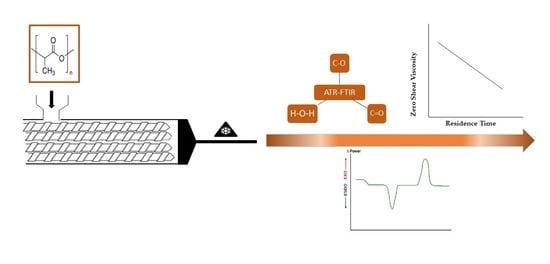
Impact of Melt Processing Conditions on the Degradation of Polylactic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. Characterization
2.3.1. Rheological Properties
2.3.2. Melt Flow Index
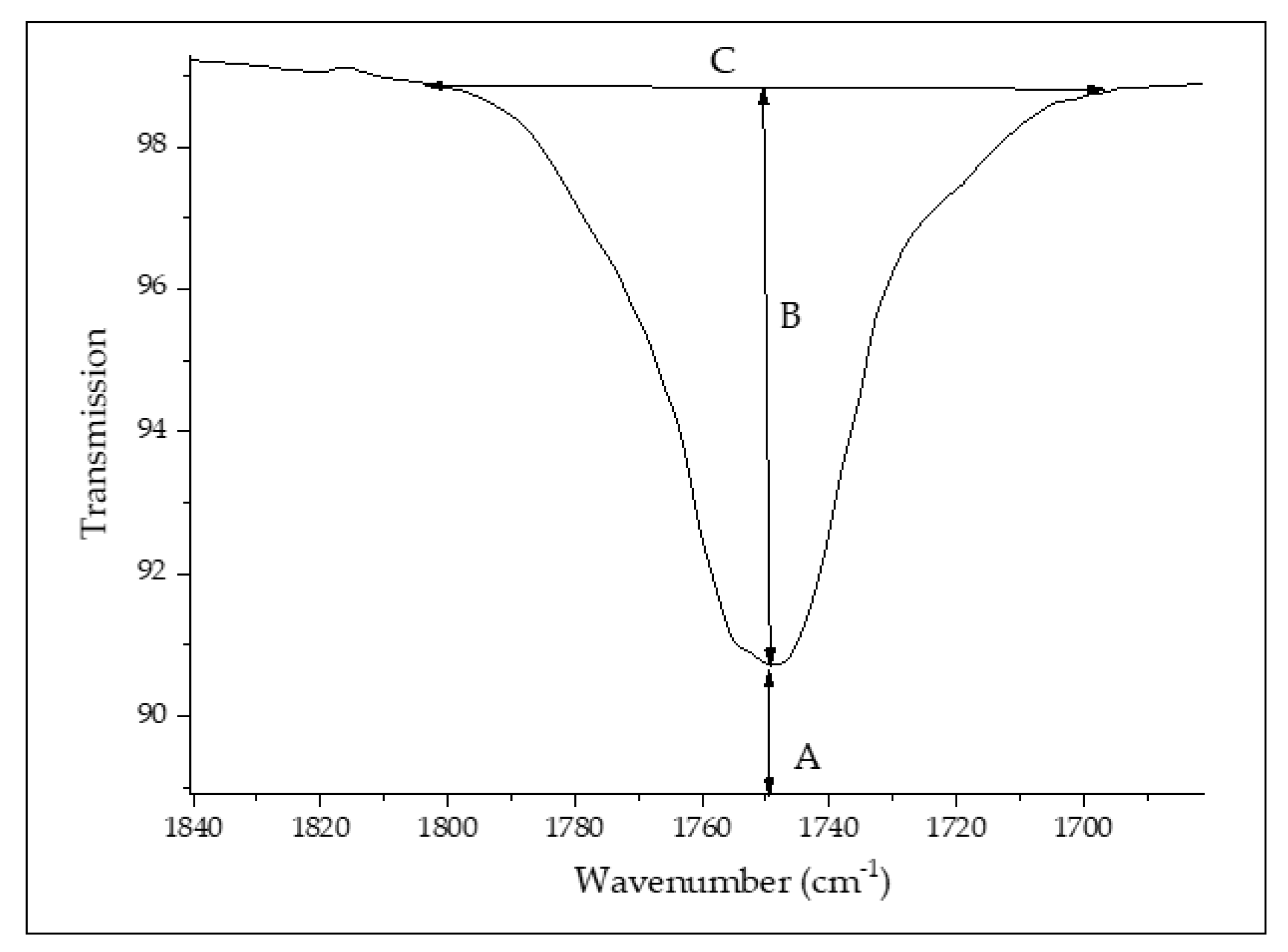
2.3.3. FTIR
2.3.4. Differential Scanning Calorimetry
3. Results and Discussion
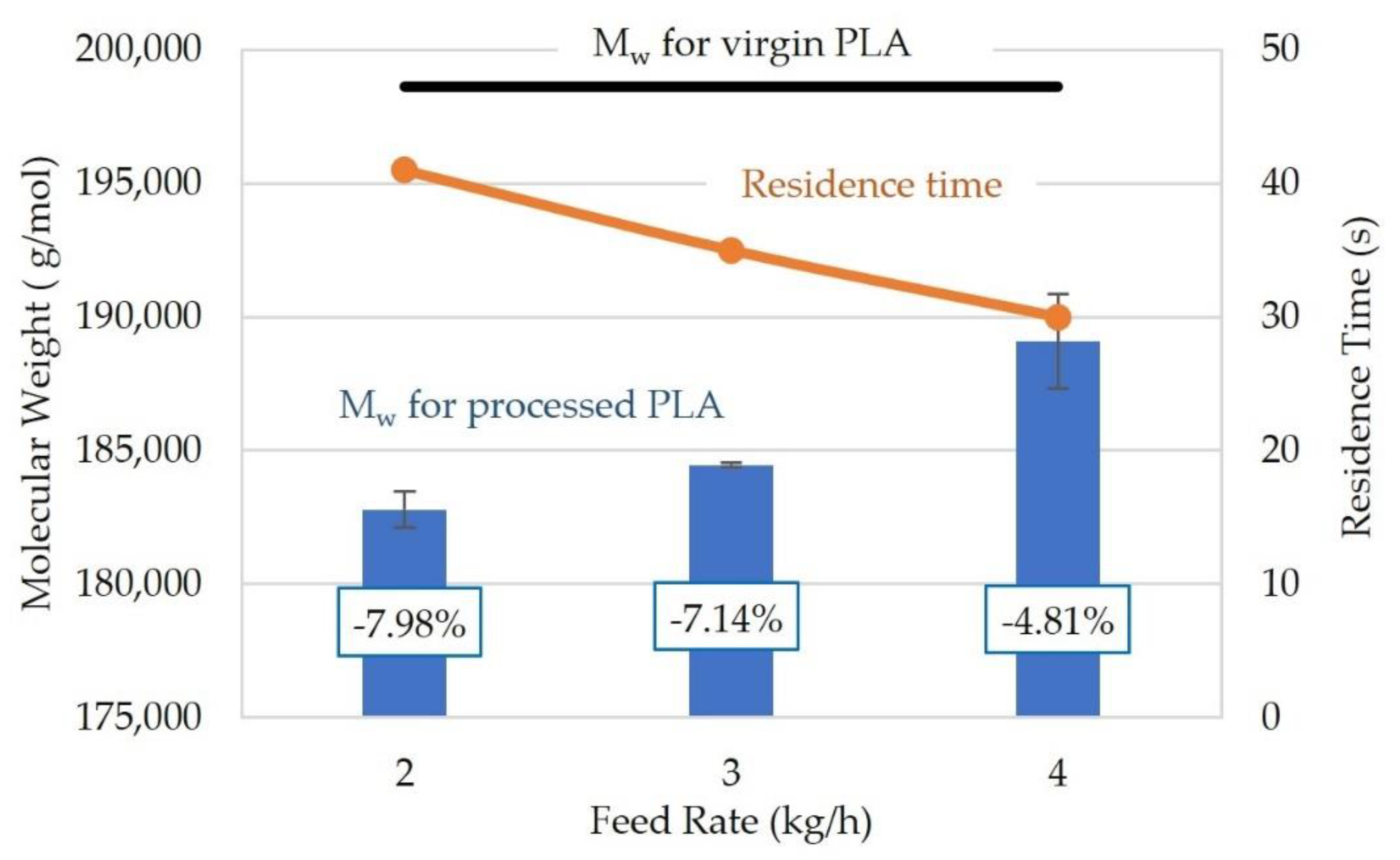
3.1. Extrusion Parameters
3.2. Rheology
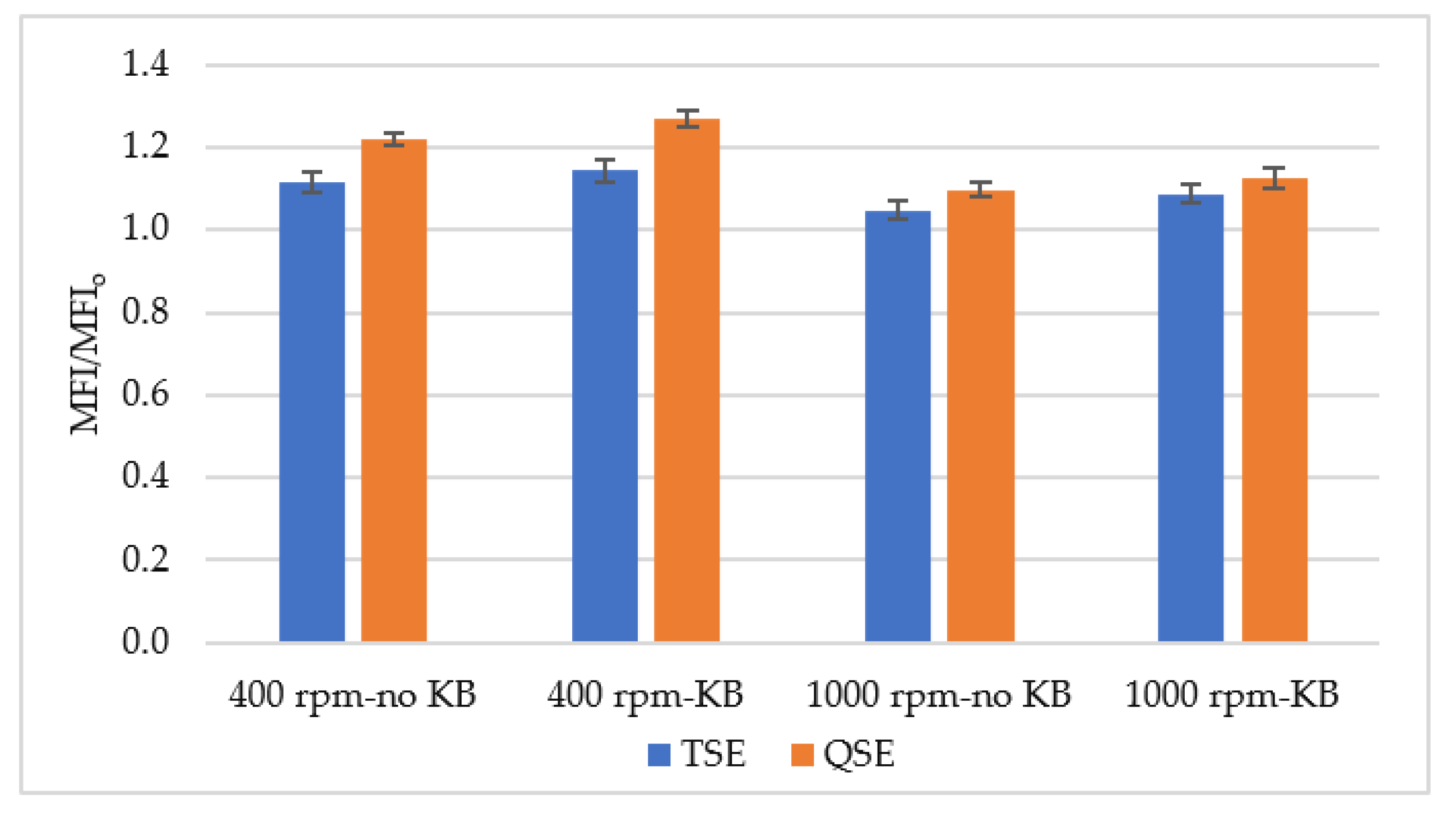
3.3. Melt Flow Index
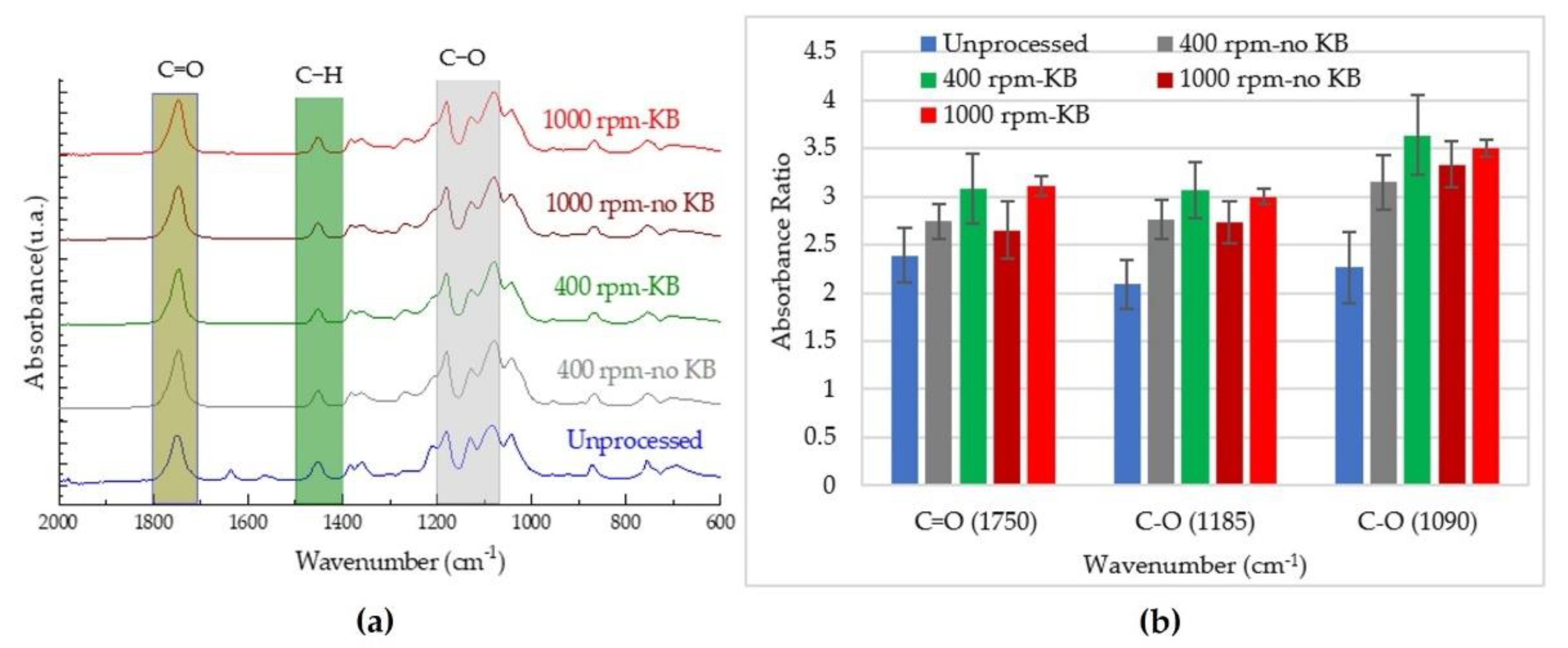
3.4. Chemical Properties
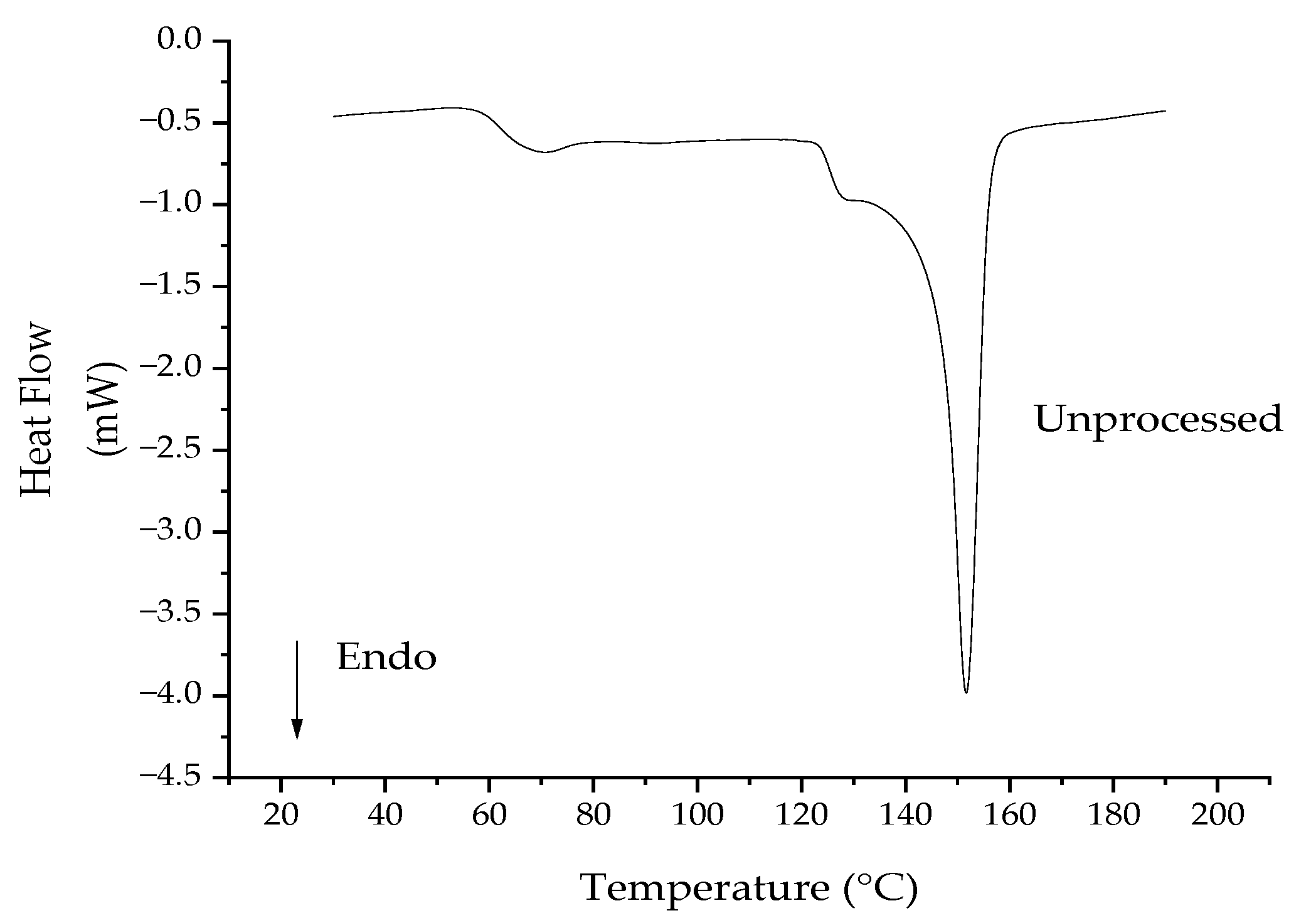
3.5. Differential Scanning Calorimetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| ATR | attenuated total reflection |
| CH3 | methyl group |
| C=O | carbonyl stretching |
| C–O–C | asymmetric vibration of the ester group |
| DSC | differential scanning calorimetry |
| FTIR | Fourier transform infrared radiation |
| G′ | storage modulus45 |
| G″ | loss modulus |
| KB | kneading block in mixing zone (zone 5), refers to screw configuration |
| no-KB | without kneading block in mixing zone (zone 5), refers to screw configuration |
| MFI | melt flow index |
| PLA | polylactic acid |
| PLLA | poly (l-lactide) |
| QSE | quad screw extruder |
| Tg | glass transition temperature |
| Tm | melt temperature |
| TSE | twin screw extrusion |
| (χc) | crystallinity |
| ho | zero shear viscosity |
| η* | complex viscosity |
