Interfacial Adhesion in Silica-Silane Filled NR Composites: A Short Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Accelerators
2.2. Silica vs. CB
2.3. Interfacial Interaction
2.4. Hydrolysis Mechanism
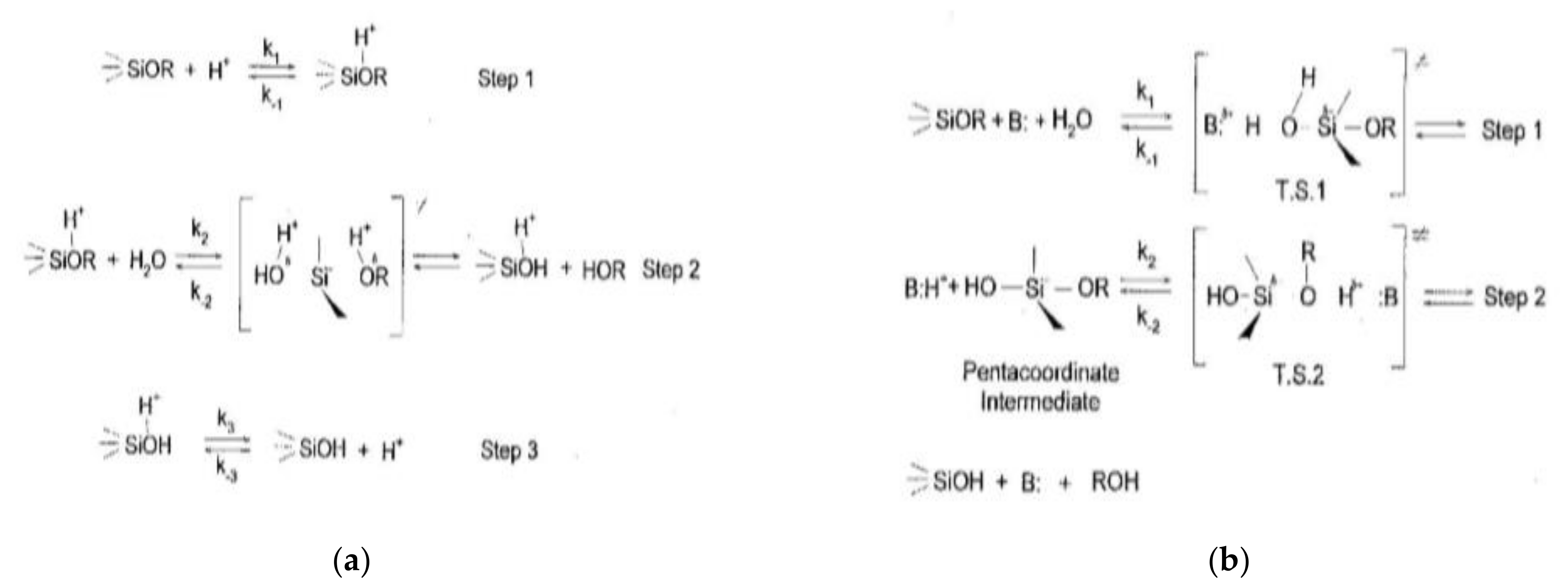
2.4.1. Acid Catalyzed Hydrolysis
2.4.2. Base Catalyzed Hydrolysis
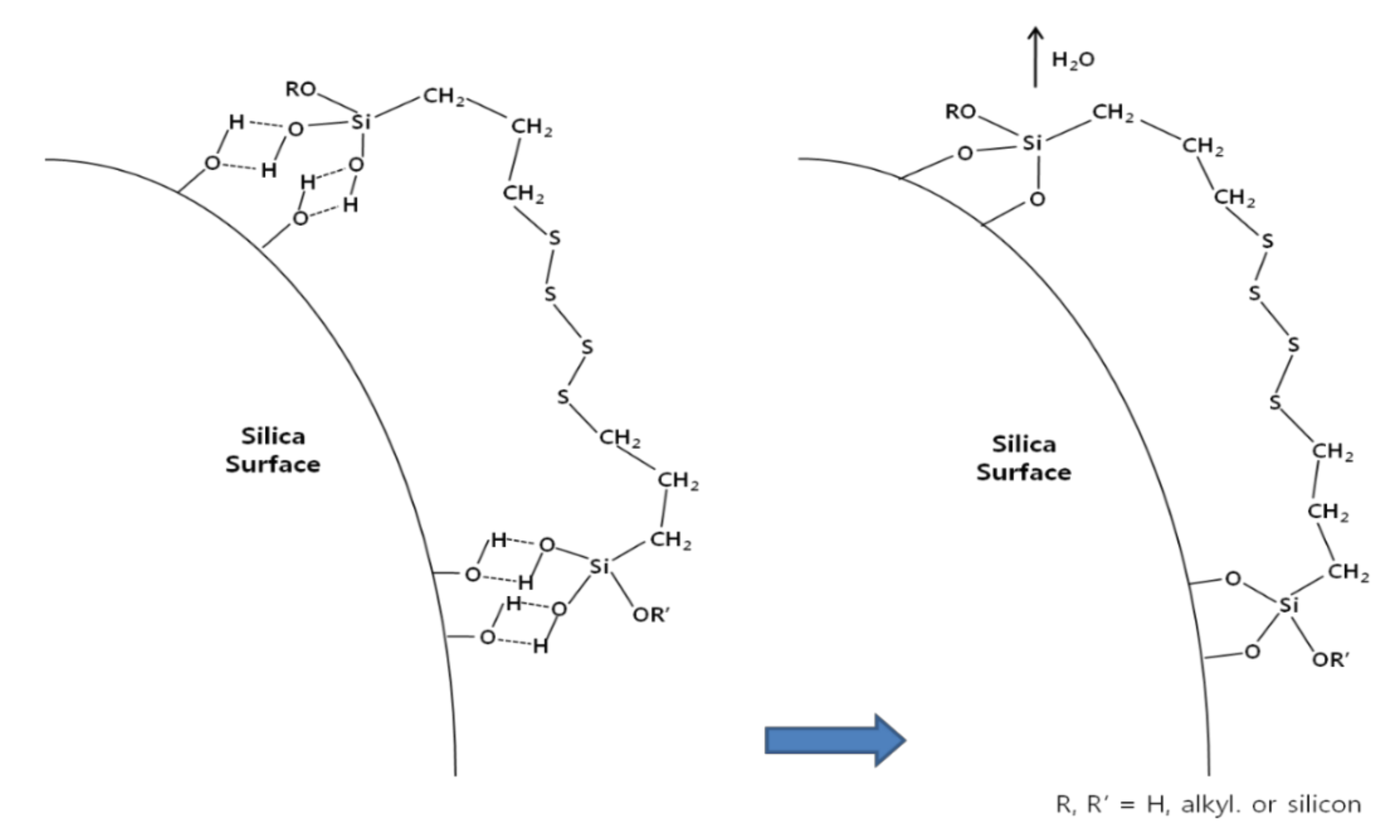
2.5. Alkoxysilane Silanization Reaction Mechanism
3. Hydrolysis Quantitative Analysis
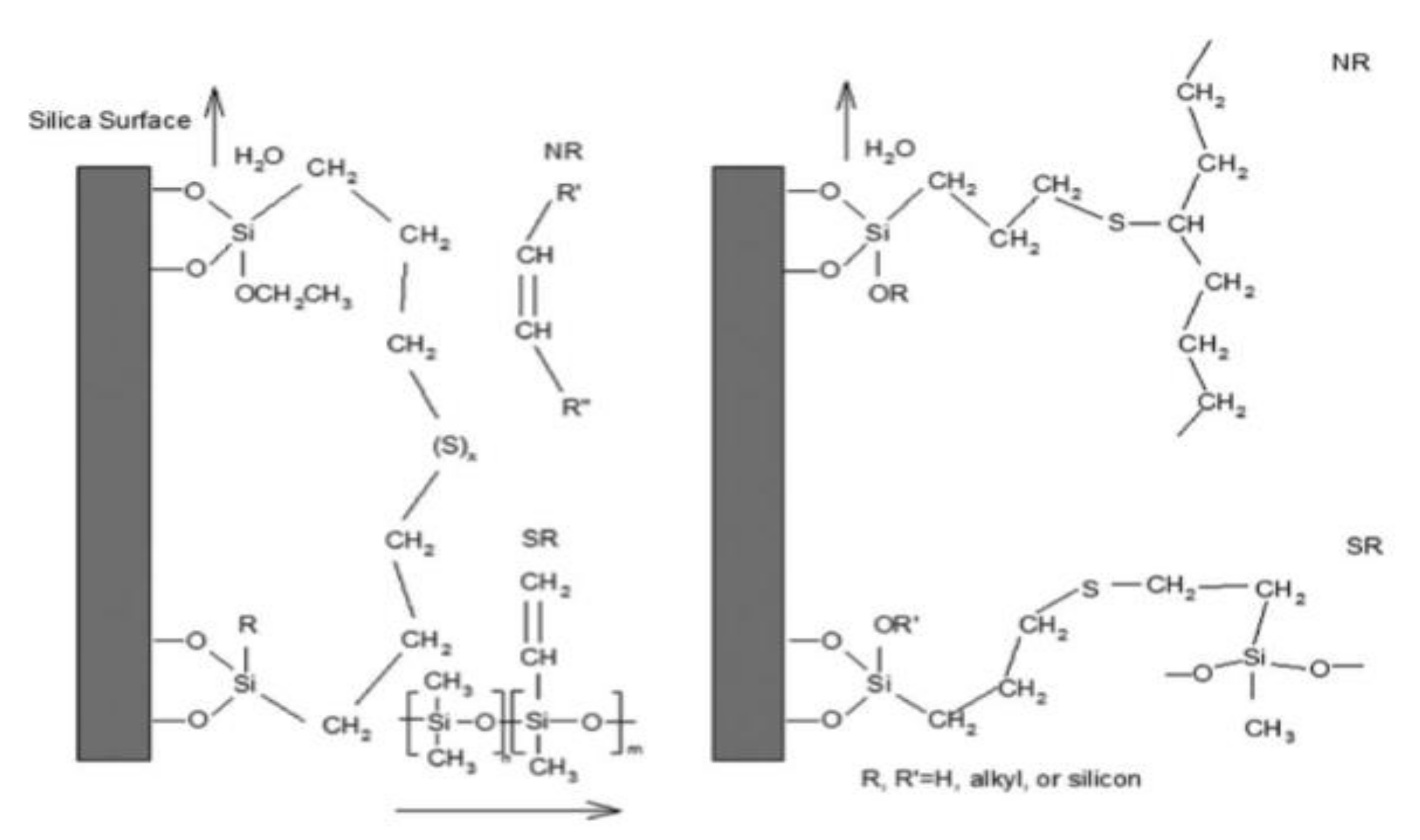
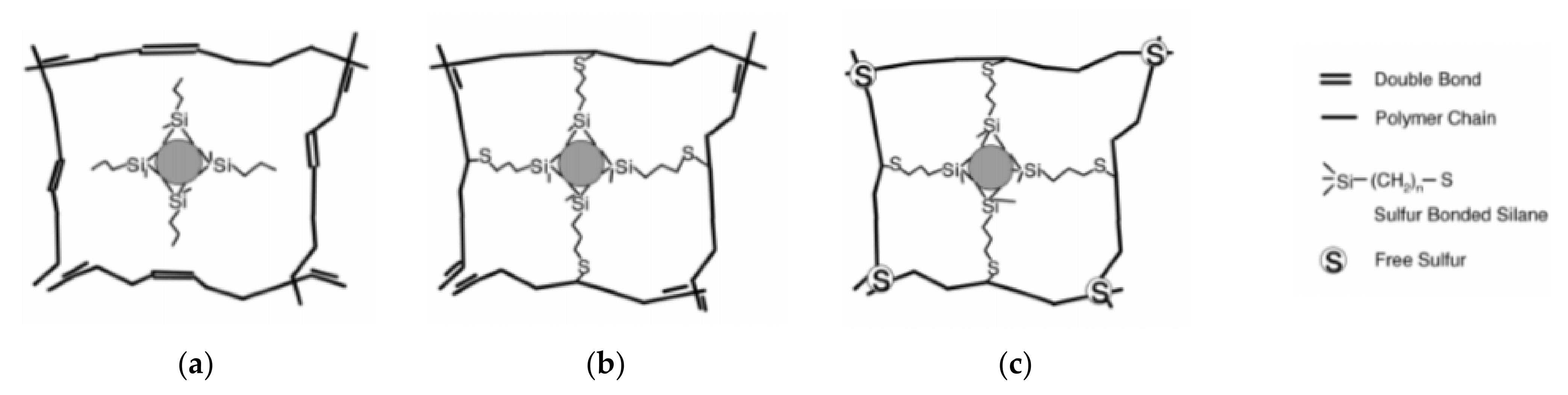
4. Effect of Sulfur Concentration of Hydrolyzed Silane
5. Zinc Ion Mechanism
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.M.; Kim, K.J. Effects of accelerator on silica vs. carbon black filled natural rubber compounds. Polym.-Korea 2013, 37, 269–275. [Google Scholar] [CrossRef][Green Version]
- Goodyear, C. Improvement in India-Rubber Fabrics. U.S. Patent 3633, 15 June 1844. [Google Scholar]
- Hancock, T. Preparation or Manufacture of Caoutchouc in Combination with Other Substances. U.K. Patent 9952, 21 November 1843. [Google Scholar]
- Bateman, L.; Moore, C.G.; Porter, M.; Saville, B. Chapter 19. In The Chemistry and Physics of Rubber like Substances; Bateman, L., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1963. [Google Scholar]
- Molony, S.B. Rubber Vulcanization and the Product Thereof. U.S. Patent 1,343,224, 9 March 1920. [Google Scholar]
- Coran, A.Y. Chapter 7. In Science and Technology of Rubber, 3rd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Bedford, C.W. Art of Vulcanizing Caoutohouc. U.S. Patent 1,371,662, 15 March 1921. [Google Scholar]
- Sebrell, L.B.; Bedford, C.W.; Bedford, C.W. Art of Vulcanizing or Curing Caoutchouc Substances. U.S. Patent 1,544,687, 18 April 1925. [Google Scholar]
- Bruni, G.; Romani, E. Mechanism of Action of certain Acceleratiors of Vulcanization. Indian Rubber J. 1921, 62, 63–66. [Google Scholar]
- Harman, M.W. Process of Vulcanizing Rubber and Product Produced Thereby. U.S. Patent 2,100,692, 11 April 1933. [Google Scholar]
