perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. Melamine cyanurate-microencapsulated red phosphorus flame retardant unreinforced and glass fiber reinforced polyamide 66. Polym. Degrad. Stab. 2006, 91, 3103–3109. [Google Scholar] [CrossRef]
- Liu, J.; Guan, H.; Song, D.M. Preparation and characterization of microcapsulated red phosphorus and kinetic analysis of its thermal oxidation. Kinet. Catal. 2017, 58, 191–197. [Google Scholar] [CrossRef]
- Zhao, P.P.; Guo, C.G.; Li, L.P. Exploring the effect of melamine pyrophosphate and aluminum hypophosphite on flame retardant wood flour/polypropylene composites. Constr. Build. Mater. 2018, 170, 193–199. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.H.; Dong, H.X.; Wang, Y.H.; Qu, H.Q.; Xu, J.Z. Synergistic flame-retardant effects of aluminum phosphate and Trimer in ethylene–vinyl acetate composites. J. Therm. Anal. Calorim. 2018, 132, 919–926. [Google Scholar] [CrossRef]
- Tian, S.H.; He, H.; Wang, D.K.; Yu, P.; Jia, Y.C.; Luo, Y.F. Study of using aluminum hypophosphite as a flame retardant for low-density polyethylene. Fire Mate 2017, 41, 983–992. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Tang, G.; Jiang, S.H.; Gui, Z.; Hu, Y. Combination effect of MoS2 with aluminum hypophosphite in flame retardant ethylene-vinyl acetate composites. RSC Adv. 2016, 6, 37672–37680. [Google Scholar] [CrossRef]
- Yang, R.; Gu, G.Z.; Tang, C.; Miao, Z.C.; Cao, H.W.; Zou, G.X.; Li, J.C. Zhicheng Miao, Super-tough and flame-retardant poly(lactic acid) materials using a phosphorus-containing malic acid-based copolyester by reactive blending. Polym. Degrad. Stab. 2022, 198, 109889. [Google Scholar] [CrossRef]
- Hajj, R.; El Hage, R.; Sonnier, R.; Otazaghine, B.; Rouif, S.; Nakhl, M.; Lopez-Cuesta, J.M. Influence of lignocellulosic substrate and phosphorus flame retardant type on grafting yield and flame retardancy. React. Funct. Polym. 2020, 153, 104612. [Google Scholar] [CrossRef]
- Dai, X.Y.; Li, P.H.; Sui, Y.L.; Zhang, C.L. Thermal and flame-retardant properties of intrinsic flame-retardant epoxy resin containing biphenyl structures and phosphorus. Eur. Polym. J. 2021, 147, 110319. [Google Scholar] [CrossRef]
- Petersen, H.; Jakubowicz, I.; Enebro, J.; Yarahmadi, N. Organic modification of montmorillonite for application in plasticized PVC nanocomposites. Appl. Clay Sci. 2015, 107, 78–84. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.T.; Sin, L.T.; Rahmat, A.R. Polymer Nanocomposites based on Silylated-Montmorillonite: A Review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Qian, Y.; Zhu, X.Y.; Li, S.S.; Chen, X.L. Flame-retardant properties of ethylene-vinyl acetate/oil sludge/ fumed silica composites. RSC Adv. 2016, 6, 63091–63098. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, P.; Gao, C.; Wang, F.; Ding, Y.F.; Zhang, S.M.; Yang, M.S. Synergistic effect of cocondensed nanosilica in intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2019, 30, 1116–1125. [Google Scholar] [CrossRef]
- Castro-Landinez, J.F.; Salcedo-Galan, F.; Medina-Perilla, J.A. Polypropylene/Ethylene-And Polar-Monomer-Based Copolymers/Montmorillonite Nanocomposites: Morphology, Mechanical Properties, and Oxygen Permeability. Polymers 2021, 13, 705. [Google Scholar] [CrossRef]
- Arjmandi, R.; Balakrishnan, H.; Hassan, A.; Jawaid, M.; Othman, A.Y. Enhanced Flame Retardancy, Thermal and Mechanical Properties of Hybrid Magnesium Hydroxide/Montmorillonite Reinforced Polyamide 6/Polypropylene Nanocomposites. Fibers Polym. 2018, 19, 914–926. [Google Scholar] [CrossRef]
- Pang, H.C.; Wang, X.S.; Zhu, X.K.; Tian, P.; Ning, G.L. Nanoengineering of brucite@SiO2 for enhanced mechanical properties and flame retardant behaviors. Polym. Degrad. Stab. 2015, 120, 410–418. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Preparation and properties of highly conductive palmitic acid/graphene oxide composites as thermal energy storage materials. Energy 2013, 58, 628–634. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Gilman, J.W.; Butler, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame Retardant Mechanism of Silica Gel/Silica. Fire Mate. 2000, 24, 277–289. [Google Scholar] [CrossRef]
- Dong, Q.X.; Liu, M.M.; Ding, Y.F.; Wang, F.; Gao, C.; Liu, P.; Wen, B.; Zhang, S.M.; Yang, M.S. Synergistic effect of DOPO immobilized silica nanoparticles in the intumescent flame retarded polypropylene composites. Polym. Adv. Technol. 2013, 24, 732–739. [Google Scholar] [CrossRef]
- Ai, L.H.; Liu, J.B.; Chen, S.S.; Xu, Z.P.; Liu, P. Synthesis of melamine phenyl hypophosphite and its synergistic flame retardance with SiO2 on polypropylene. J. Therm. Anal. Calorim. 2021, 147, 6207–6217. [Google Scholar] [CrossRef]
- Zhou, S.J.; Qian, Y.; Chen, X.L.; Li, L. In situ synthesis of layered double hydroxides-silicon dioxide hybrids and its flame retardancy in EVA composites. J. Therm. Anal. Calorim. 2018, 134, 1071–1082. [Google Scholar] [CrossRef]
- Fu, M.Z.; Qu, B.J. Synergistic flame retardant mechanism of fumed silica in ethylene-vinyl acetate/magnesium hydroxide blends. Polym. Degrad. Stab. 2004, 85, 633–639. [Google Scholar] [CrossRef]
- Jiao, C.M.; Chen, X.L. Influence of fumed silica on the flame-retardant properties of ethylene vinyl acetate/aluminum hydroxide composites. J. Appl. Polym. Sci. 2011, 120, 1285–1289. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, G.L.; Jiang, P.K. Research on the related properties of EVM/Al(OH)3/SiO2 composites applied for halogen-free flame retardant cable insulation and jacket. J. Appl. Polym. Sci. 2011, 120, 368–378. [Google Scholar] [CrossRef]
- Ureyen, M.E.; Kaynak, E.; Yuksel, G. Flame-retardant effects of cyclic phosphonate with HALS and fumed silica in polypropylene. J. Appl. Polym. Sci. 2020, 137, 48308. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Wang, S.F.; Gui, Z.; Chen, Z.Y.; Fan, W.C. Intumescent flame retardant–montmorillonite synergism in polypropylene-layered silicate nanocomposites. Polym. Int. 2003, 52, 1396–1400. [Google Scholar] [CrossRef]
- Biao, Y.; Zeng, X.S.; Dai, G.J.; Cai, X.; Lin, Z.D.; Zhang, X.J.; Tan, S.Z.; Liu, Y.L. Combustion Behaviour and Morphology of Phosphonium Montmorillonite Filled Polypropylene/Magnesium Hydroxide Composites. Asian J. Chem. 2011, 23, 2069–2071. [Google Scholar]
- Xu, J.L.; Liu, X.Q.; Yang, W.L.; Niu, L.; Zhao, J.Q.; Ma, B.X.; Kang, C.H. Influence of montmorillonite on the properties of halogen–antimony flame retardant polypropylene composites. Polym. Compos. 2019, 40, 1930–1938. [Google Scholar] [CrossRef]
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.C. Polypropylene/Montmorillonite Nanocomposites. Review of the Synthetic Routes and Materials Properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Li, B.G.; Liu, L.; Wang, Z.Z.; Chen, Z.Y.; Fan, W.C. Polypropylene/montmorillonite nanocomposites and intumescent, flame-retardant montmorillonite synergism in polypropylene nanocomposites. J. Polym. Sci. A Polym. Chem. 2004, 42, 6163–6173. [Google Scholar] [CrossRef]
- Liu, L.; Wei, S.L.; Lai, X.J. In situ synthesis and characterization of polypropylene/polyvinyl acetate-organophilic montmorillonite nanocomposite. J. Appl. Polym. Sci. 2012, 124, 4107–4113. [Google Scholar] [CrossRef]
- Younis, A.A. Flammability properties of polypropylene containing montmorillonite and some of silicon compounds. Egypt. J. Pet. 2017, 26, 1–7. [Google Scholar] [CrossRef]
- Scarfato, P.; Incarnato, L.; Di Maio, L.; Dittrich, B.; Schartel, B. Influence of a novel organo-silylated clay on the morphology, thermal and burning behavior of low density polyethylene composites. Compos. B Eng. 2016, 98, 444–452. [Google Scholar] [CrossRef]
- Chang, M.K.; Hwang, S.S.; Liu, S.P. Flame retardancy and thermal stability of ethylene-vinyl acetate copolymer nanocomposites with alumina trihydrate and montmorillonite. J. Ind. Eng. Chem. 2014, 20, 1596–1601. [Google Scholar] [CrossRef]
