Understanding the Influence of Gypsum upon a Hybrid Flame Retardant Coating on Expanded Polystyrene Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Gypsum-Based HFR Formulation
2.3. Preparation of the Flame-Retardant EPS Foam
2.4. Characterization
3. Results and Discussion
3.1. Thermogravimetric Analysis (TGA)
3.2. Microstructural Study
3.3. Combustion Behavior
3.4. Cone Calorimetry
3.5. Char Residue Analysis
3.6. Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, W.; Yao, Y.; Guo, J.; Fei, B.; Gu, X.; Li, H.; Sun, J.; Zhang, S. Toward an understanding of how red phosphorus and expandable graphite enhance the fire resistance of expandable polystyrene foams. J. Appl. Polym. Sci. 2020, 137, 49045. [Google Scholar] [CrossRef]
- Shao, X.; Du, Y.; Zheng, X.; Wang, J.; Wang, Y.; Zhao, S.; Xin, Z.; Li, L. Reduced fire hazards of expandable polystyrene building materials via intumescent flame-retardant coatings. J. Mater. Sci. 2020, 55, 7555–7572. [Google Scholar] [CrossRef]
- Demirel, B. Optimization of the composite brick composed of expanded polystyrene and pumice blocks. Constr. Build. Mater. 2013, 40, 306–313. [Google Scholar] [CrossRef]
- Raps, D.; Hossieny, N.; Park, C.B.; Altstädt, V. Past and present developments in polymer bead foams and bead foaming technology. Polymer 2015, 56, 5–19. [Google Scholar] [CrossRef]
- Cao, B.; Gu, X.; Song, X.; Jin, X.; Liu, X.; Liu, X.; Sun, J.; Zhang, S. The flammability of expandable polystyrene foams coated with melamine modified urea formaldehyde resin. J. Appl. Polym. Sci. 2017, 134, 44423. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Liu, P.; Jing, Z.; Ge, X.; Jiang, Y. The flame resistance properties of expandable polystyrene foams coated with a cheap and effective barrier layer. Constr. Build. Mater. 2018, 176, 403–414. [Google Scholar] [CrossRef]
- Lu, H.; Wilkie, C.A. Study on intumescent flame retarded polystyrene composites with improved flame retardancy. Polym. Degrad. Stab. 2010, 95, 2388–2395. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Liu, N. Ignition of expandable polystyrene foam by a hot particle: An experimental and numerical study. J. Hazard. Mater. 2015, 283, 536–543. [Google Scholar] [CrossRef]
- Antonatus, E. Fire safety of etics with EPS material properties and relevance for fire safety during transport, construction and under end use conditions in external thermal insulation component systems. MATEC Web Conf. 2013, 9, 02008. [Google Scholar] [CrossRef]