- Coran, A.Y.; Kerwood, J.E. Inhibiting Premature Vulcanization of Diene Rubbers. U.S. Patent 3,546,185, 8 December 1970. [Google Scholar]
- Thurn, F.; Burmester, K.; Pochert, J.; Wolff, S. Rubber Mixtures Giving Reversion Free Vulcanizates and Process of Vulcanization. U.S. Patent 4,517,336, 14 May 1985. [Google Scholar]
- Rauline, R. Composition de Caoutchouc et Enveloppes de Pneumatiques à Base de Ladite Composition. European Patent EP 501,227, 2 September 1992. [Google Scholar]
- Wagner, M.P. Reinforcing silicas and silicates. Rubber Chem. Technol. 1976, 49, 703–774. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kennal, E.; Kim, K.J. Polymer Nanocomposites Handbook; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- White, J.L.; Kim, K.J. Thermoplastic and Rubber Compounds Technology and Physical Chemistry; Hanser Publisher: Munich, Germany; Cincinnati, OH, USA, 2008. [Google Scholar]
- Isayev, A.I.; Hong, C.K.; Kim, K.J. Continuous Mixing and Compounding of Polymer/Filler and Polymer/Polymer Mixtures with the Aid of Ultrasound. Rubber Chem. Technol. 2003, 76, 923–947. [Google Scholar] [CrossRef]
- Kim, K.J.; White, J.L. Silica Agglomerate Breakdown In Three-Stage Mix Including A Continuous Ultrasonic Extrude. J. Ind. Eng. Chem. 2000, 6, 372–379. [Google Scholar]
- Kim, S.M.; Cho, H.W.; Kim, J.W.; Kim, K.J. Effects of processing geometry on the mechanical properties and silica dispersion of silica-filled isobutylene-isoprene rubber (IIR) compounds. Elastomers Compos. 2010, 45, 223–229. [Google Scholar]
- Wolff, S. Reinforcing and Vulcanization Effects of Silane Si 69 in Silica-Filled Compounds. Kautsch. Gummi Kunstst. 1981, 34, 280–284. [Google Scholar]
- Wolff, S. Optimization of Silane-Silica OTR Compounds. Part 1: Variations of Mixing Temperature and Time during the Modification of Silica with Bis-(3-Triethoxisilylpropyl)-Tetrasulfide. Rubber Chem. Technol. 1982, 55, 967–989. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents; Plenum Press: New York, NY, USA, 1982. [Google Scholar]