- Yi, D.Q.; Ye, C.X.; Yang, R.J. Ammonium Polyphosphate-Calcium Montmorillonite Nanocompounds Flame Retarded Polypropylene. Polym. Mater. Sci. Eng. 2014, 30, 51–56. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, Q.; Kong, Q.H.; Zhang, X.G.; Li, Y.J.; Zhang, J.H. Synergistic effect of organophilic Fe-montmorillonite on flammability in polypropylene/intumescent flame retardant system. J. Therm. Anal. Calorim. 2014, 117, 693–699. [Google Scholar] [CrossRef]
- Zhu, S.P.; Chen, J.Y.; Li, H.L.; Cao, Y.; Yang, Y.H.; Feng, Z.H. Preparation and properties of montmorillonite/poly(ethylene glycol) grafted polypropylene/polypropylene nanocomposites. Appl. Clay Sci. 2014, 87, 303–310. [Google Scholar] [CrossRef]
- Liu, S.P. Flame retardant and mechanical properties of polyethylene/magnesium hydroxide/montmorillonite nanocomposites. J. Ind. Eng. Chem. 2014, 20, 2401–2408. [Google Scholar] [CrossRef]
- Lenza, J.; Merkel, K.; Rydarowski, H. Comparison of the effect of montmorillonite, magnesium hydroxide and a mixture of both on the flammability properties and mechanism of char formation of HDPE composite. Polym. Degrad. Stab. 2012, 97, 2581–2593. [Google Scholar] [CrossRef]
- Yi, D.Q.; Yang, R.J. Ammonium polyphosphate/montmorillonite nanocompounds in polypropylene. J. Appl. Polym. Sci. 2010, 118, 834–840. [Google Scholar] [CrossRef]
- Du, B.X.; Guo, Z.H.; Liu, H.; Fang, Z.P.; Wu, Y. Flame retardant mechanism of organo-bentonite in polypropylene. Appl. Clay Sci. 2009, 45, 178–184. [Google Scholar] [CrossRef]
- Haurie, L.; Fernandez, A.I.; Velasco, J.I.; Chimenos, J.M.; Cuesta, J.M.; Espiell, F. Thermal stability and flame retardancy of LDPE/EVA blends filled with synthetic hydromagnesite/aluminium hydroxide/montmorillonite and magnesium hydroxide/aluminium hydroxide/montmorillonite mixtures. Polym. Degrad. Stab. 2007, 92, 1082–1087. [Google Scholar] [CrossRef]
- Li, D.S.; Liu, L.B.; Zhang, Z.Y.; Xu, M.J.; Xu, Y.; Qian, L.J. An urethane-based phosphonate ester for improving flame retardancy and smoke suppression of thermoplastic polyurethane. Polym. Degrad. Stab. 2020, 188, 109568. [Google Scholar] [CrossRef]
- Tian, N.N.; Wen, X.; Jiang, Z.W.; Gong, J.; Wang, Y.H.; Xue, J.; Tang, T. Synergistic Effect between a Novel Char Forming Agent and Ammonium Polyphosphate on Flame Retardancy and Thermal Properties of Polypropylene. Ind. Eng. Chem. Res. 2013, 52, 10905–10915. [Google Scholar] [CrossRef]
- Lv, Q.; Huang, J.Q.; Chen, M.J.; Zhao, J.; Tan, Y.; Chen, L.; Wang, Y.Z. An Effective Flame Retardant and Smoke Suppression Oligomer for Epoxy Resin. Ind. Eng. Chem. Res. 2013, 52, 9397–9404. [Google Scholar] [CrossRef]
- Qian, L.J.; Ye, L.; Xu, G.Z.; Liu, J.; Guo, J.Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym. Degrad. Stab. 2011, 96, 1118–1124. [Google Scholar] [CrossRef]
- Tao, K.; Li, J.; Xu, L.; Zhao, X.L.; Xue, L.X.; Fan, X.Y.; Yan, Q. A novel phosphazene cyclomatrix network polymer: Design, synthesis and application in flame retardant polylactide. Polym. Degrad. Stab. 2011, 96, 1248–1254. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, X.; Yu, B.; Yuan, H.X.; Song, L.; Hu, Y.; Yuen, R.K.K.; Yeoh, G.H. A novel polyurethane prepolymer as toughening agent: Preparation, characterization, and its influence on mechanical and flame retardant properties of phenolic foam. J. Appl. Polym. Sci. 2013, 128, 2720–2728. [Google Scholar] [CrossRef]
- Ye, J.H.; Liang, G.Z.; Gu, A.J.; Zhang, Z.Y.; Han, J.P.; Yuan, L. Novel phosphorus-containing hyperbranched polysiloxane and its high performance flame retardant cyanate ester resins. Polym. Degrad. Stab. 2013, 98, 597–608. [Google Scholar] [CrossRef]
- Duquesne, S.; Lefebvre, J.; Seeley, G.; Camino, G.; Delobel, R.; Le Bras, M. Vinyl acetate/butyl acrylate copolymers-Part 2: Fire Retardancy using Phosphorus. Polym. Degrad. Stab. 2004, 85, 883–892. [Google Scholar] [CrossRef]
- Laoutid, F.; Ferry, L.; Lopez-Cuesta, J.M.; Crespy, A. Red phosphorus/aluminium oxide compositions as flame retardants in recycled poly(ethylene terephthalate). Polym. Degrad. Stab. 2003, 82, 357–363. [Google Scholar] [CrossRef]
- Verkade, J. Reynolds, L. Notes-The Synthesis of a Novel Ester of Phosphorus and of Arsenic. J. Org. Chem. 1960, 25, 663–665. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.Y.; Lu, H.D.; Lv, P.; Jie, G.X. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Li, X.; Ou, Y.X.; Zhang, Y.H.; Lian, D.J. Synthesis and structure of a novel caged bicyclic phosphate flame retardant. Chin. Chem. Lett. 2000, 10, 887–890. [Google Scholar]
- Jiang, W.Z.; Hao, J.W.; Han, Z.D. Study on the thermal degradation of mixtures of ammonium polyphosphate and a novel caged bicyclic phosphate and their flame retardant effect in polypropylene. Polym. Degrad. Stab. 2012, 97, 632–637. [Google Scholar] [CrossRef]
- Wang, L.C.; Jiang, J.Q.; Jiang, P.K.; Yu, J.H. Synthesis, characteristic of a novel flame retardant containing phosphorus and its application in poly(ethylene-co-vinyl acetate). Fire Mater. 2011, 35, 193–207. [Google Scholar] [CrossRef]
- Rothemund, S.; Teasdale, I. Preparation of polyphosphazenes: A tutorial review. Chem. Soc. Rev. 2016, 45, 5200–5215. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, W.H.; Qu, H.Q.; Xu, J.Z. Synthesis of a novel phosphazene derivative and its application in intumescent flame retardant-EVA copolymer composites. Mater. Lett. 2015, 160, 282–285. [Google Scholar] [CrossRef]
- Ciesielski, M.; Burk, B.; Heinzmann, C.; Doring, M. Fire-retardant high-performance epoxy-based materials. In Novel Fire Retardant Polymers and Composite Materials; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–51. [Google Scholar] [CrossRef]
- Chen, C.H.; Chiang, C.L. Preparation and Characteristics of an Environmentally Friendly Hyperbranched Flame-Retardant Polyurethane Hybrid Containing Nitrogen, Phosphorus, and Silicon. Polymers 2019, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-Cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Novel flame retardancy effects of DOPO-POSS on epoxy resins. Polym. Degrad. Stab. 2011, 96, 2167–2173. [Google Scholar] [CrossRef]
- Liu, L.C.; Zhang, W.C.; Yang, R.J. Flame retardant epoxy composites with epoxy-containing polyhedral oligomeric silsesquioxanes. Polym. Adv. Technol. 2020, 31, 2058–2074. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Vu, C.M.; Thi, H.V. MWCNT grafted with POSS-based novel flame retardant-filled epoxy resin nanocomposite: Fabrication, mechanical properties, and flammability. Compos. Interf. 2020, 28, 843–858. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Han, Z.Q.; Wang, Q. A coating method combined with bulk addition for efficient flame retardant thermoplastic polyolefin sheet material. Polym. Degrad. Stab. 2020, 174, 109093. [Google Scholar] [CrossRef]
- Wang, X.; Feng, N.; Chang, S.Q.; Zhang, G.X.; Li, H.; Lv, H. Intumescent flame retardant TPO composites: Flame retardant properties and morphology of the charred layer. J. Appl. Polym. Sci. 2012, 124, 2071–2079. [Google Scholar] [CrossRef]
- Guo, K.Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.D.; Zhao, L.; Gao, J.F.; Song, P.G.; Tang, L.C. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response. Compos. B Eng. 2020, 193, 108017. [Google Scholar] [CrossRef]
- Liu, L.B.; Xu, Y.; Li, S.; Xu, M.J.; He, Y.T.; Shi, Z.X.; Li, B. A novel strategy for simultaneously improving the fire safety, water resistance and compatibility of thermoplastic polyurethane composites through the construction of biomimetic hydrophobic structure of intumescent flame retardant synergistic system. Compos. B Eng. 2019, 176, 107218. [Google Scholar] [CrossRef]
- Liu, X.D.; Sun, J.; Zhang, S.; Guo, J.; Tang, W.F.; Li, H.F.; Gu, X.Y. Effects of carboxymethyl chitosan microencapsulated melamine polyphosphate on the flame retardancy and water resistance of thermoplastic polyurethane. Polym. Degrad. Stab. 2019, 160, 168–176. [Google Scholar] [CrossRef]
- Jia, Y.W.; Zhao, X.; Fu, T.; Li, D.F.; Guo, Y.; Wang, X.L.; Wang, Y.Z. Synergy effect between quaternary phosphonium ionic liquid and ammonium polyphosphate toward flame retardant PLA with improved toughness. Compos. B Eng. 2020, 197, 108192. [Google Scholar] [CrossRef]
