Thermal Decomposition Behavior of Polyimide Containing Flame Retardant SiO2 and Mg(OH)2
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Silica/Polyimide (SPI), Magnesium Hydroxide/Polyimide (MPI)
2.3. Characterization
3. Results and Discussion
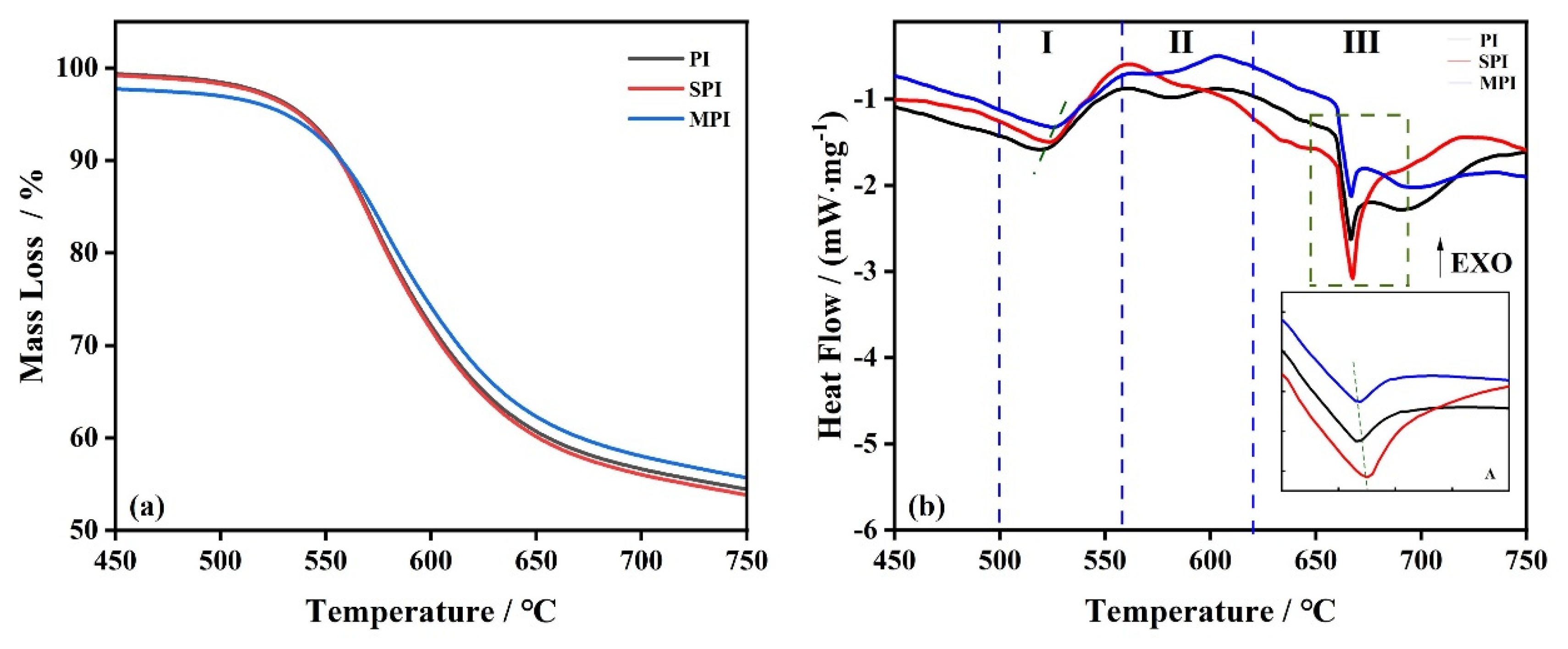
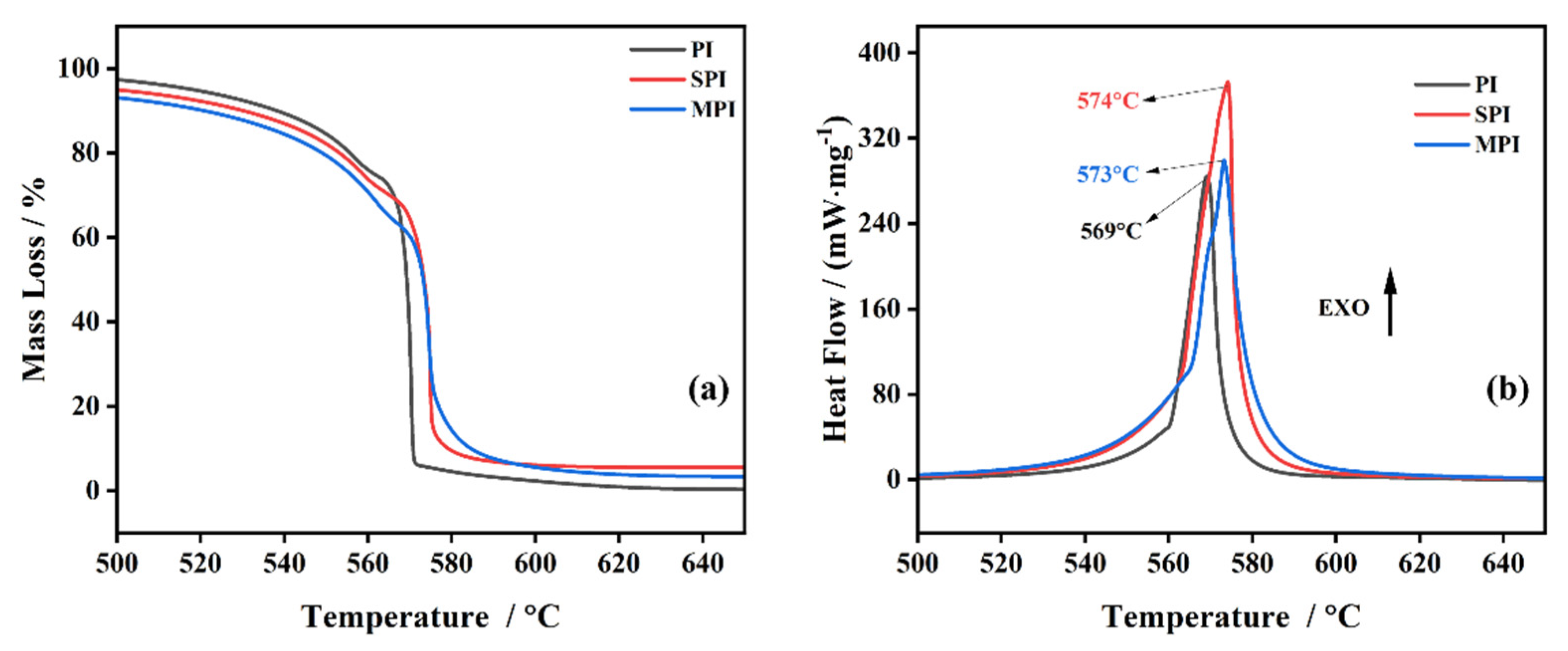
3.1. Thermal Stability of Polyimide
3.2. The Influence of SiO2 and Mg(OH)2 on the Thermal Stability of Polyimide
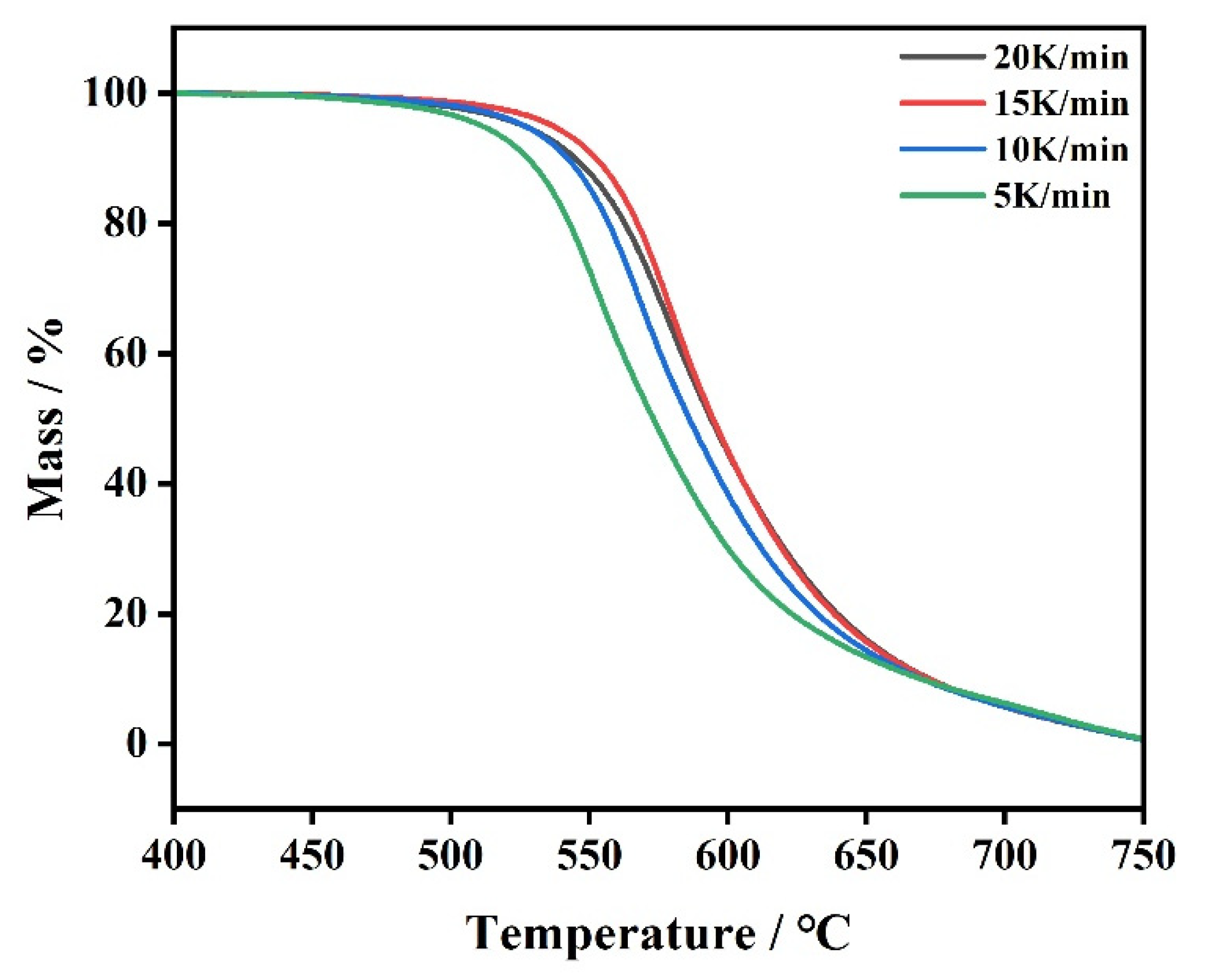
3.3. Kinetic Analysis of Polyimide
3.3.1. Calculation of Activation Energy
3.3.2. Multivariate Nonlinear Fitting
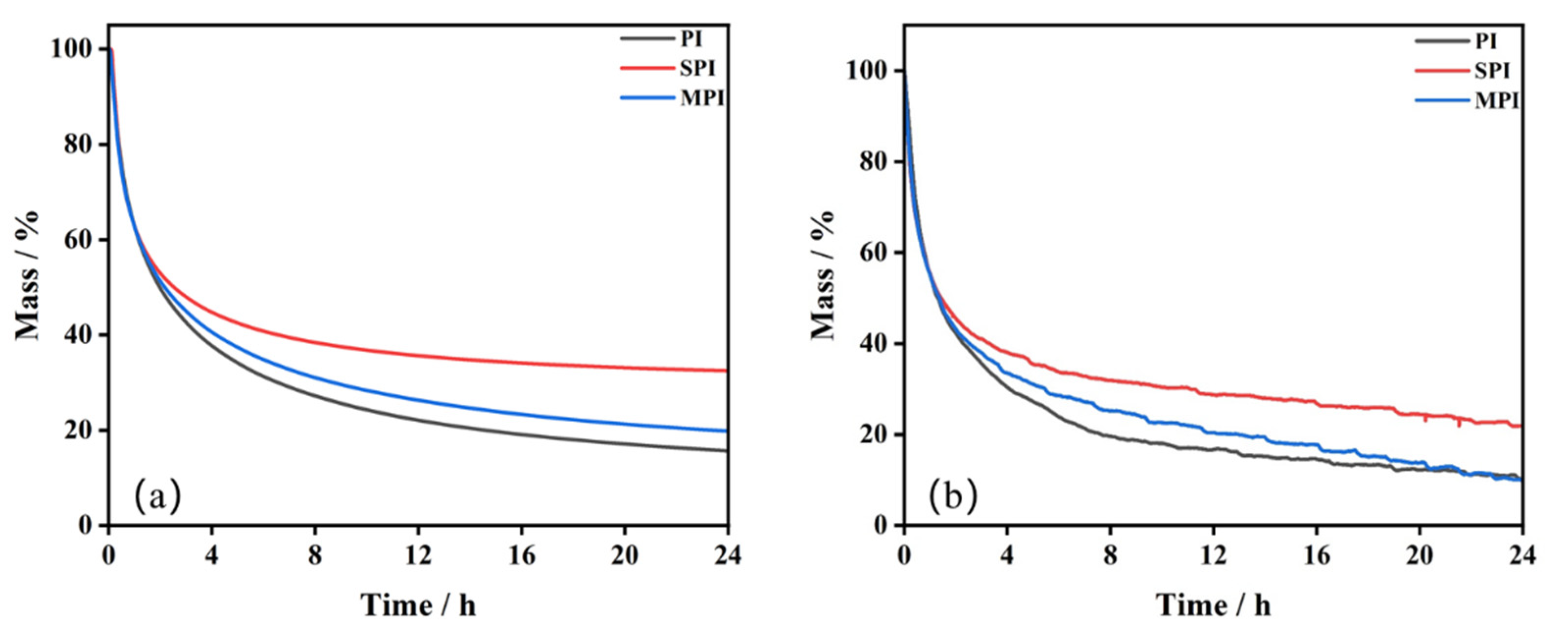
3.3.3. Lifetime Prediction of Three Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantor, Z.; Wu, T.; Zeng, Z.; Gaan, S.; Lehner, S.; Jovic, M.; Bonnin, A.; Pan, Z.; Mazrouei-Sebdani, Z.; Opris, D.M.; et al. Heterogeneous silica-polyimide aerogel-in-aerogel nanocomposites. Chem. Eng. J. 2022, 443, 136401–136412. [Google Scholar] [CrossRef]
- Yunhan, X.; Leilei, W.; Aijun, H.; Lili, Y.; Zhiyuan, W.; Shiyong, Y. Polyimide foams for high temperature applications. Prog. Chem. 2018, 30, 684–691. [Google Scholar]
- Chen, C.K.; Lin, Y.C.; Hsu, L.C.; Ho, J.C.; Ueda, M.; Chen, W.C. High performance biomass-based polyimides for flexible electronic applications. ACS Sustain. Chem. Eng. 2021, 9, 3278–3288. [Google Scholar] [CrossRef]