- Kim, K.J.; VanderKooi, J. TESPT Treated Silica Compounds on and TESPD Rheological Property and Silica Break Down in Natural Rubber. Kautsch. Gummi Kunstst. 2002, 55, 518–528. [Google Scholar]
- Kim, K.J.; White, J.L. TESPT and Different Aliphatic Silane Treated Silica Compounds Effects on Silica Agglomerate Dispersion and on Processability During Mixing in EPDM. J. Ind. Eng. Chem. 2001, 7, 50–57. [Google Scholar]
- Kim, K.J. Bifunctional silane (TESPD) effects on silica containing elastomer compound: Part I: Natural Rubber (NR). Elastomers Compos. 2009, 44, 134–142. [Google Scholar]
- Jeon, D.K.; Kim, K.J. Bifunctional silane (TESPD) effects on silica containing elastomer compound: Part II: Styrene-co-Butadiene Rubber (SBR). Elastomers Compos. 2009, 44, 252–259. [Google Scholar]
- Kim, K.J. Bifunctional silane (TESPD) effects on improved mechanical properties of silica containing nitrile-butadiene rubber/poly(vinyl chloride) compound. J. Appl. Polym. Sci. 2012, 124, 2937. [Google Scholar] [CrossRef]
- Dang, T.T.N.; Kim, J.K.; Kim, K.J. Concentration effects of organosilane (TESPD) on mechanical properties of silica filled Silicone Rubber/Natural Rubber compounds. Int. Polym. Proc. 2011, 26, 368–374. [Google Scholar] [CrossRef]
- Kim, K.J. Amino silane, vinyl silane, TESPD, ZS (TESPD/zinc complex) effects on carbon black/clay filled chlorobutyl rubber (CIIR) compounds; Part I: Effects on hard clay/carbon black filled compounds. Carbon Lett. 2009, 10, 101–108. [Google Scholar] [CrossRef]
- Kim, K.J. Amino silane, vinyl silane, TESPD, ZS (TESPD/zinc complex) effects on carbon black/clay filled chlorobutyl rubber (CIIR) compounds; Part II: Effects on soft clay/carbon black filled compounds. Carbon Lett. 2009, 10, 109–113. [Google Scholar] [CrossRef][Green Version]
- Kim, K.J. Amino silane, vinyl silane, TESPD, ZS (TESPD/zinc complex) effects on carbon black/clay filled chlorobutyl rubber (CIIR) compounds; Part III: Comparative studies on hard clay and soft clay filled compounds. Carbon Lett. 2009, 10, 190–197. [Google Scholar] [CrossRef][Green Version]