- Li, Y.C.; Xue, B.Q.; Qi, P.; Gu, X.Y.; Sun, J.; Li, H.F.; Lin, J.Y.; Zhang, S. The synergistic effect between bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate and polysiloxane on the photo-aging resistance and flame retardancy of polypropylene. Compos. B Eng. 2022, 234, 109666. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Lu, X.; Yang, H.Y.; Xin, Z. Vinyl polysiloxane microencapsulated ammonium polyphosphate and its application in flame retardant polypropylene. J. Polym. Res. 2018, 25, 107. [Google Scholar] [CrossRef]
- Xu, J.C.; Ou, H.X.; Shan, X.Y.; Liu, B.; Jiang, J.C.; Xu, G.G. Investigation of novel intumescent flame retardant low-density polyethylene based on SiO2@MAPP and double pentaerythritol. J. Appl. Polym. Sci. 2020, 137, e49242. [Google Scholar] [CrossRef]
- Chen, X.S.; Ma, Y.H.; Cheng, Y.J.; Zhang, A.Q.; Liu, W.; Zhou, H.F. Synergistic effect between a novel silane-containing hyperbranched polyphosphamide and ammonium polyphosphate on the flame retardancy and smoke suppression of polypropylene composites. Polym. Degrad. Stab. 2020, 181, 109348. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The effects of POSS particles on the flame retardancy of intumescent polypropylene composites and the structure-property relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Jin, Q.; Zhang, N.E.; Guo, X.R.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Lai, X.J.; Yin, C.Y.; Li, H.Q.; Zeng, X.R. Synergistic effect between silicone-containing macromolecular charring agent and ammonium polyphosphate in flame retardant polypropylene. J. Appl. Polym. Sci. 2015, 132, 41580. [Google Scholar] [CrossRef]
- Deng, C.L.; Du, S.L.; Zhao, J.; Shen, Z.Q.; Deng, C.; Wang, Y.Z. An intumescent flame retardant polypropylene system with simultaneously improved flame retardancy and water resistance. Polym. Degrad. Stab. 2014, 108, 97–107. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.K.; Hao, J.W.; Du, J.X. Preparation of hybrid phosphamide containing polysilsesquioxane and its effect on flame retardancy and mechanical properties of polypropylene composites. Compos. B Eng. 2013, 45, 1541–1547. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.K.; Zhao, X.M.; Yu, H.Z. Synthesis of a novel hybrid synergistic flame retardant and its application in PP/IFR. Polym. Degrad. Stab. 2011, 96, 1134–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cao, Z.H.; Peng, M.; Fang, Z.P. Synthesis of novel poly(aminophosphonate ester)s flame retardants and their applications in EVA copolymer. Polym. Adv. Technol. 2013, 24, 197–203. [Google Scholar] [CrossRef]
- Chiu, S.H.; Wang, W.K. Dynamic flame retardancy of polypropylene filled with ammonium polyphosphate, pentaerythritol and melamine additives. Polymer 1998, 39, 1951–1955. [Google Scholar] [CrossRef]
- Camino, G.; Martinasso, G.; Costa, L.; Gobetto, R. Thermal degradation of pentaerythritol diphosphate, model compound for fire retardant intumescent systems: Part II-Intumescence step. Polym. Degrad. Stab. 1990, 28, 17–38. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers: Part II-Mechanism of action in polypropylene-ammonium polyphosphate-pentaerythritol mixtures. Polym. Degrad. Stab. 1984, 7, 25–31. [Google Scholar] [CrossRef]
- Dong, L.P.; Deng, C.; Li, R.M.; Cao, Z.J.; Lin, L.; Chen, L.; Wang, Y.Z. Poly(piperazinyl phosphamide): A novel highly-efficient charring agent for an EVA/APP intumescent flame retardant system. RSC Adv. 2016, 6, 30436–30444. [Google Scholar] [CrossRef]
- Fukuda, T.; Nakatani, K.; Suzuki, J.; Endo, S.; Ochiai, G. Fire-Resistant Polyolefin Compositions with Reduced Smoke. Patent Number JP 06025476, 28 July 2003. [Google Scholar]
- Ye, L.; Meng, X.Y.; Ji, X.; Li, Z.M.; Tang, J.H. Synthesis and characterization of expandable graphite–poly(methyl methacrylate) composite particles and their application to flame retardation of rigid polyurethane foams. Polym. Degrad. Stab. 2009, 94, 971–979. [Google Scholar] [CrossRef]
- Delobel, R.; Bras, M.L.; Ouassou, N.; Alistiqsa, F. Thermal behaviours of ammonium polyphosphate-pentaerythritol and ammonium pyrophosphate-pentaerythritol in-tumescent additives in polypropylene formulations. J. Fire Sci. 1990, 8, 85–108. [Google Scholar] [CrossRef]
- Santosh, K.; Zhang, W.P.; Saad, A.; Muhammad, A.; Xu, S.A. Effects of intumescent flame retardant system consisting of tris (2-hydroxyethyl) isocyanurate and ammonium polyphosphate on the flame retardant properties of high-density polyethylene composites. Compos. A Appl. Sci. Manuf. 2018, 112, 444–451. [Google Scholar] [CrossRef]
- Wang, B.B.; Sheng, H.B.; Shi, Y.Q.; Hu, W.Z.; Hong, N.N.; Zeng, W.R.; Ge, H.; Yu, X.J.; Song, L.; Hu, Y. Recent advances for microencapsulation of flame retardant. Polym. Degrad. Stab. 2015, 113, 96–109. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.N.; Li, L.; Wang, Z.Z. Microencapsulated ammonium polyphosphate with epoxy resin shell: Preparation, characterization, and application in EP system. Polym. Adv. Technol. 2011, 22, 2403–2408. [Google Scholar] [CrossRef]
- Li, R.M.; Deng, C.; Deng, C.L.; Dong, L.P.; Di, H.W.; Wang, Y.Z. An efficient method to improve simultaneously the water resistance, flame retardancy and mechanical properties of POE intumescent flame-retardant systems. RSC Adv. 2015, 5, 16328–16339. [Google Scholar] [CrossRef]
- Cao, K.; Wu, S.L.; Wang, K.L.; Yao, Z. Kinetic Study on Surface Modification of Ammonium Polyphosphate with Melamine. Ind. Eng. Chem. Res. 2011, 50, 8402–8406. [Google Scholar] [CrossRef]
- Wang, B.B.; Tang, Q.B.; Hong, N.N.; Song, L.; Wang, L.; Shi, Y.Q.; Hu, Y. Effect of Cellulose Acetate Butyrate Microencapsulated Ammonium Polyphosphate on the Flame Retardancy, Mechanical, Electrical, and Thermal Properties of Intumescent Flame-Retardant Ethylene-Vinyl Acetate Copolymer/Microencapsulated Ammonium Polyphosphate/Polyamide-6 Blends. ACS Appl. Mater. Interfaces 2011, 3, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Giraud, S.; Salaün, F.; Bourbigot, S. Polypropylene fabrics padded with microencapsulated ammonium phosphate: Effect of the shell structure on the thermal stability and fire performance. Polym. Degrad. Stab. 2010, 95, 1716–1720. [Google Scholar] [CrossRef]
- Lai, X.J.; Zeng, X.R.; Li, H.Q.; Yin, C.Y.; Zhang, H.L.; Liao, F. Synergistic effect of phosphorus-containing nanosponges on intumescent flame-retardant polypropylene. J. Appl. Polym. Sci. 2012, 125, 1758–1765. [Google Scholar] [CrossRef]
- Zhang, N.E.; Zhang, J.; Yan, H.; Guo, X.R.; Sun, Q.; Guo, R.J. A novel organic-inorganic hybrid K-HBPE@APP performing excellent flame retardancy and smoke suppression for polypropylene. J. Hazard. Mater. 2019, 373, 856–865. [Google Scholar] [CrossRef]
- Wang, S.H.; Jiang, Y.C.; Meng, Y.J.; Li, Y.C.; Han, Z.Q.; Liu, X.D.; Li, H.F.; Sun, J.; Fei, B.; Gu, X.Y. The encapsulation of intumescent flame retardants by poly-siloxane for thermoplastic polyolefin: Fire safety and water resistance. Polym. Degrad. Stab. 2021, 188, 109561. [Google Scholar] [CrossRef]
- Demir, H.; Balkose, D.; Ulku, S. Influence of surface modification of fillers and polymer on flammability and tensile behaviour of polypropylene-composites. Polym. Degrad. Stab. 2006, 91, 1079–1085. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiao, C.M.; Zhang, J. Microencapsulation of ammonium polyphosphate with hydroxyl silicone oil and its flame retardance in thermoplastic polyurethane. J. Therm. Anal. Calorim. 2011, 104, 1037–1043. [Google Scholar] [CrossRef]
- Shi, X.; Pan, Y.; Wang, Y.; Jia, Z.M.; Chen, T.T.; Gong, J.H.; Jiang, J.C. Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymer 2021, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Yan, H.; Liu, B.; Wei, L.Q.; Xu, B.S. The influence of KH-550 on properties of ammonium polyphosphate and polypropylene flame retardant composites. Polym. Degrad. Stab. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, D.D.; Li, W.M.; Cai, X.F. Flame retardant efficiency of KH-550 modified urea-formaldehyde resin cooperating with ammonium polyphosphate on polypropylene. Polym. Degrad. Stab. 2018, 151, 160–171. [Google Scholar] [CrossRef]