- Flores-Bonano, S.; Vargas-Martinez, J.; Suarez, O.M.; Silva-Araya, W. Tortuosity index based on dynamic mechanical properties of polyimide foam for aerospace applications. Materials 2019, 12, 1851. [Google Scholar] [CrossRef] [Green Version]
- Irina, G.; Eitan, G.; Ronen, V.; Nurit, A.; Asaf, B.; Noam, E. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar]
- Kimoto, M.; Mizutani, K. Blends of thermoplastic polyimide with epoxy resin. J. Mater. Sci. 1997, 32, 2479–2483. [Google Scholar] [CrossRef]
- Qian, G.; Wang, L.; Shang, Y.; He, X.; Tang, S.; Liu, M.; Li, T.; Zhang, G.; Wang, J. Polyimide binder: A facile way to improve safety of lithium-ion batteries. Electrochim. Acta. 2016, 187, 113–118. [Google Scholar] [CrossRef]
- Satoshi, U.; Megumi, M.; Masaki, Y.; Masashi, I. Electrochemical properties of non-nano-silicon negative electrodes prepared with a polyimide binder. J. Power Sources 2015, 273, 118–122. [Google Scholar]
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W.G. Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl. Mater. Inter. 2013, 5, 2677–2684. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, X.; Zhu, Y.; Hui, D.; Qiu, Y. Mechanical, electrical and thermal properties of aligned carbon nanotube/polyimide composites. Compos. Part B Eng. 2014, 56, 408–412. [Google Scholar] [CrossRef]