- Kim, K.J.; VanderKooi, J. Rheological Effects of Zinc Surfactant on the TESPT-Silica Mixture in NR and S-SBR Compounds. Int. Polym. Proc. 2002, 17, 192–200. [Google Scholar] [CrossRef]
- Kim, K.J.; VanderKooi, J. Zinc Surfactant Effects on Processability and Mechanical Properties of Silica Filled Natural Rubber Compounds. J. Ind. Eng. Chem. 2004, 10, 772–781. [Google Scholar]
- Kim, K.J. Ethylene–propylene–diene terpolymer/silica compound modification with organosilane [bis(triethoxysilylpropyl)disulfide] and improved processability and mechanical properties. J. Appl. Polym. Sci. 2010, 116, 237–244. [Google Scholar] [CrossRef]
- Dang, T.T.N.; Kim, J.K.; Kim, K.J. Zinc Surfactant Effects on Improved Mechanical Properties of SR/NR/Silica/TESPD Compounds. Int. Polym. Proc. 2009, 24, 359–367. [Google Scholar] [CrossRef]
- Kim, K.J.; VanderKooi, J. Moisture Effects on Improved Hydrolysis Reaction for TESPT and TESPD-Silica Compounds. Compos. Interfaces 2004, 11, 471–488. [Google Scholar] [CrossRef]
- Kim, K.J.; VanderKooi, J. Moisture Effects on TESPDSilica/CB/SBR Compounds. Rubber Chem. Technol. 2005, 78, 84–104. [Google Scholar] [CrossRef]
- Kim, K.J.; VanderKooi, J. Temperature Effects of Silane Coupling on Moisture Treated Silica Surface. J. Appl. Polym. Sci. 2005, 95, 623–633. [Google Scholar] [CrossRef]
- Kim, S.M.; Nam, C.S.; Kim, K.J. TMTD, MBTS, and CBS accelerator effects on a silica filled natural rubber compound upon vulcanization properties. Appl. Chem. Eng. 2011, 22, 144–148. [Google Scholar]
- Choi, C.Y.; Kim, S.M.; Park, Y.H.; Jang, M.K.; Nah, J.W.; Kim, K.J. Effects of Thiuram, thiazole, and sulfenamide accelerators on silica filled natural rubber compound upon vulcanization and mechanical properties. Appl. Chem. Eng. 2011, 22, 411–415. [Google Scholar]
- Dang, T.T.N.; Kim, J.K.; Lee, S.H.; Kim, K.J. Vinyl functional group effects on mechanical and thermal properties of silica-filled Silicone Rubber/Natural Rubber blends. Compos. Interf. 2011, 18, 151–168. [Google Scholar] [CrossRef]