- Guo, X.R.; Geng, J.T.; Sun, B.; Xu, Q.; Li, Y.B.; Xie, S.W.; Xue, Y.Y.; Yan, H. Great enhancement of efficiency of intumescent flame retardants by titanate coupling agent and polysiloxane. Polym. Adv. Technol. 2020, 32, 41–53. [Google Scholar] [CrossRef]
- Sun, Y.R.; Yuan, B.H.; Shang, S.; Zhang, H.M.; Shi, Y.Q.; Yu, B.; Qi, C.R.; Dong, H.R.; Chen, X.F.; Yang, X.L. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. B Eng. 2019, 181, 107588. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Liao, C.C.; Xia, Y.R.; Liu, Y.H.; Dai, B.Y.; Li, A.B. Co-microencapsulation of biomass-based char source and melamine polyphosphate and investigation for their synergistic action in flame-retarding polypropylene. Polym. Test. 2020, 90, 106741. [Google Scholar] [CrossRef]
- Yu, S.W.; Xiao, S.J.; Zhao, Z.W.; Huo, X.W.; Wei, J.F. Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene. Chin. J. Chem. Eng. 2019, 27, 1735–1743. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, Z.L.; Wang, Y.H.; Jin, Q.; Zhang, X.Y. Structural modification of ammonium polyphosphate by DOPO to achieve high water resistance and hydrophobicity. Powder Technol. 2017, 320, 14–21. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Qiang, L.H.; Yang, T.; Wang, B.N.; Cui, X.J.; Wang, H.Y. Preparation of microencapsulated ammonium polyphosphate with carbon source- and blowing agent-containing shell and its flame retardance in polypropylene. J. Polym. Res. 2014, 21, 1–15. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, W.L.; Zhou, J.; Li, H.L.; Wang, X.L.; Chen, X.D.; Zhang, Z.Y. Effects of microencapsulated APP-II on the microstructure and flame retardancy of PP/APP-II/PER composites. Polym. Degrad. Stab. 2014, 105, 150–159. [Google Scholar] [CrossRef]
- Shao, Z.B.; Deng, C.; Tan, Y.; Chen, M.J.; Chen, L.; Wang, Y.Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Feng, C.M.; Zhang, Y.; Liu, S.W.; Chi, Z.G.; Xu, J.R. Synergistic effect of La2O3 on the flame retardant properties and the degradation mechanism of a novel PP/IFR system. Polym. Degrad. Stab. 2012, 97, 707–714. [Google Scholar] [CrossRef]
- Feng, C.M.; Zhang, Y.; Liang, D.; Liu, S.W.; Chi, Z.G.; Xu, J. Flame retardancy and thermal degradation behaviors of polypropylene composites with novel intumescent flame retardant and manganese dioxide. J. Anal. Appl. Pyrolysis 2013, 104, 59–67. [Google Scholar] [CrossRef]
- Feng, C.M.; Liang, M.Y.; Jiang, J.L.; Zhang, Y.K.; Huang, J.G.; Liu, H.B. Synergism effect of CeO2 on the flame retardant performance of intumescent flame retardant polypropylene composites and its mechanism. J. Anal. Appl. Pyrolysis 2016, 122, 405–414. [Google Scholar] [CrossRef]
- Qin, Z.L.; Li, D.H.; Li, Q.; Yang, R.J. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Zhu, X.D.; Xin, F.; Qian, L.J.; Liu, J.P. Research Progress of Catalytic Flame Retardant Polymers. J. Mater. Eng. 2018, 46, 14–22. [Google Scholar] [CrossRef]
- Chen, K.; Cheng, J.W.; Wu, B.; Liu, C.J.; Guo, J.N. Synergistic effects of strontium carbonate on a novel intumescent flame-retardant polypropylene system. Polym. Adv. Technol. 2021, 32, 3018–3027. [Google Scholar] [CrossRef]
- Feng, J.B.; Sun, Y.Q.; Song, P.G.; Lei, W.W.; Wu, Q.; Liu, L.N.; Yu, Y.M.; Wang, H. Fire-Resistant, Strong, and Green Polymer Nanocomposites Based on Poly(lactic acid) and Core–Shell Nanofibrous Flame Retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Song, P.A.; Wang, C.; Chen, L.; Zheng, Y.Q.; Liu, L.N.; Wu, Q.; Huang, G.B.; Yu, Y.M.; Wang, H. Thermally stable, conductive and flame-retardant nylon 612 composites created by adding two-dimensional alumina platelets. Compos. A Appl. Sci. Manuf. 2017, 97, 100–110. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Groth, K.; Harris, R.; Butler, K.; Shields, J.; Kharchenko, S.; Douglas, J. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymer 2004, 45, 4227–4239. [Google Scholar] [CrossRef]
- Song, P.A.; Liu, L.N.; Huang, G.B.; Fu, S.Y.; Yu, Y.M.; Guo, Q.P. Facile Fabrication of Polyolefin/Carbon Nanotube Composites via in Situ Friedel-Crafts Polyalkylation: Structure and Properties. Ind. Eng. Chem. Res. 2013, 52, 14384–14395. [Google Scholar] [CrossRef]
- Fang, F.; Ran, S.Y.; Fang, Z.P.; Song, P.A.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Composites 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Song, P.A.; Cao, Z.H.; Cai, Y.Z.; Zhao, L.P.; Fang, Z.P.; Fu, S.Y. Fabrication of exfoliated graphene-based polypropylene nanocomposites with enhanced mechanical and thermal properties. Polymer 2011, 52, 4001–4010. [Google Scholar] [CrossRef]
- Xia, L.; Lv, Y.; Miao, Z.X.; Luo, L.L.; Luo, W.A.; Xu, Y.T.; Yuan, C.H.; Zeng, B.R.; Dai, L.Z. A flame retardant fabric nanocoating based on nanocarbon black particles@polymer composite and its fire-alarm application. Chem. Eng. J. 2021, 433, 133501. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Hofmann, D.; Mulhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stab. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Yang, H.F.; Gong, J.; Wen, X.; Xue, J.; Chen, Q.; Jiang, Z.W.; Tian, N.N.; Tang, T. Effect of carbon black on improving thermal stability, flame retardancy and electrical conductivity of polypropylene/carbon fiber composites. Compos. Sci. Technol. 2015, 113, 31–37. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.J.; Gong, J.; Liu, J.; Tian, N.N.; Wang, Y.H.; Jiang, Z.W.; Qiu, J.; Tang, T. Thermal and flammability properties of polypropylene/carbon black nanocomposites. Polym. Degrad. Stab. 2012, 97, 793–801. [Google Scholar] [CrossRef]
- He, W.T.; Song, P.A.; Yu, B.; Fang, Z.P.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Yang, H.F.; Guan, Y.Y.; Ye, L.; Wang, S.; Li, S.X.; Wen, X.; Chen, X.C.; Mijowska, E.; Tang, T. Synergistic effect of nanoscale carbon black and ammonium polyphosphate on improving thermal stability and flame retardancy of polypropylene: A reactive network for strengthening carbon layer. Compos. B Eng. 2019, 174, 107038. [Google Scholar] [CrossRef]
- Wen, X.; Szymanska, K.; Chen, X.C.; Mijowska, E. Nanosized carbon black as synergist in PP/POE-MA/IFR system for simultaneously improving thermal, electrical and mechanical properties. J. Therm. Anal. Calorim. 2019, 139, 1091–1098. [Google Scholar] [CrossRef]
- Walong, A.; Thongnuanchan, B.; Uthaipan, N.; Sakai, T.; Lopattananon, N. Enhancing cellular structure, mechanical properties, thermal stability and flame retardation of EVA/NR blend nanocomposite foams by silicon dioxide-based flame retardant. Prog. Rubber Plast. Recycl. Technol. 2021, 38, 70–88. [Google Scholar] [CrossRef]
- Wu, Z.H.; Qu, J.P.; Zhao, Y.Q.; Tang, H.L.; Wen, J.S. Flammable and mechanical effects of silica on intumescent flame retardant/ethylene-octene copolymer/polypropylene composites. J. Therm. Compos. Mater. 2015, 28, 981–994. [Google Scholar] [CrossRef]
- Yu, G.X.; Liu, Y.F.; Li, J. Preparation of urea formaldehyde microsphere and its effect on flame retardant ethylene vinyl acetate composites. Polym. Adv. Technol. 2018, 29, 1804–1814. [Google Scholar] [CrossRef]
- Wang, X.B.; Bi, B.; Liu, J.W.; Yang, S.S.; Zhou, L.; Lu, L.G.; Wang, Y.; Xu, F.; Huang, R. Halogen-free intumescent flame-retardant ethylene-vinyl acetate copolymer system based on organic montmorillonite and graphene nanosheets. J. Appl. Polym. Sci. 2018, 135, 46361. [Google Scholar] [CrossRef]
- Gao, S.L.; Li, B.; Bai, P.; Zhang, S.Q. Effect of polysiloxane and silane-modified SiO2 on a novel intumescent flame retardant polypropylene system. Polym. Adv. Technol. 2011, 22, 2609–2616. [Google Scholar] [CrossRef]
- Wen, P.Y.; Wang, D.; Liu, J.J.; Zhan, J.; Hu, Y.; Yuen, R.K.K. Organically modified montmorillonite as a synergist for intumescent flame retardant against the flammable polypropylene. Polym. Adv. Technol. 2016, 28, 679–685. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Zhang, M.; Yuan, G.W. Synergistic and Compatibilizing Effect of Octavinyl Polyhedral Oligomeric Silsesquioxane Nanoparticles in Polypropylene/Intumescent Flame Retardant Composite System. Compos. A Appl. Sci. Manuf. 2019, 123, 46–58. [Google Scholar] [CrossRef]