- Khanal, S.; Zhang, W.; Ahmed, S.; Ali, M.; Xu, S. Effects of intumescent flame retardant system consisting of tris (2-hydroxyethyl) isocyanurate and ammonium polyphosphate on the flame retardant properties of high-density polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 444–451. [Google Scholar] [CrossRef]
- Bensabath, T.; Sarazin, J.; Jimenez, M.; Samyn, F.; Bourbigot, S. Intumescent polypropylene: Interactions between physical and chemical expansion. Fire Mater. 2021, 45, 387–395. [Google Scholar] [CrossRef]
- Da Silveira, M.R.; Peres, R.S.; Moritz, V.F.; Ferreira, C.A. Intumescent coatings based on tannins for fire protection. Mater. Res. 2019, 22, e20180433. [Google Scholar] [CrossRef]
- Maqsood, M.; Langensiepen, F.; Seide, G. The efficiency of biobased carbonization agent and intumescent flame retardant on flame retardancy of biopolymer composites and investigation of their melt-spinnability. Molecules 2019, 24, 1513. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Tang, M.; Wang, N.; Liu, N.; Chen, X.; Zhang, Z.; Zhang, K.; Lu, X. Efficient organic-inorganic intumescent interfacial flame retardants to prepare flame retarded polypropylene with excellent performance. RSC Adv. 2017, 7, 31696–31706. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, F.H.; Jian, R.K.; Ai, Y.F.; Ma, J.L.; Hui, G.J.; Wang, D.Y. Influence of eco-friendly calcium gluconate on the intumescent flame-retardant epoxy resin: Flame retardancy, smoke suppression and mechanical properties. Compos. Part B Eng. 2019, 176, 107200. [Google Scholar] [CrossRef]
- Bhoite, S.P.; Kim, J.; Jo, W.; Bhoite, P.H.; Mali, S.S.; Park, K.H.; Hong, C.K. Expanded polystyrene beads coated with intumescent flame retardant material to achieve fire safety standards. Polymers 2021, 13, 2662. [Google Scholar] [CrossRef]
- Beh, J.H.; Yew, M.C.; Saw, L.H.; Yew, M.K. Fire resistance and mechanical properties of intumescent coating using novel bioash for steel. Coatings 2020, 10, 1117. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Zhao, Z.; Liu, X.; Jiang, L.; Huang, K.; Zhao, S. Synergistic Fire Hazard Effect of a Multifunctional Flame Retardant in Building Insulation Expandable Polystyrene through a Simple Surface-Coating Method. ACS Omega 2020, 5, 799–807. [Google Scholar] [CrossRef]
- Choi, J.; Lee, G.; Kim, S.; Choi, K. Investigation on sex hormone-disruption effects of two novel brominated flame retardants (Dbdpe and btbpe) in male zebrafish (danio rerio) and two human cell lines (h295r and mvln). Appl. Sci. 2021, 11, 3837. [Google Scholar] [CrossRef]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef]