- Dang, T.T.N.; Kim, J.K.; Kim, K.J. Organo bifunctional silane effects on the vibration, thermal, and mechanical properties of a vinyl-group-containing silicone rubber/natural rubber/silica compound. J. Vinyl Addit. Technol. 2010, 16, 254–260. [Google Scholar] [CrossRef]
- Lorenz, O.; Echte, E. The Vulcanization of Elastomers. 13. The Vulcanization of Natural Rubber with Sulfur in the Presence of Mercaptobenzothiazole. II. Rubber Chem. Technol. 1958, 31, 117–131. [Google Scholar] [CrossRef]
- Scheele, W.; Cherubim, M. Vulcanization of Elastomers. 30. Kinetics of the Decrease of Sulfur Concentration during Vulcanization. Rubber Chem. Technol. 1961, 34, 606–628. [Google Scholar] [CrossRef]
- Morita, E.; Young, E.J. A Study of Sulfenamide Acceleration. Rubber Chem. Technol. 1963, 36, 844–862. [Google Scholar] [CrossRef]
- Bhatnagar, S.K.; Banerjee, S. Kinetics of Accelerated Vulcanization—III N-Cyclohexyl-Benzothiazole-2-Sulfenamide Accelerated Sulfur Vulcanization of Rubbers. Rubber Chem. Technol. 1969, 42, 1366–1382. [Google Scholar] [CrossRef]
- Wolff, S. Empirisch ermittelte Zusammenhange Zwischen Kautschuk/Fullstoff-Wechselwirkung und Prufwerten statischer und dynamischer Vulkanisat-Untersuchungen. Kautsch. Gummi. Kunstst. 1969, 22, 367. [Google Scholar]
- Wolff, S. Moeglichkeit einer neuen Charakterisierrung der Traction Resistance, Brevet Wirkungsweise von Russen in 1, 5-Polyenen. Kautsch. Gummi. Kunstst. 1970, 23, 7–14. [Google Scholar]
- Wolff, S. Chemical Aspects of Rubber Reinforcement by Fillers. Rubber Chem. Technol. 1996, 69, 325–346. [Google Scholar] [CrossRef]
- Wolff, S.; Wang, M.J. Filler—Elastomer Interactions. Part IV. The Effect of the Surface Energies of Fillers on Elastomer Reinforcement. Rubber Chem. Technol. 1992, 65, 329–342. [Google Scholar] [CrossRef]
- Tan, E.H.; Wolff, S.; Haddeman, M.; Grewatta, H.P.; Wang, M.J. Filler—Elastomer Interactions. Part IX. Performance of Silicas in Polar Elastomers. Rubber Chem. Technol. 1993, 66, 594–604. [Google Scholar] [CrossRef]