- Wang, S.H.; Li, J.S.; Wang, W.J.; Wang, X.G.; Li, H.F.; Sun, J.; Fei, B.; Gu, X.Y.; Zhang, S. Silicone filled halloysite nanotubes for polypropylene composites: Flame retardancy, smoke suppression and mechanical property. Part A Appl. Sci. Manuf. 2021, 140, 106170. [Google Scholar] [CrossRef]
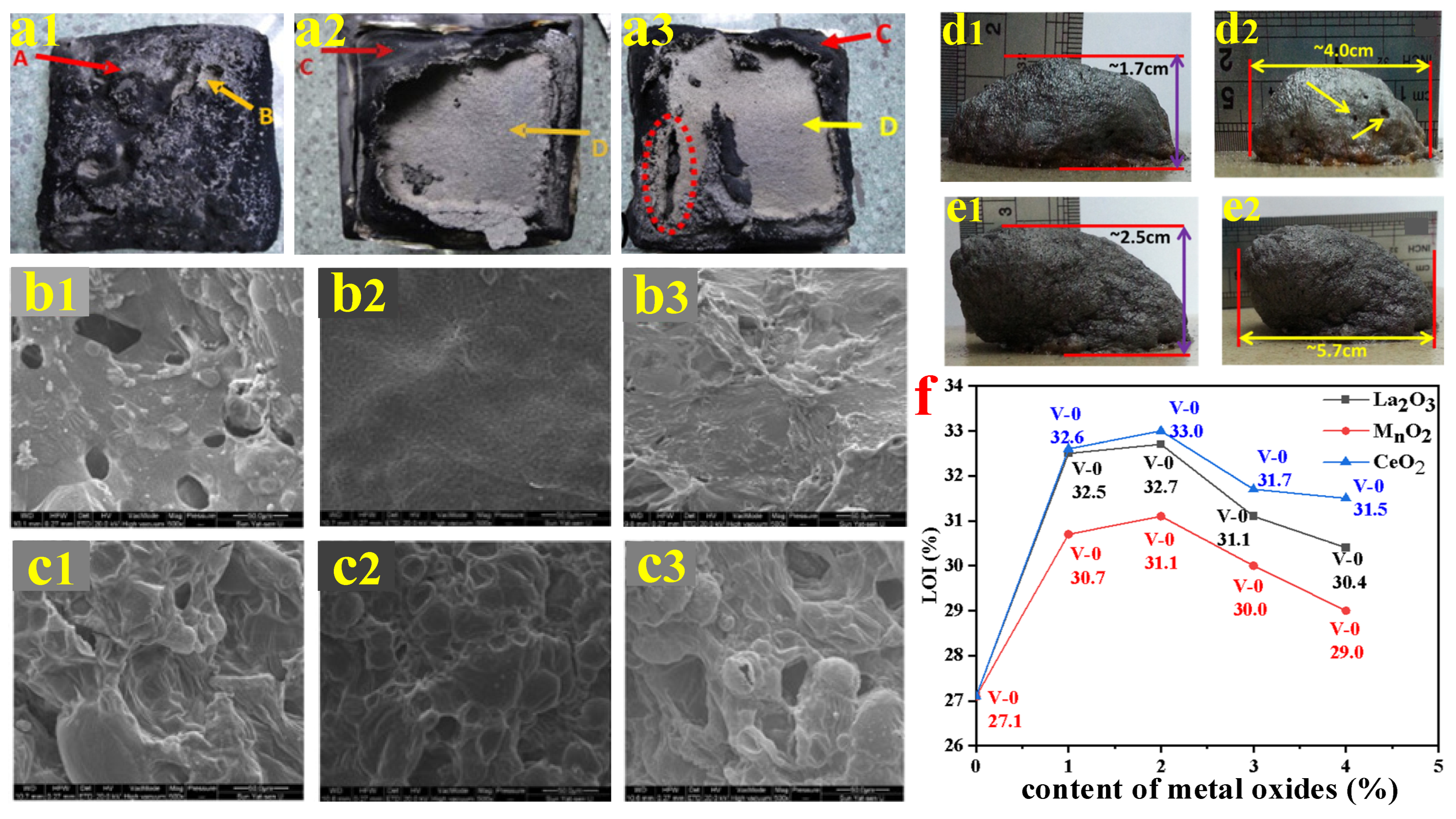
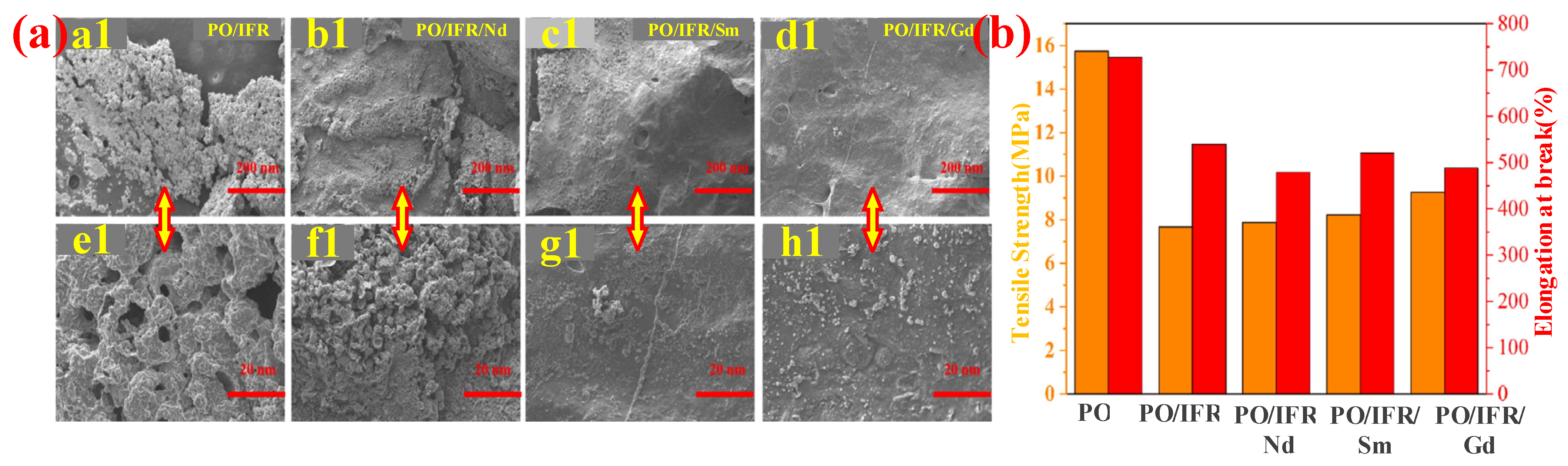
- Jia, P.F.; Yu, X.L.; Lu, J.Y.; Zhou, X.D.; Yin, Z.T.; Tang, G.; Lu, T.T.; Guo, L.; Song, L.; Wang, B.B. The Re2Sn2O7 (Re=Nd, Sm, Gd) on the enhancement of fire safety and physical performance of Polyolefin/IFR cable materials. J. Colloid Interface Sci. 2021, 608, 1652–1661. [Google Scholar] [CrossRef]
- Lu, J.Y.; Zhang, Y.; Tao, Y.J.; Wang, B.B.; Cheng, W.H.; Jie, G.X.; Song, L.; Hu, Y. Self-healable Castor Oil-based Waterborne Polyurethane/MXene Film with Outstanding Electromagnetic Interference Shielding Effectiveness and Excellent Shape Memory Performance. J. Colloid Interface Sci. 2020, 588, 164–174. [Google Scholar] [CrossRef]
- Lu, J.Y.; Cheng, W.H.; Shi, Y.Q.; Jia, P.F.; Liao, C.; Zhang, K.; Song, L.; Wang, B.B.; Hu, Y. A Facile Strategy for Lightweight, Anti-dripping, Flexible Polyurethane Foam with Low Smoke Emission Tendency and Superior Electromagnetic Wave Blocking. J. Colloid Interface Sci. 2021, 603, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, R.; Mu, J.J.; Ding, Y.M.; Jiang, J.C. Synergistic flame retardancy of linear low-density polyethylene with surface modified intumescent flame retardant and zinc borate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128400. [Google Scholar] [CrossRef]
- Tang, W.F.; Song, L.X.; Liu, F.; Dessie, W.; Qin, Z.D.; Zhang, S.; Gu, X.Y. Improving the flame retardancy and thermal stability of polypropylene composites via introducing glycine intercalated kaolinite compounds. Appl. Clay Sci. 2022, 217, 106411. [Google Scholar] [CrossRef]
- Zhang, M.D.; Zhang, W.C.; Chen, Y.H.; Yang, B. Preparation of efficiently intumescent-flame-retarded polypropylene composite: Synergistic effect of novel phosphorus-containing polyhedral oligomeric silsesquioxane. Plastics Rubber Compos. 2021, 50, 464–476. [Google Scholar] [CrossRef]
- Dang, L.; Lv, Z.H.; Liu, X. Influences of 4ZnO·B2O3·H2O whisker based intumescent flame retardant on the mechanical, flame retardant and smoke suppression properties of polypropylene composites. J. Appl. Polym. Sci. 2021, 138, e51016. [Google Scholar] [CrossRef]
- Xu, S.Y.; Zhou, C.; Li, J.X.; Shen, L.G.; Lin, H.J. Simultaneously improving mechanical strength, hydrophobic property and flame retardancy of ethylene vinyl acetate copolymer/intumescent flame retardant/FeOOH by introducing modified fumed silica. Mater. Today Commun. 2021, 26, 102114. [Google Scholar] [CrossRef]
- Ou, H.X.; Xu, J.C.; Liu, B.; Xue, H.L.; Weng, Y.X.; Jiang, J.C.; Xu, G.G. Study on synergistic expansion and flame retardancy of modified kaolin to low density polyethylene. Polymer 2021, 211, 123586. [Google Scholar] [CrossRef]
- Li, W.X.; Zhang, H.J.; Hu, X.P.; Yang, W.X.; Cheng, Z.; Xie, C.Q. Highly Efficient Replacement of Traditional Intumescent Flame Retardants in Polypropylene by Manganese Ions Doped Melamine Phytate Nanosheets. J. Hazard. Mater. 2020, 398, 123001. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.F.; Guo, J.; Jin, X.D.; Li, H.F.; Gu, X.Y.; Sun, J. Improvement of flame retardancy and thermal stability of polypropylene by P-type hydrated silica aluminate containing lanthanum. Polym. Degrad. Stab. 2018, 154, 276–284. [Google Scholar] [CrossRef]
- Ran, S.Y.; Guo, Z.H.; Fang, Z.P.; Li, J.; Wang, H. Improved thermal stability of polyethylene with rare earth trifluoromethanesulfonate. Compos. Commun. 2018, 8, 19–23. [Google Scholar] [CrossRef]
- Kong, Q.H.; Wu, T.; Zhang, H.K.; Zhang, Y.; Zhang, M.M.; Si, T.Y.; Yang, L.; Zhang, J. Improving flame retardancy of IFR/PP composites through the synergistic effect of organic montmorillonite intercalation cobalt hydroxides modified by acidified chitosan. Appl. Clay Sci. 2017, 146, 230–237. [Google Scholar] [CrossRef]
- Huang, P.K.; Pang, Y.Y.; Zhang, L.H.; Wu, F.; Zhang, S.H.; Zheng, W.G. A new approach designed for improving flame retardancy of intumescent polypropylene via continuous extrusion with supercritical CO2. RSC Adv. 2016, 6, 112184–112192. [Google Scholar] [CrossRef]
- Dong, X.; Nie, S.B.; Liu, Z.G.; Wang, D.Y. Study of the synergistic effect of nickel phosphate nanotubes (NiPO-NT) on intumescent flame retardant polypropylene composites. J. Therm. Anal. Calorim. 2016, 126, 1323–1330. [Google Scholar] [CrossRef]
- Shen, Y.L.; Gong, W.G.; Zheng, B.C.; Meng, X.; Gao, L. Synergistic effect of Ni-based bimetallic catalyst with intumescent flame retardant on flame retardancy and thermal stability of polypropylene. Polym. Degrad. Stab. 2016, 129, 114–124. [Google Scholar] [CrossRef]
- Makhlouf, G.; Hassan, M.; Nour, M.; Abdelmonem, Y.; Abdelkhalik, A. A Novel Intumescent Flame Retardant: Synthesis and Its Application for Linear Low-Density Polyethylene. Arab. J. Sci. Eng. 2017, 42, 4339–4349. [Google Scholar] [CrossRef]
- Wu, K.; Shen, M.M.; Hu, Y.A. Synthesis of a novel intumescent flame retardant and its flame retardancy in polypropylene. J. Polym. Res. 2011, 18, 425–433. [Google Scholar] [CrossRef]
- Song, P.A.; Fang, Z.P.; Tong, L.F.; Xu, Z.B. Synthesis of a novel oligomeric intumescent flame retardant and its application in polypropylene. Polym. Eng. Sci. 2009, 49, 1326–1331. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Liu, Y.H.; Dai, B.Y.; Meng, C.Y.; Guo, Z.X. Fabrication of cellulose-based halogen-free flame retardant and its synergistic effect with expandable graphite in polypropylene. Carbohydr. Polym. 2019, 213, 257–265. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Z.Y.; Deng, C.; Lu, P.; Zhao, P.P.; He, S.; Chen, S.W.; Lin, W. Facile Synthesis of Phytic Acid and Aluminum hydroxide Chelate-Mediated Hybrid Complex toward Fire Safety of Ethylene-Vinyl Acetate Copolymer. Polym. Degrad. Stab. 2021, 190, 109659. [Google Scholar] [CrossRef]
- Qi, H.S.; Liu, S.W.; Chen, X.L.; Shen, C.H.; Gao, S.J. The flame retardant and thermal performances of polypropylene with a novel intumescent flame retardant. J. Appl. Polym. Sci. 2020, 137, e49047. [Google Scholar] [CrossRef]
- Xia, S.Y.; Zhang, Z.Y.; Leng, Y.; Li, B.; Xu, M.J. Synthesis of a novel mono-component intumescent flame retardant and its high efficiency for flame retardant polyethylene. J. Anal. Appl. Pyrolysis 2018, 134, 632–640. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G.S. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2016, 138, 106–114. [Google Scholar] [CrossRef]
- Zhu, C.J.; He, M.S.; Liu, Y.; Cui, J.G.; Tai, Q.L.; Song, L.; Hu, Y. Synthesis and application of a mono-component intumescent flame retardant for polypropylene. Polym. Degrad. Stab. 2018, 151, 144–151. [Google Scholar] [CrossRef]
- Qin, Y.L.; Li, M.; Huang, T.K.; Shen, C.H.; Gao, S.J. A study on the modification of polypropylene by a star-shaped intumescent flame retardant containing phosphorus and nitrogen. Polym. Degrad. Stab. 2022, 195, 09801. [Google Scholar] [CrossRef]
- Pan, Y.T.; Luo, Z.L.; Wang, B.B. Synergistic flame retardant effect of piperazine salt and ammonium polyphosphate as intumescent flame retardant system for polypropylene. J. Appl. Polym. Sci. 2021, 138, e49813. [Google Scholar] [CrossRef]
- Yu, G.X.; Ma, C.; Li, J. Flame retardant effect of cytosine pyrophosphate and pentaerythritol on polypropylene. Compos. B Eng. 2020, 180, 107520. [Google Scholar] [CrossRef]
- Chen, X.S.; Ma, Y.H.; Cheng, Y.J.; Zhang, A.Q.; Liu, W. Enhanced mechanical and flame-resistant properties of polypropylene nanocomposites with reduced graphene oxide-functionalized ammonium polyphosphate and pentaerythritol. J. Appl. Polym. Sci. 2019, 136, 48036. [Google Scholar] [CrossRef]