- Dong, J.; Yin, C.; Zhao, X.; Li, Y.; Zhang, Q. High strength polyimide fibers with functionalized graphene. Polymer 2013, 54, 6415–6424. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Luo, L.; Wang, H.; Wang, X.; Li, K.; Zhang, C.; Liu, X. Releasing silica-confined macromolecular crystallization to enhance mechanical properties of polyimide/silica hybrid fibers. Compos. Sci. Technol. 2014, 101, 24–31. [Google Scholar] [CrossRef]
- Yin, C.; Dong, J.; Li, Z.; Zhang, Z.; Zhang, Q. Large-scale fabrication of polyimide fibers containing functionalized multiwalled carbon nanotubes via wet spinning. Compos. Part B Eng. 2014, 58, 430–437. [Google Scholar] [CrossRef]
- Huang, C. Study on Preparation and Properties of Multi-Walled Carbon Nanotubes/Polyimide Composites Films. Master’s Thesis, Lanzhou University, Lanzhou, China, 2008. [Google Scholar]
- He, G.; Xie, L.; Tan, K.; Li, H. Preparation of MWNTs/PI nanocomposite materials and their dynamic mechanical properties and dielectric properties. Chin. J. Nonferrous Metals 2011, 21, 1123–1130. [Google Scholar]
- Wang, X.; Fan, Y.; Chen, H.; Yang, R.; Zhao, W. Electrical, mechanical, and thermal properties of Mg(OH)2/PI nanocomposite films. J. Inorg. Organomet. Polym. 2017, 27, 1778–1786. [Google Scholar] [CrossRef]
- Shadpour, M.; Mina, N. Polymer/SiO2 nanocomposites: Production and applications. Prog. Mater. Sci. 2018, 97, 409–447. [Google Scholar]