| λ | relaxation time |
References
- Najafi, N.; Heuzey, M.C.; Carreau, P.J.; Wood-Adams, P.M. Control of thermal degradation of polylactide (PLA)-clay nanocomposites using chain extenders. Polym. Degrad. Stab. 2012, 97, 554–565. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of poly (lactic acid): A review. J. Macromol. Sci. Part. C Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Speranza, V.; De Meo, A.; Pantani, R. Thermal and hydrolytic degradation kinetics of PLA in the molten state. Polym. Degrad. Stab. 2014, 100, 37–41. [Google Scholar] [CrossRef]
- Jaszkiewicz, A.; Meljon, A.; Bledzki, A.K. Mechanical and thermomechanical properties of PLA/Man-made cellulose green composites modified with functional chain extenders—A comprehensive study. Polym. Compos. 2018, 39, 1716–1723. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Espiritu, M.; Bilyk, A.; Kurniawan, L. Morphological behaviour of poly (lactic acid) during hydrolytic degradation. Polym. Degrad. Stab. 2008, 93, 1964–1970. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.H.; Jérôme, R. Polylactide (PLA)—A new way of production. Polym. Eng. Sci. 1999, 39, 1311–1319. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- Ruiz-Silva, E.; Rodríguez-Ortega, M.; Rosales-Rivera, L.C.; Moscoso-Sánchez, F.J.; Rodrigue, D.; González-Núñez, R. Rotational molding of poly (lactic acid)/polyethylene blends: Effects of the mixing strategy on the physical and mechanical properties. Polymers 2021, 13, 217. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Carrasco, F.; Pérez-Maqueda, L.A.; Sánchez-Jiménez, P.E.; Perejón, A.; Santana, O.O.; Maspoch, M.L. Enhanced general analytical equation for the kinetics of the thermal degradation of poly (lactic acid) driven by random scission. Polym. Test. 2013, 32, 937–945. [Google Scholar] [CrossRef]
- Liu, W.-C.; Halley, P.J.; Gilbert, R.G. Mechanism of degradation of starch, a highly branched polymer, during extrusion. Macromolecules 2010, 43, 2855–2864. [Google Scholar] [CrossRef]
- Correia, C.; Gomes, T.E.; Gonçalves, I.; Neto, V. Reprocessability of PLA through Chain Extension for Fused Filament Fabrication. J. Manuf. Mater. Process. 2022, 6, 26. [Google Scholar] [CrossRef]
- Li, R.; Hu, X. Study on discoloration mechanism of polyamide 6 during thermo-oxidative degradation. Polym. Degrad. Stab. 1998, 62, 523–528. [Google Scholar] [CrossRef]
- Gzyra-Jagie\la, K.; Sulak, K.; Draczyński, Z.; Podzimek, S.; Ga\lecki, S.; Jagodzińska, S.; Borkowski, D. Modification of Poly (lactic acid) by the Plasticization for Application in the Packaging Industry. Polymers 2021, 13, 3651. [Google Scholar] [CrossRef]