- Dong, X.; Yang, J.N.; Hua, X.Z.; Nie, S.B.; Kong, F.B. Synthesis of a novel char-forming agent (PEIC): Improvement in flame retardancy, thermal stability, and smoke suppression for intumescent flame-retardant polypropylene composites. J. Appl. Polym. Sci. 2020, 137, 48296. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Y.J.; Zhou, F.; Sheng, H.B.; Qiu, S.L.; Ma, C.; Hu, Y. Synthesis of a hyperbranched phosphorus-containing polyurethane as char forming agent combined with ammonium polyphosphate for reducing fire hazard of polypropylene. Polym. Degrad. Stab. 2019, 165, 207–219. [Google Scholar] [CrossRef]
- Xu, B.; Wu, X.; Ma, W.; Qian, L.J.; Xin, F.; Qiu, Y. Synthesis and characterization of a novel organic-inorganic hybrid char-forming agent and its flame-retardant application in polypropylene composites. J. Anal. Appl. Pyrolysis 2018, 134, 231–242. [Google Scholar] [CrossRef]
- Lu, L.G.; Guo, N.; Qian, X.D.; Yang, S.S.; Wang, X.B.; Jin, J.; Shao, G.S. Thermal degradation and combustion behavior of intumescent flame-retardant polypropylene with novel phosphorus-based flame retardants. J. Appl. Polym. Sci. 2017, 135, 45962. [Google Scholar] [CrossRef]
- Wang, W.; Wen, P.Y.; Zhan, J.; Hong, N.N.; Cai, W.; Gui, Z.; Hu, Y. Synthesis of a novel charring agent containing pentaerythritol and triazine structure and its intumescent flame retardant performance for polypropylene. Polym. Degrad. Stab. 2017, 144, 454–463. [Google Scholar] [CrossRef]
- Ye, X.M.; Wang, Y.H.; Zhao, Z.L.; Yan, H. A novel hyperbranched poly(phosphorodiamidate) with high expansion degree and carbonization efficiency used for improving flame retardancy of APP/PP composites. Polym. Degrad. Stab. 2017, 142, 29–41. [Google Scholar] [CrossRef]
- Feng, C.M.; Liang, M.Y.; Jiang, J.; Huang, J.G.; Liu, H.B. Synergistic effect of a novel triazine charring agent and ammonium polyphosphate on the flame retardant properties of halogen-free flame retardant polypropylene composites. Thermochim. Acta 2016, 627, 83–90. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.J.; Li, B. Synthesis of N-methyl triazine-ethylenediamine copolymer charring foaming agent and its enhancement on flame retardancy and water resistance for polypropylene composites. Polym. Degrad. Stab. 2016, 131, 20–29. [Google Scholar] [CrossRef]
- Duan, L.J.; Yang, H.Y.; Song, L.; Hou, Y.B.; Wang, W.; Gui, Z.; Hu, Y. Hyperbranched phosphorus/nitrogen-containing polymer in combination with ammonium polyphosphate as a novel flame retardant system for polypropylene. Polym. Degrad. Stab. 2016, 134, 179–185. [Google Scholar] [CrossRef]
- Qin, Z.L.; Li, D.H.; Yang, R.J. Study on inorganic modified ammonium polyphosphate with precipitation method and its effect in flame retardant polypropylene. Polym. Degrad. Stab. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Feng, C.M.; Liang, M.Y.; Jiang, J.L.; Huang, J.G.; Liu, H.B. Preparation and characterization of oligomeric char forming agent and its effect on the thermal degradation and flame retardant properties of LDPE with ammonium polyphosphate. J. Anal. Appl. Pyrolysis 2016, 119, 75–86. [Google Scholar] [CrossRef]
- Feng, C.M.; Liang, M.Y.; Jiang, J.L.; Liu, H.B.; Huang, J.G. Synergistic effect of ammonium polyphosphate and triazine-based charring agent on the flame retardancy and combustion behavior of ethylene-vinyl acetate copolymer. J. Anal. Appl. Pyrolysis 2016, 119, 259–269. [Google Scholar] [CrossRef]
- Xie, H.L.; Lai, X.J.; Li, H.Q.; Zeng, X.R. Synthesis of a novel macromolecular charring agent with free-radical quenching capability and its synergism in flame retardant polypropylene. Polym. Degrad. Stab. 2016, 130, 68–77. [Google Scholar] [CrossRef]
- Wen, P.Y.; Feng, X.M.; Kan, Y.C.; Hu, Y.; Yuen, R.K.K. Synthesis of a novel triazine-based polymeric flame retardant and its application in polypropylene. Polym. Degrad. Stab. 2016, 134, 202–210. [Google Scholar] [CrossRef]
- Yang, R.; Qiao, H.; Hu, W.; Xu, L.; Song, Y.; Li, J. Synthesis, physical-mechanical properties and fire behaviors of polyurethane foam with reactive flame retardant and expandable graphite. CIESC J. 2016, 67, 2169–2175. [Google Scholar] [CrossRef]
- Li, G.X.; Liang, G.Z.; He, T.S.; Yang, Q.L.; Song, X.F. Effects of EG and MoSi2 on thermal degradation of intumescent coating. Polym. Degrad. Stab. 2007, 92, 569–579. [Google Scholar] [CrossRef]
- Li, J.; Mo, X.H.; Li, Y.; Zou, H.W.; Liang, M.; Chen, Y. Influence of expandable graphite particle size on the synergy flame retardant property between expandable graphite and ammonium polyphosphate in semi-rigid polyurethane foam. Polym. Bull. 2018, 75, 5287–5304. [Google Scholar] [CrossRef]
- Guo, B.Y.; Zhang, T.Q.; Zhang, W.X.; Dou, Y.L. Influence of surface flame-retardant layer containing ammonium polyphosphate and expandable graphite on the performance of jute/polypropylene composites. J. Therm. Anal. Calorim. 2018, 135, 2367–2375. [Google Scholar] [CrossRef]
- Wang, S.W.; Shi, M.N.; Yang, W.M.; Yan, H.; Zhang, C.; An, Y.; Zhang, F.H. Experimental investigation of flame retardancy and mechanical properties of APP/EG/TPU multilayer composites prepared by microlayer coextrusion technology. J. Appl. Polym. Sci. 2020, 138, e50219. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, F.; Dong, Q.X.; Yuan, W.J.; Liu, P.; Ding, Y.F.; Zhang, S.M.; Yang, M.S.; Zheng, G.Q. Expandable graphite encapsulated by magnesium hydroxide nanosheets as an intumescent flame retardant for rigid polyurethane foams. J. Appl. Polym. Sci. 2018, 135, 46749. [Google Scholar] [CrossRef]
- Zhou, R.; Ming, Z.; He, J.P.; Ding, Y.M.; Jiang, J.C. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/HDPE Phase Change Blends. Polymer 2020, 12, 180. [Google Scholar] [CrossRef]
- Ning, H.Z.; Ma, Z.Y.; Zhang, Z.H.; Zhang, D.; Wang, Y.H. Core–shell expandable graphite@layered double hydroxide as a flame retardant for polyvinyl alcohol. J. Therm. Anal. Calorim. 2021, 147, 6249–6258. [Google Scholar] [CrossRef]
- Ye, L.; Ding, P.; Zhang, M.; Qu, B.J. Synergistic effects of exfoliated LDH with some halogen-free flame retardants in LDPE/EVA/HFMH/LDH nanocomposites. J. Appl. Polym. Sci. 2010, 107, 3694–3701. [Google Scholar] [CrossRef]
- Yang, J.J.; Chen, X.H.; Zhou, H.L.; Guo, W.C.; Zhang, J.; Miao, Z.; He, D.H. Synergistic effect of expandable graphite and aluminum hypophosphite in flame-retardant ethylene vinyl acetate composites. Polym. Adv. Technol. 2021, 33, 638–646. [Google Scholar] [CrossRef]
- Guo, X.C.; Liu, N.; Li, L.T.; Bai, Z.Y.; Chen, X.L.; Zhou, D.F.; Qin, J.; Zhang, K.; Lu, Z.C. The synergistic flame retardancy of modified expandable graphite and metal hydroxides on HDPE/EVA composites. J. Therm. Compos. Mater. 2020, 35, 782–798. [Google Scholar] [CrossRef]
- Thi, N.H.; Nguyen, T.N.; Oanh, H.T.; Trang, N.T.T.; Tham, D.Q.; Nguyen, H.T.; Nguyen, T.V.; Hoang, M.H. Synergistic effects of aluminum hydroxide, red phosphorus, and expandable graphite on the flame retardancy and thermal stability of polyethylene. J. Appl. Polym. Sci. 2020, 138, 50317. [Google Scholar] [CrossRef]
- Liu, N.; Wang, N.; Li, L.T.; He, W.D.; Guo, J.B.; Chen, X.L.; Zhang, K.; Wu, H. Modified expandable graphite as an effective flame retardant for LLDPE/EVA composites filled with Mg(OH)2/Al(OH)3. J. Therm. Compos. Mater. 2019, 33, 938–955. [Google Scholar] [CrossRef]
- Qin, J.; Liu, N.; Wang, N.; Li, L.T.; He, W.D.; Guo, J.B.; Chen, X.L.; Zhang, K.; Yu, J. Synergistic Effect of Modified Expanded Graphite and Zinc Borate on the Flammability, Thermal Stability and Crystallization Behavior of LLDPE/EVA Composites with Mg(OH)2/Al(OH)3. Polym. Compos. 2018, 40, E687–E694. [Google Scholar] [CrossRef]
- Qi, F.; Tang, M.Q.; Wang, N.; Liu, N.; Chen, X.L.; Zhang, Z.B.; Zhang, K.; Lu, X. Efficient organic–inorganic intumescent interfacial flame retardants to prepare flame retarded polypropylene with excellent performance. RSC Adv. 2017, 7, 31696–31706. [Google Scholar] [CrossRef]
- Yang, Z.W.; Liang, X.X.; Xu, X.Q.; Lei, C.; He, X.L.; Song, T.; Huo, W.Y.; Ma, H.C.; Lei, Z.Q. PGS@B-N: An Efficient Flame Retardant to Improve Simultaneously the Interfacial Interaction and the Flame Retardancy of EVA. RSC Adv. 2016, 6, 65921–65929. [Google Scholar] [CrossRef]