- Covaci, A.; Harrad, S.; Abdallah, M.A.E.; Ali, N.; Law, R.J.; Herzke, D.; de Wit, C.A. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 2011, 37, 532–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Nie, X.; Tian, M.; Luo, X.; An, T.; Mai, B. Photolytic degradation of decabromodiphenyl ethane (DBDPE). Chemosphere 2012, 89, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Huhtala, S. In vivo and vitro toxicity of decabromodiphenyl ethane, a flame retardant. Environ. Toxicol. 2009, 25, 333–338. [Google Scholar] [CrossRef]
- Hornsby, P.R. Fire retardant fillers for polymers. Int. Mater. Rev. 2001, 46, 199–210. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame retardant mechanisms of red phosphorus and magnesium hydroxide in high impact polystyrene. Macromol. Chem. Phys. 2004, 205, 2185–2196. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Y.; Qiu, R.; Xu, W.; Hou, Y. Investigation of UiO-66 as Flame Retardant and Its Application in Improving Fire Safety of Polystyrene. Macromol. Res. 2020, 28, 42–50. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2014, 51, 28–73. [Google Scholar] [CrossRef]
- Rajaei, M.; Wang, D.Y.; Bhattacharyya, D. Combined effects of ammonium polyphosphate and talc on the fire and mechanical properties of epoxy/glass fabric composites. Compos. Part B Eng. 2017, 113, 381–390. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of chicken eggshell on the flame-retardant and smoke suppression properties of an epoxy-based traditional APP-PER-MEL system. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
- Pedersen, B.F.; Semmingsen, D. Neutron Diffraction Refinement of the Structure of Gypsum, CaSO4·2H2O. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 1074–1077. [Google Scholar]
- Thomas, G. Thermal properties of gypsum plasterboard at high temperatures. Fire Mater. 2002, 26, 37–45. [Google Scholar] [CrossRef]
- Charola, A.E.; Pühringer, J.; Steiger, M. Gypsum: A review of its role in the deterioration of building materials. Environ. Geol. 2007, 52, 207–220. [Google Scholar] [CrossRef]
- Javangula, H.; Lineberry, Q. Comparative studies on fire-rated and standard gypsum wallboard. J. Therm. Anal. Calorim. 2014, 116, 1417–1433. [Google Scholar] [CrossRef]
- Ballirano, P.; Melis, E. Thermal behaviour and kinetics of dehydration of gypsum in air from in situ real-time laboratory parallel-beam X-ray powder diffraction. Phys. Chem. Miner. 2009, 36, 391–402. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Luz Sánchez, M.; Valverde, J.L.; Carmona, M.; Rodríguez, J.F. Thermal testing and numerical simulation of gypsum wallboards incorporated with different PCMs content. Appl. Energy 2011, 88, 930–937. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Effect of cenospheres on the char formation and fire protective performance of water-based intumescent coatings on structural steel. Prog. Org. Coatings 2016, 92, 8–15. [Google Scholar] [CrossRef]