- Boufarguine, M.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C. PLA/PHBV films with improved mechanical and gas barrier properties. Macromol. Mater. Eng. 2013, 298, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Matykiewicz, D. Correlation between processing parameters and degradation of different polylactide grades during twin-screw extrusion. Polymers 2020, 12, 1333. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Atkinson, H. A simplified theory of crystallisation induced by polymer chain scissions for biodegradable polyesters. Polym. Degrad. Stab. 2012, 97, 1616–1620. [Google Scholar] [CrossRef] [Green Version]
- Patti, A.; Acierno, D.; Latteri, A.; Tosto, C.; Pergolizzi, E.; Recca, G.; Cristaudo, M.; Cicala, G. Influence of the processing conditions on the mechanical performance of sustainable bio-based PLA compounds. Polymers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Taubner, V.; Shishoo, R. Influence of processing parameters on the degradation of poly (L-lactide) during extrusion. J. Appl. Polym. Sci. 2001, 79, 2128–2135. [Google Scholar] [CrossRef]
- Le Marec, P.E.; Ferry, L.; Quantin, J.-C.; Bénézet, J.-C.; Bonfils, F.; Guilbert, S.; Bergeret, A. Influence of melt processing conditions on poly(lactic acid) degradation: Molar mass distribution and crystallization. Polym. Degrad. Stab. 2014, 110, 353–363. [Google Scholar] [CrossRef]
- Albareeki, M.M.; Driscoll, S.B.; Barry, C.F. Compounding of polyethylene composites using high speed twin and quad screw extruders. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: College Park, MD, USA, 2019; Volume 2139, p. 020006. [Google Scholar]