- Pang, X.Y.; Tian, Y.; Shi, X.Z. Synergism between hydrotalcite and silicate-modified expandable graphite on ethylene vinyl acetate copolymer combustion behavior. J. Appl. Polym. Sci. 2016, 134, 44634. [Google Scholar] [CrossRef]
- Pang, X.Y.; Tian, Y.; Weng, M.Q. Preparation of expandable graphite with silicate assistant intercalation and its effect on flame retardancy of ethylene vinyl acetate composite. Polym. Compos. 2015, 36, 1407–1416. [Google Scholar] [CrossRef]
- Sun, Z.D.; Ma, Y.H.; Xu, Y.; Chen, X.L.; Chen, M.; Yu, J.; Hu, S.C.; Zhang, Z.B. Effect of the Particle Size of Expandable Graphite on the Thermal Stability, Flammability, and Mechanical Properties of High-Density Polyethylene/Ethylene Vinyl-Acetate/Expandable Graphite Composites. Polym. Eng. Sci. 2014, 54, 1162–1169. [Google Scholar] [CrossRef]





| PO Matrix | SiO2 (wt.%) a | Other FRs (wt.%) | Flame Retardancy () b | Mechanical Property () b | Reference |
|---|---|---|---|---|---|
| EVA | SiO2 (1.5) | layered double hydroxides (LDH) (48.5) | LOI: 30.8% (28.3%) | [63] | |
| EVA | SiO2 (5.0) | MH (55.0) | UL-94 (3.0 mm) c: V-0 (V-0) LOI: 39.0% (35%) | σt: 11.1 MPa (10.4 MPa) εb: 70.0% (75.0%) | [64] |
| EVA | SiO2 (2.0) | ATH (53.0) | UL-94 (3.0 mm): No rating, nodripping (No rating, dripping) LOI: 33.2% (35.2%) | [65] | |
| EVA | SiO2 (5.0) | ATH (120) + DCP (2.0) | UL-94 (3.0 mm): V-0 LOI: 34.0% | σt: 21.0 MPa εb: 420.0% | [66] |
| PP | SiO2 (1.0) | PCO-900 (3.5) + NOR-116 (1.5) | LOI: 25.7% (25.0%) | [67] |
| PO Matrix | MMT/OMMT (wt.%) | Other FRs (wt.%) | Flame Retardancy () a | Mechanical Property () a | Reference |
|---|---|---|---|---|---|
| LDPE | MMT (2.25) | MH (55.0) | UL-94 (3.0 mm): NC b(NC) LOI: 29.3% (31.9%) | [76] | |
| EVA | MMT (1.0) | ATH (49.0) | UL-94: V-0 LOI: 26.0% (33.0%) | σt: 11.2 MPa (17.4 MPa) εb: 61.0% (21.0%) | [77] |
| EVA | OMMT (1.0) | ATH (49.0) | UL-94: V-0; LOI: 28.0% (33.0%) | σt: 11.7 MPa (17.4 MPa) εb: 61.0% (21.0%) | [77] |
| PP | APP-CaMMT (APP:CaMMT = 19:1) (14.3) | Dipentaerythritol (DPER) (5.7) | UL-94 (3.2 mm): V-0 (NC) LOI: 27.5% (21.9%) | [78] | |
| PP | Fe-OMT (4.0) | IFR (APP: PER: melamine polyphosphate (MPP) = 9:4:7) (24.0) | UL-94 (3.0 mm): V-0 (NC) LOI: 30.0% (23.0%) | [79] | |
| PP | Ca-MMT (0.5) | Poly(ethylene glycol) grafted polypropylene (0.5) | σt: 33.5 MPa εb: 132.5% | [80] | |
| LDPE | MMT (4.0) + LDPE grafted maleic anhydride (12.0) | MH (48.0) | LOI: 26.0% (25.0%) | σt: 13.1 MPa (10.8 MPa) εb: 2.8% (1.6%) | [81] |
| HDPE/ EVA | MMT (5.0) | MH (45.0) | UL-94 (3.0 mm): V-0 (V-0) LOI: 28.3% (28.4%) | [82] | |
| HDPE/ EVA | OMMT (5.0) | MH (45.0) | UL-94 (3.0 mm): V-0 (V-0) LOI: 29.6% (28.4%) | [82] | |
| PP | MMT (1.2) | APP (10.8) + DPER (4.0) + melamine (MEL) (4.0) | UL-94 (3.2 mm): V-0 LOI: 29.8% | σt: 30.0 MPa | [83] |
| PP | OMMT (2.6) | IFR (28.0) + PP-g-MAH (4.0) | UL-94 (4.0 mm): V-0 (V-0) LOI: 32.8% (30.7%) | σt: 28.1 MPa (30.8 MPa) | [84] |
| LDPE/ EVA | OMMT (5.0) | ATH (30.0) + MH (15.0) | LOI: 23.3% | σt: 15.6 MPa εb: 33.5% | [85] |
| PO Matrix | FRs Additives (wt.%) | Mode of Action | Results () a | Reference |
|---|---|---|---|---|
| LDPE | MAPP (28.6) (APP covered with KH-570 (3-(Methylacryl-oxyl) propyltrimethoxy silane) and SiO2) + DPER (11.4) | MAPP composites are better dispersed and have good compatibility with the matrix. | UL-94 (10.0 mm): V-0 (V-0); LOI: 28.1% (26.7%); σt: 2.7 MPa (2.4 MPa); εb: 33.8% (24.1%) | [116] |
| PP | HBPPA-Si (12.5) (hyperbranched polyphosphamide with terminal groups of silane) + APP (12.5) | HBPPA-Si has higher thermal stability and more excellent char formation. | UL-94 (3.2 mm): V-0 (NC); LOI: 27.5% (21.3%); σt: 28.3 MPa (26.3 MPa); εb: 28.0% (24.0%) | [117] |
| PP | OA-POSS (octa-ammonium-POSS) (1.0) + IFR(APP:PER = 3:1) (19.0) | OA-POSS acts as a plasticizer in the melt. | UL-94 (2.0 mm): V-1 (NC); LOI: 29.7% (24.5%); εb: 24.0% (23.0%) | [118] |
| PP | TS-POSS (trissulfonic acid propyl-POSS) (1.0) + IFR (APP:PER = 3:1) (19.0) | TS-POSS acts as a plasticizer in the melt. | UL-94 (2.0 mm): V-1 (NC); LOI: 32.4% (24.5%); εb: 27.0% (23.0%) | [118] |
| PP | APID (polysiloxane containing phosphorus, nitrogen and benzene rings) (10.0) + APP (15.0) | APID acts as blowing agent and carbonization agent. | UL-94 (1.6 mm): V-0 (NC); LOI: 29.8% (24.1%); σt: 31.8 MPa (34.7 MPa); εb: 72.3% (109.9%) | [119] |
| PP | Si-MCA (6.3) (silicone-containing macromolecular) + APP (18.7) | Si-MCA helps to form a compact and thermostable intumescent char. | UL-94 (3.2 mm): V-0 (NC); LOI: 33.5% (26.0%); σt: 27.4 MPa (25.6 MPa) | [120] |
| PP | Si-APP (APP modified with polysiloxane) (18.75) + CA (charring agent) (6.25) | Polysiloxane shell can enhance thermal stability. | UL-94 (3.2 mm): V-0 (V-0); LOI: 35.0% (32.7%); | [121] |
| PP | Polysilsesquioxane (5.0) + IFR (APP:PER = 3:1) (25.0) | The synergism between IFR and polysilsesquioxane enhances char yield and form stable C-Si bonds. | UL-94 (3.0 mm): V-0 (NC); LOI: 36.0% (30.0%); σt: 21.0 MPa (20.5 MPa); εb: 33.0% (39.0%) | [122] |
| PP | Polysilsesquioxane (5.0) + IFR (APP:PER = 3:1) (30.0) | The synergism between IFR and polysilsesquioxane enhances char yield and form stable C-Si bonds. | UL-94 (3.0 mm): V-0 (V-0); LOI: 39.5% (32.0%); σt: 16.0 MPa (20.0 MPa); εb: 25.0% (32.0%) | [122] |
| PP | HFR (prepared with γ-Aminopropyltriethoxysilane and other agents) (5.0) + IFR (APP:PER = 3:1) (25.0) | HFR helps to produce more compact intumescent char. | UL-94 (3.0 mm): V-0 (V-0) LOI: 36.0% (32.0%); | [123] |
| PO Matrix | Methods | IFR Formulation (wt.%) | LOI () a UL-94 () a | σt () a εb () a | Reference |
|---|---|---|---|---|---|
| PP | Modify traditional IFRs with a titanate coupling agent NDZ-201 by ball milling to obtain MIFRs | MIFRs (APP + PER + MEL) (25.0) | 31.2% (29.0%) (3.2 mm) b V-0 (V-2) | 29.0 MPa (23.0 MPa) 100.0% (15.0%) | [147] |
| PP | Use phytic acid (PA) and MF resin to modified APP by supramolecular assembly method to obtain APP@MF-PA | APP@MF-PA (20.0) + CFA (5.0) | 35.0% (34.0%) (3.0 mm) V-0 (V-0) | [148] | |
| PP | Decorate the surface of MPP and dialdehyde starch (DAS) by co-microencapsulation technology to obtain M-MPP and M-DAS | M-MPP (15 phr) + M-DAS (15 phr) | 28.2% (27.1%) (3.0 mm) V-1 (V-1) | [149] | |
| PP | Microencapsulate APP with HBPE by KH-550 to obtain K-HBPE@APP | K-HBPE@APP (25.0) | 34.2% (31.0%) (3.2 mm) V-0 (V-1) | 21.0 MPa (24.0 MPa) 375.0% (83.0%) | [140] |
| PP | Use DPER, 4, 4′-diphenylmethane diisocyanate (MDI) and MEL to microencapsulate APP in situ polymerization to obtain MAPP | MAPP (30.0) | 32.1% (22.0%) (3.2 mm) V-0 (NC) | [150] | |
| PP | Modify UF by KH-550 to obtain M-UF | APP (20.0) + M-UF (10.0) | 29.5% (22.0%) (3.2 mm) V-0 (NC) | 19.4 MPa (17.9 MPa) 11.4% c (5.6%) | [146] |
| PP | Introduce DOPO into the molecular structure of APP to obtain DOPO-modified APP | DOPO-modified APP (30.0) | 30.1% (24.2%) (1.6 mm) V-0 (NC) | 31.6 MPa (29.8 MPa) - | [151] |
| PP | Microencapsulate APP with MEL, PER, and MDI via in situ two-step surface polymerization to obtain MAPP | MAPP (30.0) | 25.0% (20.0%) (3.0 mm) V-1 (NC) | [152] | |
| PP | Microencapsulate APP-II with MF resin via in situ polymerization to obtain MFAPP-II | MFAPP-II (30.0) + PER (8.3) | 39.7% (39.0%) (3.0 mm) V-0 (V-0) | [153] | |
| PP | Modify APP-I with ethylenediamine via ion exchange reaction to obtain MAPP | MAPP (40.0) | 32.5% (20.9%) (3.2 mm) V-0 (NC) | [154] |
| PO Matrix | IFRs (wt.%) | Adjuvants (wt.%) | LOI () a UL-94 () a | σt () a εb () a | Reference |
|---|---|---|---|---|---|
| LLDPE | IFRs (ADP@KH-560: neopentyl glycol:MEL = 1.5:1:1) (25.0) ADP@KH-560: aluminum diethylphosphinate modified with KH-560; | ZB (5.0) | 28.7% (28.5%); (3.0 mm) b V-0 (V-0) | [185] | |
| PP | IFRs (APP:DPER = 3:1) (23.5) DPER: double pentaerythritol | Kaol-GLY (1.5) Kaol-GLY: introduced glycine into layers of kaolinite. | 32.9% (27.3%); (3.0 mm) V-0 (NC) | [186] | |
| PP | Single-component IFR (APP + PER + MEL) (24.0) | polyhedral oligomeric silsesquioxane (1.0) | 31.2% (29.7%); (1.6 mm) V-0 (V-1) | 29.0 MPa (26.0 MPa) - | [187] |
| PP | IFRs (APP:PER = 3:1) (20phr) + PP-g-MAH (4phr) | 4ZnO·B2O3·H2O (1 phr) | 31.2% (28.9%); (4.0 mm) V-2 (V-2) | - 1084.0% (1146.0%) | [188] |