- Kannan, P.; Biernacki, J.J.; Visco, D.P.; Lambert, W. Kinetics of thermal decomposition of expandable polystyrene in different gaseous environments. J. Anal. Appl. Pyrolysis 2009, 84, 139–144. [Google Scholar] [CrossRef]
- Chiang, C.L.; Hsu, S.W. Novel epoxy/expandable graphite halogen-free flame retardant composites-preparation, characterization, and properties. J. Polym. Res. 2010, 17, 315–323. [Google Scholar] [CrossRef]
- Duquesne, S.; Le Bras, M.; Bourbigot, S.; Delobel, R.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T. Thermal degradation of polyurethane and polyurethane/expandable graphite coatings. Polym. Degrad. Stab. 2001, 74, 493–499. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2017, 138, 106–114. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.; Li, L.; Wang, J.; He, W.; Chen, X. Effects of thermal-oxidative aging on the flammability, thermal degradation kinetics and mechanical properties of DBDPE flame retardant long glass fiber reinforced polypropylene composites. Polym. Compos. 2018, 39, E1733–E1741. [Google Scholar] [CrossRef]
- Chen, X.S.; Yu, Z.Z.; Liu, W.; Zhang, S. Synergistic effect of decabromodiphenyl ethane and montmorillonite on flame retardancy of polypropylene. Polym. Degrad. Stab. 2009, 94, 1520–1525. [Google Scholar] [CrossRef]
- Luo, X.; He, M.; Guo, J.B.; Wu, B. Flame retardancy and mechanical properties of brominated flame retardant for long glass fiber reinforced polypropylene composites. Adv. Mater. Res. 2013, 750–752, 85–89. [Google Scholar] [CrossRef]
- Deodhar, S.; Shanmuganathan, K.; Fan, Q.; Wilkie, C.A.; Costache, M.C.; Dembsey, N.A.; Patra, P.K. Calcium carbonate and ammonium polyphosphate-based flame retardant composition for polypropylene. J. Appl. Polym. Sci. 2011, 120, 1866–1873. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Yan, L. Comparative study of fire resistance and char formation of intumescent fire-retardant coatings reinforced with three types of shell bio-fillers. Polymers 2021, 13, 4333. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Saini, P.; Dhawan, S.K. composite—Super paramagnetic behavior and variable range hopping 1D conduction mechanism—Synthesis and characterization. Polym. Adv. Technol. 2008, 19, 229–236. [Google Scholar] [CrossRef]
- Ghazi Wakili, K.; Hugi, E.; Wullschleger, L.; Frank, T. Gypsum board in fire—Modeling and experimental validation. J. Fire Sci. 2007, 25, 267–282. [Google Scholar] [CrossRef]