- Zhang, X.; Ni, X.; Li, C.; You, B.; Sun, G. Co-gel strategy for preparing hierarchically porous silica/polyimide nanocomposite aerogel with thermal insulation and flame retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- Inagaki, M.; Harad, S.; Sato, T. Carbonnization of polyimide film “Kapton”. Carbon 1989, 27, 253–257. [Google Scholar] [CrossRef]
- Inagaki, M.; Meng, L.J.; Ibuki, T.; Sakai, M.; Hishiyama, Y. Carbonization and graphitization of polyimide film “Novax”. Carbon 1991, 29, 1239–1243. [Google Scholar] [CrossRef]
- Zhao, G.X.; Zhu, C.F. Study on pyrolysis behavior of precursor of kapton film used for graphite film with high crystallinity. Carbon Tech. 2003, 22, 13–16. [Google Scholar]
- Ning, R.; Jianjun, Z. Progress in datum treatment methods of thermal analysis kinetics. Prog. Chem. 2006, 18, 410–416. [Google Scholar]
- Xi, G.; Song, S.; Liu, Q. New progress in thermal analysis kinetics studies. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2004, 32, 78–82. [Google Scholar]
- Yu, J.; Yang, W.; Xu, W.; Ding, Y.; He, W. Study on properties IFR copolymer polypropylene reinforced and toughened by nano-SiO2. Plast. Addit. 2015, 19, 33–39. [Google Scholar]