- Kim, K.J. Silane effects on in-rubber silica dispersion and silica structure (alpha(F)): A Review. Asian J. Chem. 2013, 25, 5119–5123. [Google Scholar] [CrossRef]
- Kim, K.J. Overview of Hydrolysis: A Review Part I- Hydrolysis Mechanism. Elast. Compos. 2020, 55, 128–136. [Google Scholar]
- Kim, K.J. Overview of Hydrolysis: A Review Part II- Hydrolysis Application. Elast. Compos. 2020, 55, 137–146. [Google Scholar]
- Arkles, B.; Steinmetz, J.R.; Zazyczny, J.; Metha, P. Silanes and Other Coupling Agents; Mittal, K.L., Ed.; VSP: Utrecht, The Netherlands, 1992; p. 93. [Google Scholar]
- Oral, U.; Hunsche, A. Advanced investigations into the silica/silane reaction system. Soc. Rubber Ind. 1998, 71, 549–561. [Google Scholar]
- Oral, U.; Hunsche, A.; Müller, A.; Koban, H.G. Investigations into the Silica/Silane Reaction System. Rubber Chem. Technol. 1997, 70, 608–623. [Google Scholar]
- Hunsche, A.; Gorl, U.; Müller, A.; Knaack, M.; Göbel, T. Investigations concerning the reaction silica/organosilane and organosilane/polymer. Part 1: Reaction mechanism and reaction model for silica/organosilane. Kautsch. Gummi Kunstat. 1997, 50, 881–889. [Google Scholar]
- Lee, J.Y.; Kim, K.J. MEG effects on hydrolysis of polyamide 66/glass fiber composites and mechanical property changes. Molecules 2019, 24, 755. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.W., Jr. Chapter. 13. In Steric Effects in Organic Chemistry; Newman, M.S., Ed.; Wiley: New York, NY, USA, 1956. [Google Scholar]
- McNeil, K.J.; DiCapri, J.A.; Walsh, D.A.; Pratt, R.F. Kinetics and mechanism of hydrolysis of a silicate triester, tris(2-methoxyethoxy)phenylsilane. J. Am. Chem. Soc. 1980, 102, 1859–1865. [Google Scholar] [CrossRef]
- Vorokonov, M.G.; Meleshkevisk, V.P.; Yuzekelvski, Y.A. The Siloxane Bond; Plenum Press: New York, NY, USA, 1978; pp. 375–380. [Google Scholar]
- DeTar, D.F. Effects of alkyl groups on rates of acyl-transfer reactions. J. Org. Chem. 1980, 45, 5166–5174. [Google Scholar] [CrossRef]