- Lehermeier, H.J.; Dorgan, J.R. Melt rheology of poly (lactic acid): Consequences of blending chain architectures. Polym. Eng. Sci. 2001, 41, 2172–2184. [Google Scholar] [CrossRef]
- Yasuda, K.Y.; Armstrong, R.C.; Cohen, R.E. Shear flow properties of concentrated solutions of linear and star branched polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Sabnis, S.; Block, L.H. Improved infrared spectroscopic method for the analysis of degree of N-deacetylation of chitosan. Polym. Bull. 1997, 39, 67–71. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Für Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Jing, Z. Controlled Rheology Study of Odor Free Polypropylene by Extrusion Processing. Ph.D. Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2012. [Google Scholar]
- Farahanchi, A.; Sobkowicz, M.J. Kinetic and process modeling of thermal and mechanical degradation in ultrahigh speed twin screw extrusion. Polym. Degrad. Stab. 2017, 138, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Rauwendaal, C.J. Analysis and experimental evaluation of twin screw extruders. Polym. Eng. Sci. 1981, 21, 1092–1100. [Google Scholar] [CrossRef]
- Lewandowski, A.; Wilczyński, K. Modeling of Twin Screw Extrusion of Polymeric Materials. Polymers 2022, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Sombatsompop, N.; Panapoy, M. Effect of screw rotating speed on polymer melt temperature profiles in twin screw extruder. J. Mater. Sci. 2000, 35, 6131–6137. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. The effect of thermal and thermo-oxidative degradation conditions on rheological, chemical and thermal properties of HDPE. Polym. Degrad. Stab. 2017, 141, 11–18. [Google Scholar] [CrossRef]
- Yu, T.-H.; Wilkes, G.L. Influence of molecular weight distribution on the melt extrusion of high density polyethylene (HDPE): Effects of melt relaxation behavior on morphology and orientation in HDPE extruded tubular films. J. Rheol. 1996, 40, 1079–1093. [Google Scholar] [CrossRef] [Green Version]
- Hammani, S.; Moulai-Mostefa, N.; Samyn, P.; Bechelany, M.; Dufresne, A.; Barhoum, A. Morphology, Rheology and Crystallization in Relation to the Viscosity Ratio of Polystyrene/Polypropylene Polymer Blends. Materials 2020, 13, 926. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.; Acosta-Ramírez, A.; Mehrkhodavandi, P.; Dorgan, J.R.; Hatzikiriakos, S.G. Solution and melt viscoelastic properties of controlled microstructure poly (lactide). J. Rheol. 2011, 55, 987–1005. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Rehovsky, C.; Bajwa, S.G.; Vahidi, G. Deterioration in the physico-mechanical and thermal properties of biopolymers due to reprocessing. Polymers 2019, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Cruz, S.A.; Zanin, M. Evaluation and identification of degradative processes in post-consumer recycled high-density polyethylene. Polym. Degrad. Stab. 2003, 80, 31–37. [Google Scholar] [CrossRef]