- Liu, Y.; Xu, X.; Mo, S.; Lan, B.; Zhu, C.; Xu, J.; Li, C.; Fan, L. Kinetic study of the multistep thermo-oxidative degradation of thermosetting siloxane-containing polyimide and unmodified polyimide. J. Appl. Polym. Sci. 2020, 137, 49021. [Google Scholar] [CrossRef]
- Liu, R. Study on the Structural Evolutions and Properties of Carbon Films Prepared from Polyimide Precursor Films via Gradient Heating. Ph.D. Thesis, Shandong University, Jinan, China, 2014. [Google Scholar]
- Inagaki, M.; Ibuki, T.; Takeichi, T. Carbonization behavior of polyimide films with various chemical structures. J. Appl. Polym. Sci. 1992, 44, 521–525. [Google Scholar] [CrossRef]
- Li, Y. Preparation and Surface Modification of Silica Nanoparticle. Master’s Thesis, Soochow University, Suzhou, China, 2011. [Google Scholar]
- Henry, F. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C 1964, 6, 183–195. [Google Scholar]




| Sample | Specification | Manufacturer |
|---|---|---|
| Polyimide (PI) | Thermoplastic, Mw: 50,000–80,000 | Nanjing WANQING chemical Glass ware & Instrument Co., Ltd., Nanjing, China |
| Silica (SiO2) | 99.5%, 30 nm | |
| Magnesium hydroxide (Mg(OH)2) | AR, 2.86 µm |
| Sample | Ⅰ (450–565 °C) | Ⅱ (565–620 °C) | Ⅲ (620–750 °C) | |||
|---|---|---|---|---|---|---|
| Tp/°C | ∆m/% | Tp/°C | ∆m/% | Tp/°C | ∆m/% | |
| PI | 520 | 21.3 | 580 | 48.1 | 667 | 29.6 |
| SPI | 524 | 27.6 | — | 46.7 | 667 | 26.1 |
| MPI | 527 | 23.8 | 583 | 46.1 | 667 | 29.3 |