- Jencks, W.P.; Salvesen, K. Equilibrium deuterium isotope effects on the ionization of thiol acids. J. Am. Chem. Soc. 1971, 93, 4433–4436. [Google Scholar] [CrossRef]
- Prassas, M.; Hench, L.L. Ultrastructure Processing of Ceramics; Hench, L., Ulrich, D., Eds.; John Wiley: New York, NY, USA, 1984; p. 100. [Google Scholar]
- Allen, K.W. Silane Coupling Agents; Mittal, K.L., Ed.; VSP: Utrechi, The Netherlands, 1992. [Google Scholar]
- Kay, B.D.; Assink, R.A. Sol-gel kinetics: II. Chemical speciation modeling. J. Non-Cryst. Solids 1988, 104, 112–122. [Google Scholar] [CrossRef]
- Osterholtz, F.D.; Pohl, E.P. Silane Coupling Agents; Mittal, K.L., Ed.; VSP: Utrechi, The Netherlands, 1992. [Google Scholar]
- Moore, M.J. Silanes improve rubber-to-metal bonding. Rubber Plast. News 2002, 31, 14–16. [Google Scholar]
- Kim, K.J.; VanderKooi, J. Reactive Batch Mixing for Improved Silica-Silane Coupling. Int. Polym. Process. 2004, 19, 364–373. [Google Scholar] [CrossRef]
- Hashim, A.S.; Zahare, B.A.; Ikeda, Y.; Ohjiya, S.K. The Effect of Bis(3-Triethoxysilylpropyl) Tetrasulfide on Silica Reinforcement of Styrene-Butadiene Rubber. Rubber Chem. Technol. 1998, 78, 289–299. [Google Scholar] [CrossRef]
- Heiken, D.; Barentsen, W. Particle dimensions in polystyrene/polyethylene blends as a function of their melt viscosity and of the concentration of added graft copolymer. Polymer 1977, 18, 69–72. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, K.J. Overview of Polyamide Resins and Composites: A Review. Elast. Compos. 2016, 51, 317–341. [Google Scholar] [CrossRef]
- Roberts, A.D. Chapter 12. In Natural Rubber Science and Technology; Oxford Science Publishers: New York, NY, USA, 1988. [Google Scholar]
- Kim, K.J.; VanderKooi, J. Effects of Zinc Ion Containing Surfactant on Bifunctional Silane Treated Silica Compounds in Natural Rubber. J. Ind. Eng. Chem. 2002, 8, 334–347. [Google Scholar]
- Farnsworth, M.; Kline, C. Zinc Chemicals; Chales Kline and Co.: New York, NY, USA, 1983. [Google Scholar]