- Oliveira, M.; Santos, E.; Araújo, A.; Fechine, G.J.; Machado, A.V.; Botelho, G. The role of shear and stabilizer on PLA degradation. Polym. Test. 2016, 51, 109–116. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative degradation of polylactide (PLA) and its effects on physical and mechanical properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Material valorisation of amorphous polylactide. Influence of thermo-mechanical degradation on the morphology, segmental dynamics, thermal and mechanical performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly (lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef] [Green Version]
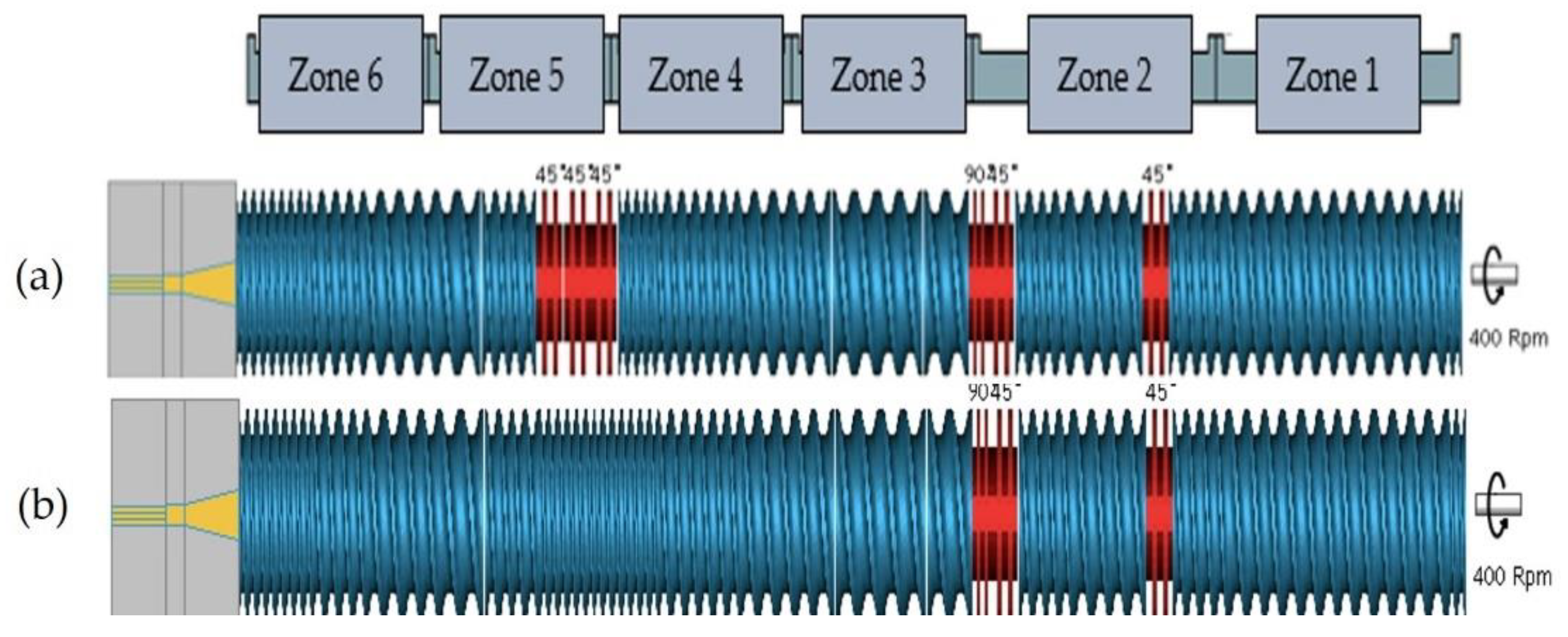





| Parameter | Trial 1 | Trial 2 |
|---|---|---|
| Extruder(s) | TSE, QSE | QSE |
| Screw Configuration | 1, 2 | 2 |
| Screw Speed (rpm) | 400, 1000 | 400 |
| Feed Rate (kg/h) | 2 | 2, 3, 4 |
| Temperature (°C) Zone 1 to Zone 8 (Die) | 165 to 180 | 165 to 180 |
| Screw Speed (rpm) | Screw Configuration | ηo (Pa·s) | λ (ms) | |
|---|---|---|---|---|
| Unprocessed PLA | - | - | 5672 ± 35 | 76.1 ± 14.0 |
| TSE | 400 | no KB | 4806 ± 216 | 56.5 ± 22.8 |
| KB | 4356 ± 57 | 51.8 ± 27.5 | ||
| 1000 | no KB | 5332 ± 68 | 75.8 ± 1.7 | |
| KB | 5137 ± 182 | 66.8 ± 16.8 | ||
| QSE | 400 | no KB | 4276 ± 53 | 47.5 ± 9.2 |
| KB | 4051 ± 42 | 35.4 ± 3.3 | ||