| Kinetic Parameters | PI (A2 a + An b + C1 c) | PI/SiO2 (A2 + An + Cn d) | PI/Mg(OH)2 (A2 + An + F2 e) | |
|---|---|---|---|---|
| Ⅰ | LogA1/s−1 | 10.1 | 6.6 | 6.1 |
| E1/(kJ/mol) | 187.1 | 134.4 | 127.6 | |
| Ⅱ | LogA2/s−1 | 15.1 | 17.6 | 15.7 |
| E2/(kJ/mol) | 279.6 | 314.1 | 290.1 | |
| Ⅲ | LogA3/s−1 | 13.6 | 21.5 | 15.1 |
| E3/(kJ/mol) | 294.4 | 380.4 | 318.3 | |
| Correlation Coefficient | R = 0.9997 | R = 0.9964 | R = 0.9998 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jiang, A.; Li, Y.; Song, D.; Li, Y.; Cheng, L. Thermal Decomposition Behavior of Polyimide Containing Flame Retardant SiO2 and Mg(OH)2. Polymers 2022, 14, 2791. https://doi.org/10.3390/polym14142791
Wang J, Jiang A, Li Y, Song D, Li Y, Cheng L. Thermal Decomposition Behavior of Polyimide Containing Flame Retardant SiO2 and Mg(OH)2. Polymers. 2022; 14(14):2791. https://doi.org/10.3390/polym14142791
Chicago/Turabian StyleWang, Jie, Aifeng Jiang, Yanchun Li, Dongming Song, Yifan Li, and Long Cheng. 2022. "Thermal Decomposition Behavior of Polyimide Containing Flame Retardant SiO2 and Mg(OH)2" Polymers 14, no. 14: 2791. https://doi.org/10.3390/polym14142791
APA StyleWang, J., Jiang, A., Li, Y., Song, D., Li, Y., & Cheng, L. (2022). Thermal Decomposition Behavior of Polyimide Containing Flame Retardant SiO2 and Mg(OH)2. Polymers, 14(14), 2791. https://doi.org/10.3390/polym14142791