- Duchacek, V.; Kuta, A.; Pribyl, P. Efficiency of metal activators of accelerated sulfur vulcanization. J. Appl. Polym. Sci. 1993, 47, 743–746. [Google Scholar] [CrossRef]
- Kokes, R. Catalysis of Hydrocarbons and Related Studies. Intra-Sci. Rept. 1972, 6, 77. [Google Scholar]



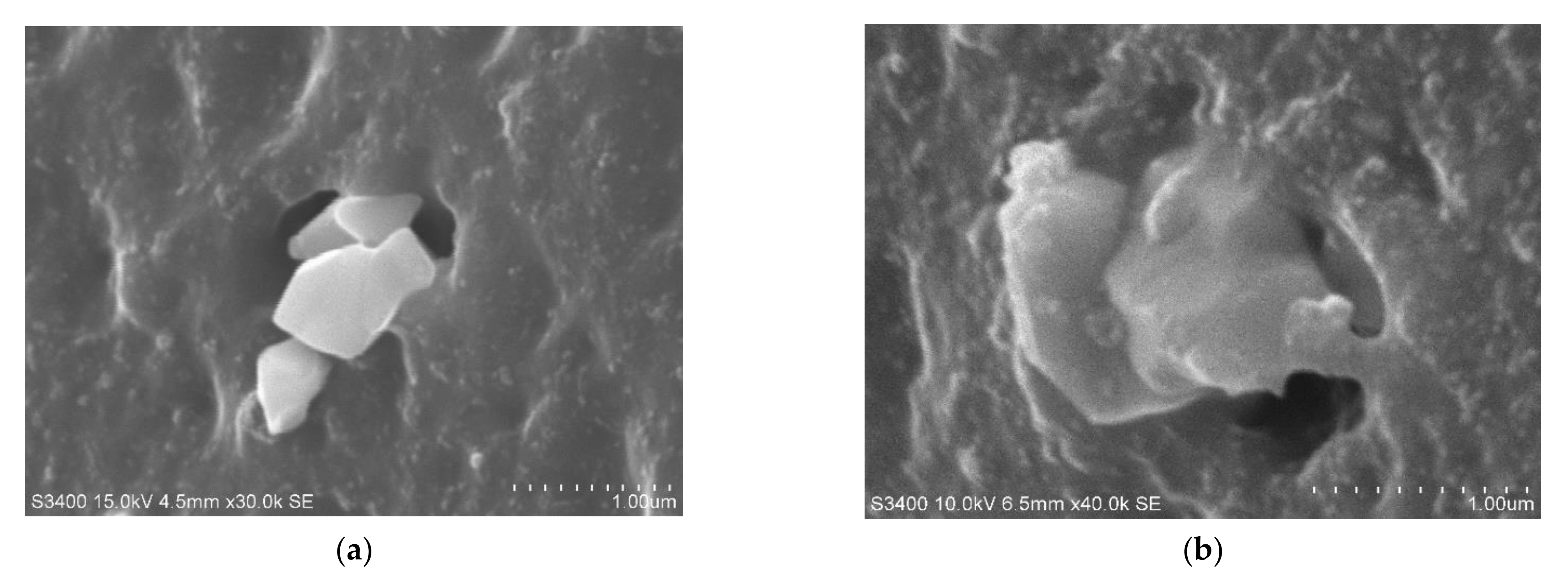
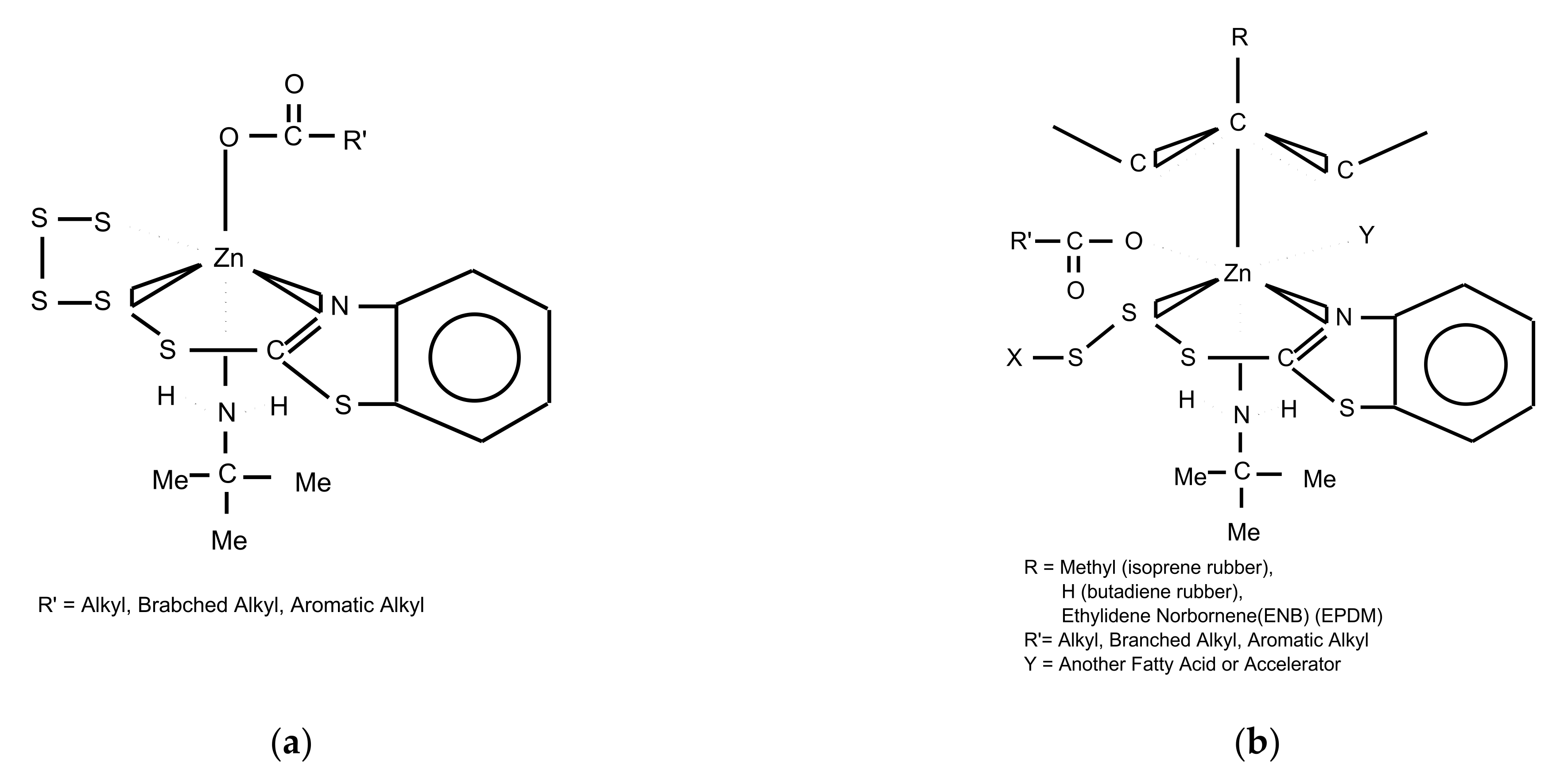
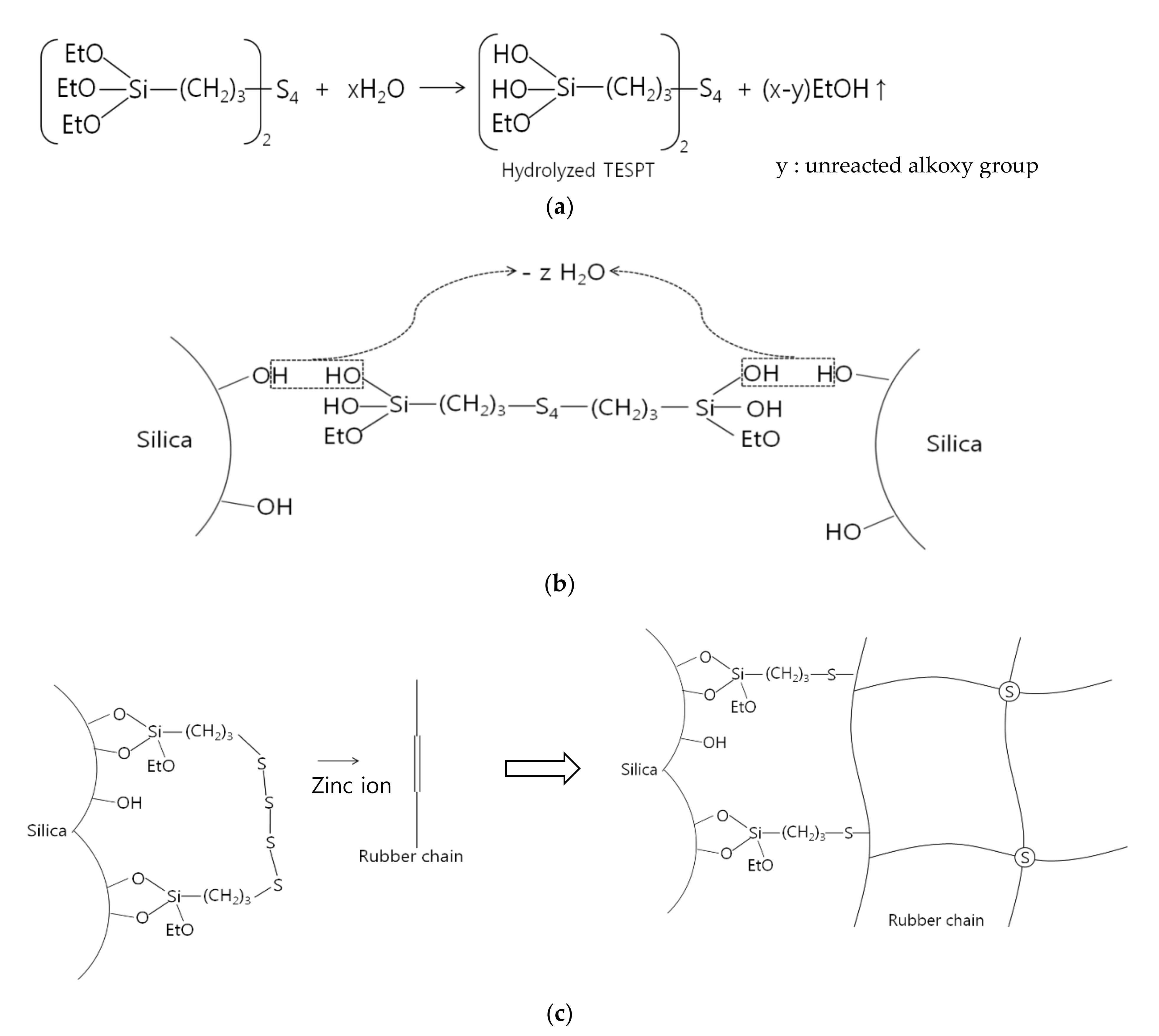
| Silane | Structure | Curing System | Application |
|---|---|---|---|
| TESPT | (C2H5O)3Si−(CH2)3−S4−(CH2)3−Si(OC2H5) | Sulfur | Tire treads, shoe soles, industrial rubber goods |
| TESPD | (C2H5O)3Si−(CH2)3−S2−(CH2)3−Si(OC2H5) | Sulfur | Tire treads, industrial rubber goods |
| TCPTEO | (C2H5O)3Si−(CH2)3−SCN | Sulfur | Shoe soles, industrial rubber goods |
| MTMO | (CH3O)3Si−(CH2)3−SH | Sulfur | Shoe soles, industrial rubber goods |
| VTEO | (C2H5O)3Si−CH=CH2 | Peroxide | Industrial rubber goods |
| VTEO | (CH3−O−C2H4O)Si−CH=CH2 | Peroxide | Industrial rubber goods |
| CPTEO | (CH3O)3Si−(CH2)3−Cl | Metal oxide | Chloroprene rubber |
| MEMO | (CH3O)3Si−(CH2)3−O−C(O)C(CH3)=CH2 | Peroxide | Textile adhesion |
| AMEO | (C2H5O)3Si−(CH2)3−NH2 | Sulfur | Special polymers, metal adhesion |
| OCTEO | (CH3−CH2−O)3Si−(CH2)7−CH3 | Sulfur, peroxide | Processing aid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, C.S.; Kim, K.-J. Interfacial Adhesion in Silica-Silane Filled NR Composites: A Short Review. Polymers 2022, 14, 2705. https://doi.org/10.3390/polym14132705
Ryu CS, Kim K-J. Interfacial Adhesion in Silica-Silane Filled NR Composites: A Short Review. Polymers. 2022; 14(13):2705. https://doi.org/10.3390/polym14132705
Chicago/Turabian StyleRyu, Chang Seok, and Kwang-Jea Kim. 2022. "Interfacial Adhesion in Silica-Silane Filled NR Composites: A Short Review" Polymers 14, no. 13: 2705. https://doi.org/10.3390/polym14132705
APA StyleRyu, C. S., & Kim, K.-J. (2022). Interfacial Adhesion in Silica-Silane Filled NR Composites: A Short Review. Polymers, 14(13), 2705. https://doi.org/10.3390/polym14132705