- Tsai, K.C. Orientation effect on cone calorimeter test results to assess fire hazard of materials. J. Hazard. Mater. 2009, 172, 763–772. [Google Scholar] [CrossRef]
- Seo, D.; Kim, D.; Kim, B.; Kwon, Y. An experimental study on the combustibles investigation and fire growth rate for predicting initial fire behavior in building. Procedia Eng. 2013, 62, 671–679. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Wu, W.; Meng, W.; Xie, W.; Cui, Y.; Xu, J.; Qu, H. Core-shell graphitic carbon nitride/zinc phytate as a novel efficient flame retardant for fire safety and smoke suppression in epoxy resin. Polymers 2020, 12, 212. [Google Scholar] [CrossRef] [PubMed]

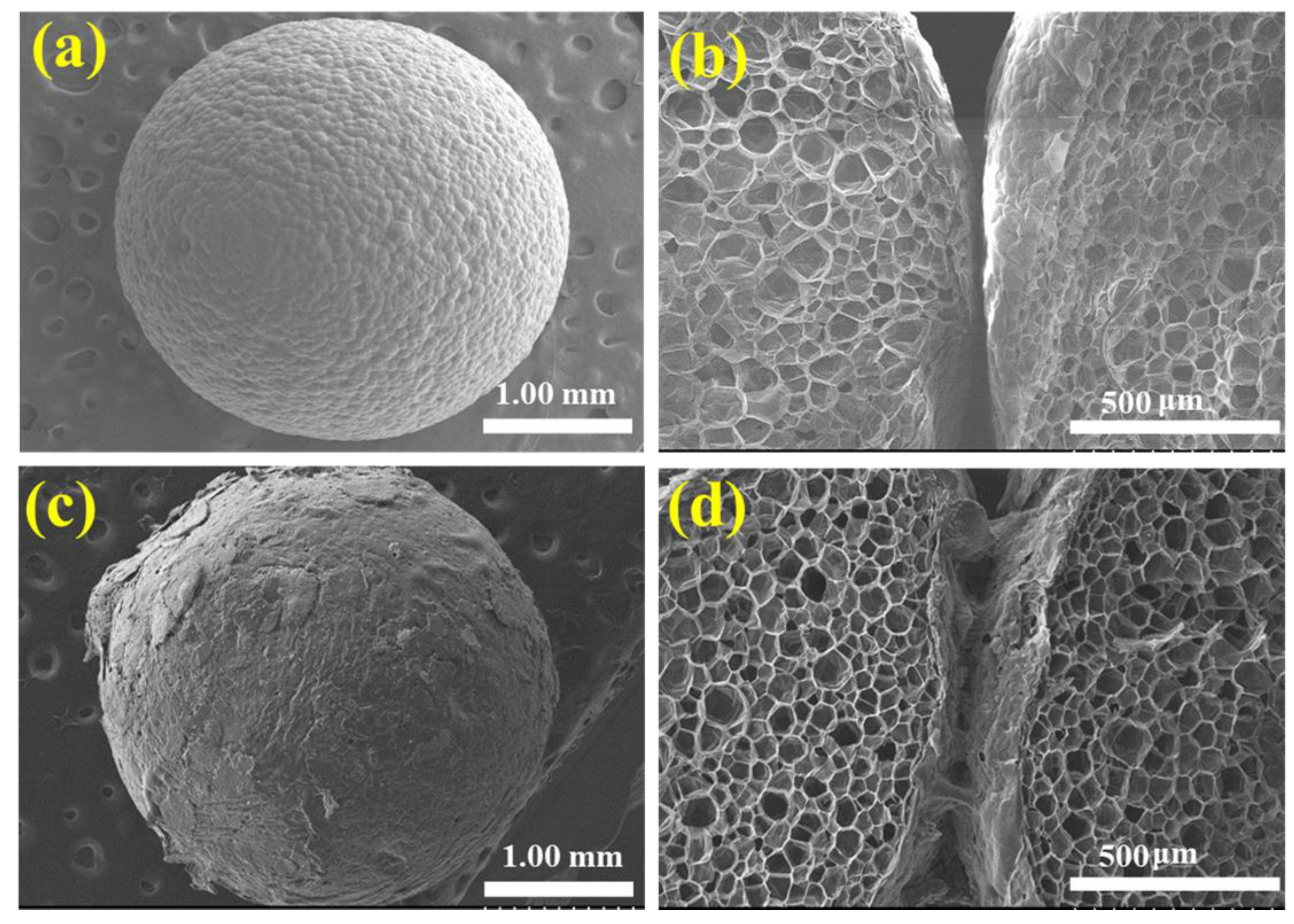


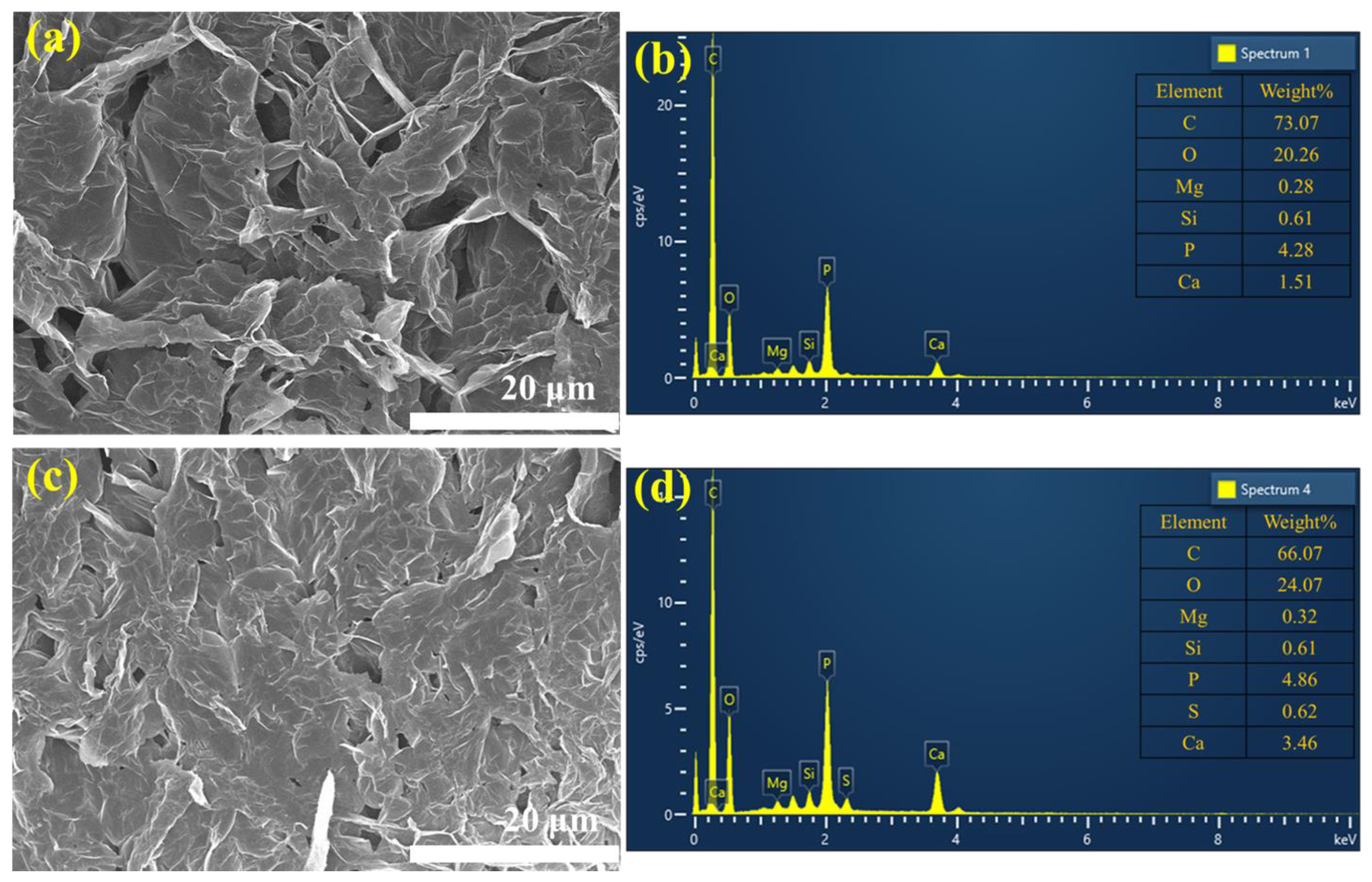
| Sample | Binder a (g) | Gypsum (g) | EG (g) | Water (mL) |
|---|---|---|---|---|
| HFR0 | 55 | 0 | 40 | 95 |
| HFR9 | 55 | 9 | 40 | 104 |
| HFR12 | 55 | 12 | 40 | 110 |
| HFR15 | 55 | 15 | 40 | 114 |
| Sample | PHRR (kW/m2) | THR (MJ/m2) | FIGRA (W/m2·s) |
|---|---|---|---|
| EPS | 310.5 | 42.1 | 6530.8 |
| EPS/HFR0 | 67.1 | 15.9 | 2764.1 |
| EPS/HFR9 | 57.5 | 13.4 | 2119.0 |
| EPS/HFR12 | 53.1 | 8.0 | 1682.9 |
| EPS/HFR15 | 55.8 | 10.6 | 2147.2 |
| Sample | Density (kg/m3) | Thermal Conductivity (W/m.K) |
|---|---|---|
| EPS | 26 | 0.028 |
| EPS/HFR0 | 68 | 0.038 |
| EPS/HFR9 | 71 | 0.038 |
| EPS/HFR12 | 72 | 0.038 |
| EPS/HFR15 | 74 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhoite, S.P.; Kim, J.; Jo, W.; Bhoite, P.H.; Mali, S.S.; Park, K.-H.; Hong, C.K. Understanding the Influence of Gypsum upon a Hybrid Flame Retardant Coating on Expanded Polystyrene Beads. Polymers 2022, 14, 3570. https://doi.org/10.3390/polym14173570
Bhoite SP, Kim J, Jo W, Bhoite PH, Mali SS, Park K-H, Hong CK. Understanding the Influence of Gypsum upon a Hybrid Flame Retardant Coating on Expanded Polystyrene Beads. Polymers. 2022; 14(17):3570. https://doi.org/10.3390/polym14173570
Chicago/Turabian StyleBhoite, Sangram P., Jonghyuck Kim, Wan Jo, Pravin H. Bhoite, Sawanta S. Mali, Kyu-Hwan Park, and Chang Kook Hong. 2022. "Understanding the Influence of Gypsum upon a Hybrid Flame Retardant Coating on Expanded Polystyrene Beads" Polymers 14, no. 17: 3570. https://doi.org/10.3390/polym14173570
APA StyleBhoite, S. P., Kim, J., Jo, W., Bhoite, P. H., Mali, S. S., Park, K.-H., & Hong, C. K. (2022). Understanding the Influence of Gypsum upon a Hybrid Flame Retardant Coating on Expanded Polystyrene Beads. Polymers, 14(17), 3570. https://doi.org/10.3390/polym14173570