| 1000 | no KB | 4713 ± 140 | 63.8 ± 11.4 | |
| KB | 4373 ± 210 | 52.8 ± 9.2 |
| Screw Speed (rpm) | Screw Configuration | Tg (°C) | Tm (°C) | χc (%) | |
|---|---|---|---|---|---|
| Unprocessed (1st cycle) | - | - | 58.9 | 145.8 | 34.45 |
| Unprocessed (2nd cycle) | - | - | 58.2 | 145.9 | 0.16 |
| TSE | 400 | no KB | 58.3 | 146.3 | 0.31 |
| KB | 57.4 | 144.1 | 0.32 | ||
| 1000 | no KB | 58.7 | 143.2 | 0.16 | |
| KB | 58.3 | 146.3 | 0.24 | ||
| QSE | 400 | no KB | 58.3 | 145.5 | 0.42 |
| KB | 57.4 | 144.3 | 1.37 | ||
| 1000 | no KB | 58.7 | 145.1 | 0.23 | |
| KB | 58.3 | 144.7 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhafeeri, T.; Alotaibi, M.; Barry, C.F. Impact of Melt Processing Conditions on the Degradation of Polylactic Acid. Polymers 2022, 14, 2790. https://doi.org/10.3390/polym14142790
Aldhafeeri T, Alotaibi M, Barry CF. Impact of Melt Processing Conditions on the Degradation of Polylactic Acid. Polymers. 2022; 14(14):2790. https://doi.org/10.3390/polym14142790
Chicago/Turabian StyleAldhafeeri, Thamer, Mansour Alotaibi, and Carol Forance Barry. 2022. "Impact of Melt Processing Conditions on the Degradation of Polylactic Acid" Polymers 14, no. 14: 2790. https://doi.org/10.3390/polym14142790
APA StyleAldhafeeri, T., Alotaibi, M., & Barry, C. F. (2022). Impact of Melt Processing Conditions on the Degradation of Polylactic Acid. Polymers, 14(14), 2790. https://doi.org/10.3390/polym14142790