| EVA | mixed FR (IFRs (APP:PER:MEL = 3:1:1) + FeOOH) (19.0) | Fumed silica (1.0) | 20.8% (21.3%); (3.0 mm) V-2 (V-2) | 19.9 MPa (14.2 MPa); 675.0% (615.0%) | [189] |
| LDPE | IFRs (SiO2@MAPP:DPER = 2:1) (23.6) | KU (1.4) KU: The intercalation of modified kaolin with urea. | 27.2% (24.1%); (3.0 mm) V-1 (NC) | 16.6 MPa (16.1 MPa); 554.0% (512.0%) | [190] |
| PP | IFRs (APP:PER = 2:1) (13.5) | PAMA-Mn (4.5) PAMA-Mn: MEL phytate supramolecular nanosheet FR incorporating manganese ion | 31.8% (26.5%); (3.2 mm) V-0 (NC) | [191] | |
| PP | IFRs (MCAPP:PEPA = 2:1) (25.0) MCAPP: APP microencapsulated with MEL | P-type hydrated silica aluminate (HSA-P) (1.5) | 35.1% (31.2%); (3.0 mm) V-0 (V-2) | [192] | |
| PP | IFRs (MCAPP:PEPA = 2:1) (25.0) MCAPP: APP microencapsulated with MEL | La-loaded for P-type hydrated silica aluminate(HSA-P-La) (1.5) | 37.5% (31.2%); (3.0 mm) V-0 (V-2) | [192] | |
| PE | IFRs (APP:PER = 3:1) (25.6) | Yb(OTf)3 (0.4) | 25.9% (24.2%); V-0 (NC) | [193] | |
| PP | IFRs (APP:PER = 3:1) (19.0) | Co-MMT (MMT intercalation cobalt compounds) (4.0) | 32.1% (26.5%); (3.2 mm) V-0 (V-2) | [194] | |
| PP | IFRs (APP:PER = 3:1) (25.0) | scCO2 (7.0) | 35.8% (32.8%); (3.2 mm) V-0 (V-2) | [195] | |
| PP | IFRs (OS-MCAPP:CFA = 3:1) (29.7) OS-MCAPP: silica-gel microencapsulated ammonium polyphosphate | NiPO-NT (0.3) NiPO-NT: nickel phosphate nanotubes | 33.9% (29.8%); (3.0 mm) V-0 (V-0) | [196] | |
| PP | IFRs (APP:PER = 2:1) (25.0) | Ni (4.0) | 34.2% (29.0%); (3.0 mm) V-0 (V-1) | [197] | |
| PP | IFRs (APP:PER = 2:1) (25.0) | Ni-Al (Ni:Al = 9:1) (4.0) | 36.8% (29.0%); (3.0 mm) V-0 (V-1) | [197] | |
| PP | IFRs (APP:PER = 2:1) (25.0) | Ni-Mg (Ni:Mg = 9:1) (2.0) | 38.1% (29.0%); (3.0 mm) V-0 (V-1) | [197] | |
| PP | IFRs (APP:PER = 2:1) (25.0) | Ni-Cu (Ni:Cu = 9:1) (4.0) | 36.6% (29.0%); (3.0 mm) V-0 (V-1) | [197] |
| PO Matrix | New IFR (wt.%) | Molecular Structure or Synthetic Method of the Acid/Carbon Sources | LOI UL-94 | σt εb | Reference |
|---|---|---|---|---|---|
| PP | Acid source: APP (16.7) Carbon source: BTETP (8.3) |  | 32.3% V-0 | [207] | |
| PP | Acid source: APP modified with piperazine (18.75) Carbon source: ATPIP (6.25) |  | 30.0% V-0 | 35.5 MPa 40.3% | [208] |
| PP | Acid source: APP (12.5) Carbon source: HBPPA-Si (12.5) | 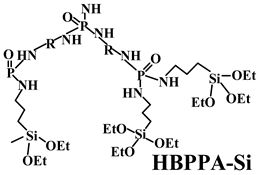 | 27.5% V-0 | 28.3 MPa 28.0% | [117] |
| PP | Acid source: PPA-C (15.0) Carbon source: PER (5.0) | PPA-C was prepared with pyrophosphoric acid (PPA) and cytosine (C) via a one-pot method. | 30.0% V-0 | [209] | |
| PP | Acid source: GO-APP (22.5) Carbon source: PER (7.5) Adjuvants: maleic anhydride-grafted polypropylene (1.0); antioxygen 1010 (1.0) | 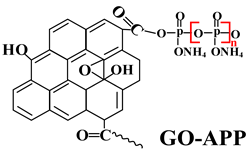 | 31.2% V-0 | 25.0 MPa 47.0% | [210] |
| PP | Acid source: silica-gel-microencapsulated APP (20.0) Carbon source: PEIC (10.0) | 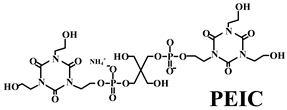 | 32.7% V-0 | 15.3 MPa 11.2% a | [211] |
| PP | Acid source: APP (5.0) Carbon source: HPPU (20.0) | 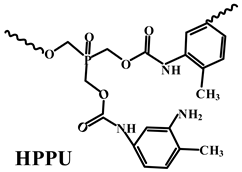 | 27.0% V-0 | [212] | |
| PP | Acid source: APP (20.0) Carbon source: SCTCFA-ZnO (10.0) | 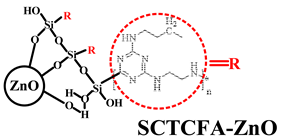 | 36.2% V-0 | [213] | |
| PP | Acid source: APP (18.0) Carbon source: NFR (12.0) |  | 31.0% V-0 | 66.5 MPa 19.7% | [214] |
| PP | Acid source: APP (15.0) Carbon source: PEPAPC (5.0) | 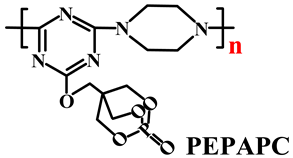 | 28.0% V-0 | [215] | |
| PP | Acid source: APP (18.75) Carbon source: HBPPDA (6.25) |  | 30.6% V-0 | [216] | |
| PP | Acid source: APP (22.5) Carbon source: CNCD-DA (7.5) | 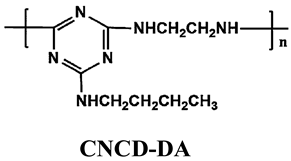 | 36.5% V-0 | [217] | |
| PP | Acid source: APP (18.24) Carbon source: MTEC (4.56) Adjuvant: SiO2 (1.2) | 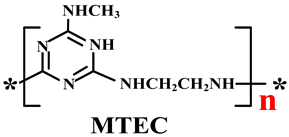 | 30.7% V-0 | [218] | |
| PP | Acid source: APP (10.0) Carbon source: PN-HBP (10.0) | 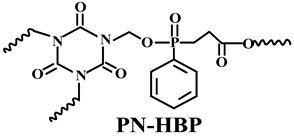 | 30.0% V-0 | [219] | |
| PP | Acid source: IMAPP (17.2) Carbon source: DPER (7.8) Adjuvants: 1. antioxidant 1010 (0.1); 2. antioxidant 168 (0.2) | IMAPP is prepared by the chemical reaction between aluminum chloride and ammonia | 32.1% V-0 | [220] | |
| LDPE | Acid source: APP (20.0) Carbon source: CNCA-DA (10.0) | 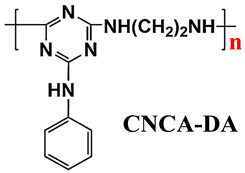 | 30.9% V-0 | [221] | |
| EVA | Acid source: APP (18.0) Carbon source: CNCO-HA (12.0) | 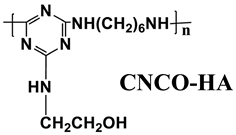 | 30.9% V-0 | [222] | |
| PP | Acid source: APP (12.5) Carbon source: CNCO-HA (12.5) |  | 29.5% V-0 | [223] | |
| PP | Acid source: APP (18.75) Carbon source: TBMC (6.25) |  | 30.5% V-0 | 21.2 MPa 36.9% | [224] |
| PO Matrix | EG FRs (wt.%) | Other Additives (wt.%) | LOI () a UL-94 () a | σt () a εb () a | Reference |
|---|---|---|---|---|---|
| HDPE/ EVA | MEG (modified by DOPO) (5.0) | MH/ATH (3/2) (45.0) | 38.4% (29.0%); (4.0 mm) V-0 (NC) | 21.5 MPa (20.3 MPa); 13.6% (50.8%) | [235] |
| HDPE/ EVA | MEG (modified by DOPO) (4.0) | 1.MH/ATH (3/2) (45.0); 2.zinc borate (1.0) | 37.1% (29.4%); (4.0 mm) V-0 (NC) | 23.0 MPa (20.8 MPa); 15.7% (54.8%) | [235] |
| LDPE | EG (5.0) | 1.RPPMHS (modified with poly(methylhydrosiloxane)) (5.25); 2.ATHMgst (modified with magnesium stearate) (5.25) | 25.4% (22.6%); (2.2 mm) V-0 (V-2) | 9.3 MPa (9.3 MPa); 64.9% (112.7%) | [236] |
| LLDPE/EVA | MEG (modified with DOPO and silane coupling agent) (10.0) | MH/ATH(3/2) (40.0) | 32.7% (29.6%); (2.7 mm) V-0 (V-2) | [237] | |
| LLDPE/EVA | MEG (modified with DOPO and KH560) (4.0) | 1.MH/ATH (45.0); 2.zinc borate (1.0) | 31.7% (29.0%); (4.0 mm) V-0 (NC) | [238] | |
| PP | MEG (modified with DOPO and silane coupling agent) (30.0) | 25.3% (18.1%); (2.7 mm) V-0 (NC) | 27.5 MPa (31.0 MPa); 9.1% (446.3%) | [239] | |
| EVA | EG (27.0) | palygorskite@boric acid@dodecylamine (PGS@B-N) (3.0) | 37.7% (21.2%); (3.0 mm) V-0 (NC) | 13.0 MPa; 1007.3% | [240] |
| EVA | EG (10.0) | LDH (20.0) | 29.7% (27.0%); (3.0 mm) V-0 (NC) | [241] | |
| EVA | EG (20.0) | APP (10.0) | 30.7% (20.3%); (3.0 mm) V-0 (V-2) | [242] | |
| HDPE/ EVA | EG (20.0) | 10.1 MPa (15.6 MPa); 315.5% (517.5%) | [243] |
| PO Matrix | LOI | UL-94 | σt | εb | Flame Retardants (wt.%) | Reference |
|---|---|---|---|---|---|---|
| PP | 31.4% | V-0 | 35.0 MPa | 132.0% | IFR (APP/PER = 3/1) (20) + nano-CB (5) + POE-MA (8) | [173] |
| 34.2% | V-0 | 21.0 MPa | 375.0% | K-HBPE@APP (use HBPE to microencapsulate APP via KH-550 to obtain K-HBPE@APP) (25) | [140] | |
| 34.3% | V-0 | 32.0 MPa | 200.0% | MIFRs (modify traditional IFRs with a titanate coupling agent NDZ-201 by ball milling to obtain MIFRs) (25) + APID (5) | [147] | |
| EVA | 34.0% | V-0 | 21.0 MPa | 420.0% | SiO2 (5.0) + ATH (120) + DCP (2) | [66] |
| 30.5% | V-0 | 12.0 MPa | 732.0% | EG (10) + AHP (5) | [234] | |
| 37.7% | V-0 | 13.0 MPa | 1007.3% | EG (27) + palygorskite@boric acid@dodecylamine(PGS@B-N) (3.0) | [242] | |
| LDPE | 28.1% | V-0 | 2.6 MPa | 33.8% | MAPP (28.6) + DPER (11.4) | [116] |
| 27.2% | V-1 | 16.6 MPa | 554.0% | IFRs (SiO2@MAPP:DPER = 2:1) (23.6) + KU(the intercalation of modified kaolin with urea) (1.4) | [190] | |
| 25.4% | V-0 | 8.3 MPa | 103.8% | EG (5) + RPPMHS (modified with poly(methylhydrosiloxane)) (5.25) + ATHMgst(modified with magnesium stearate) (5.25) + POE (4) | [238] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qi, L.; Liu, Y.; Qiao, J.; Wang, M.; Liu, X.; Li, S. Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials. Polymers 2022, 14, 2876. https://doi.org/10.3390/polym14142876
Li Y, Qi L, Liu Y, Qiao J, Wang M, Liu X, Li S. Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials. Polymers. 2022; 14(14):2876. https://doi.org/10.3390/polym14142876
Chicago/Turabian StyleLi, Yan, Leijie Qi, Yifan Liu, Junjie Qiao, Maotao Wang, Xinyue Liu, and Shasha Li. 2022. "Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials" Polymers 14, no. 14: 2876. https://doi.org/10.3390/polym14142876
APA StyleLi, Y., Qi, L., Liu, Y., Qiao, J., Wang, M., Liu, X., & Li, S. (2022). Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials. Polymers, 14(14), 2876. https://doi.org/10.3390/polym14142876






