Ionic Liquid-Cured Epoxy/PCL Blends with Improved Toughness and Adhesive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
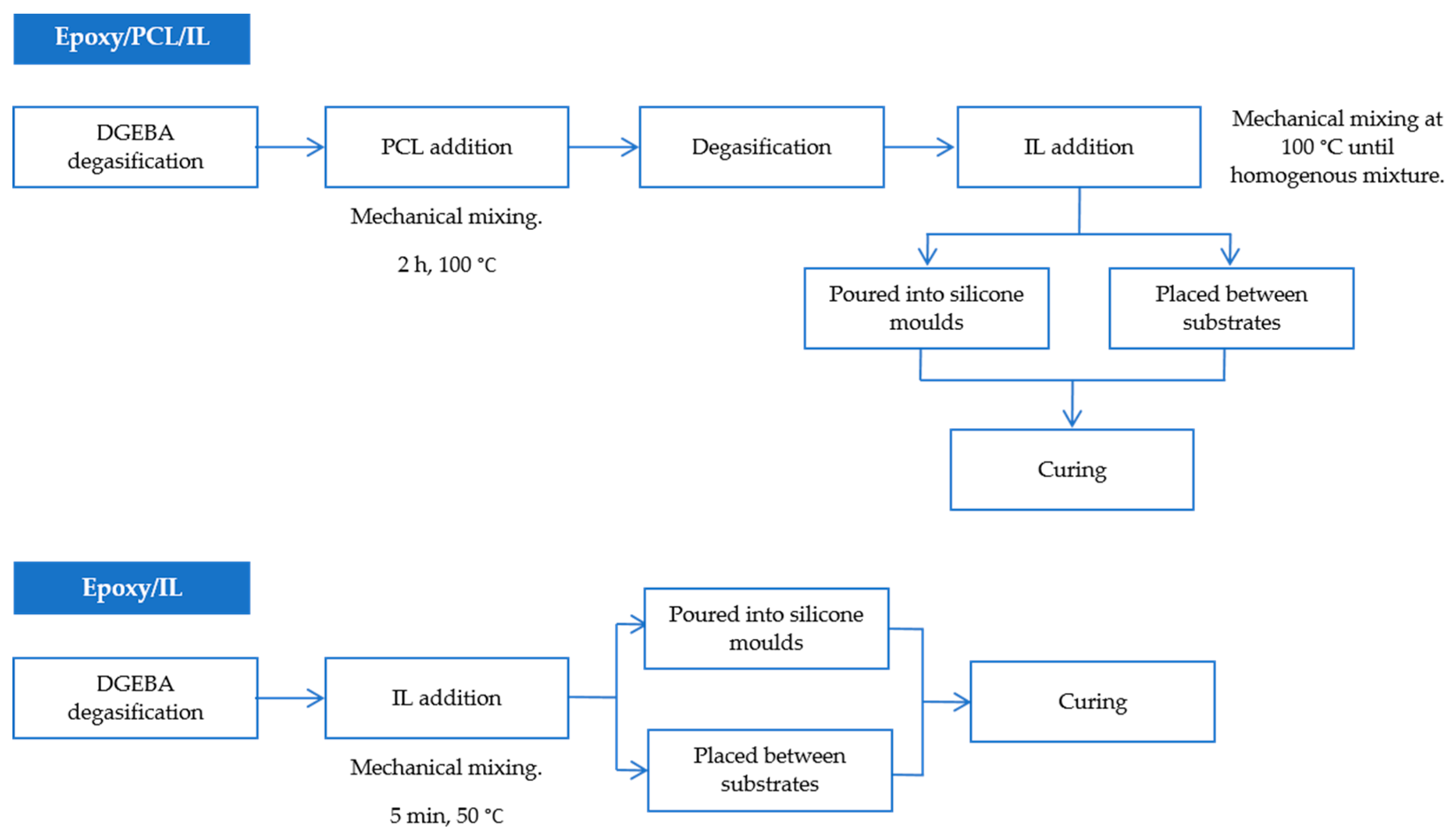
2.2. Preparation of Samples
2.3. Characterization
2.3.1. Phase Behaviour
2.3.2. Microstructure
2.3.3. Mechanical Properties
2.3.4. Adhesive Properties
3. Results and Discussion
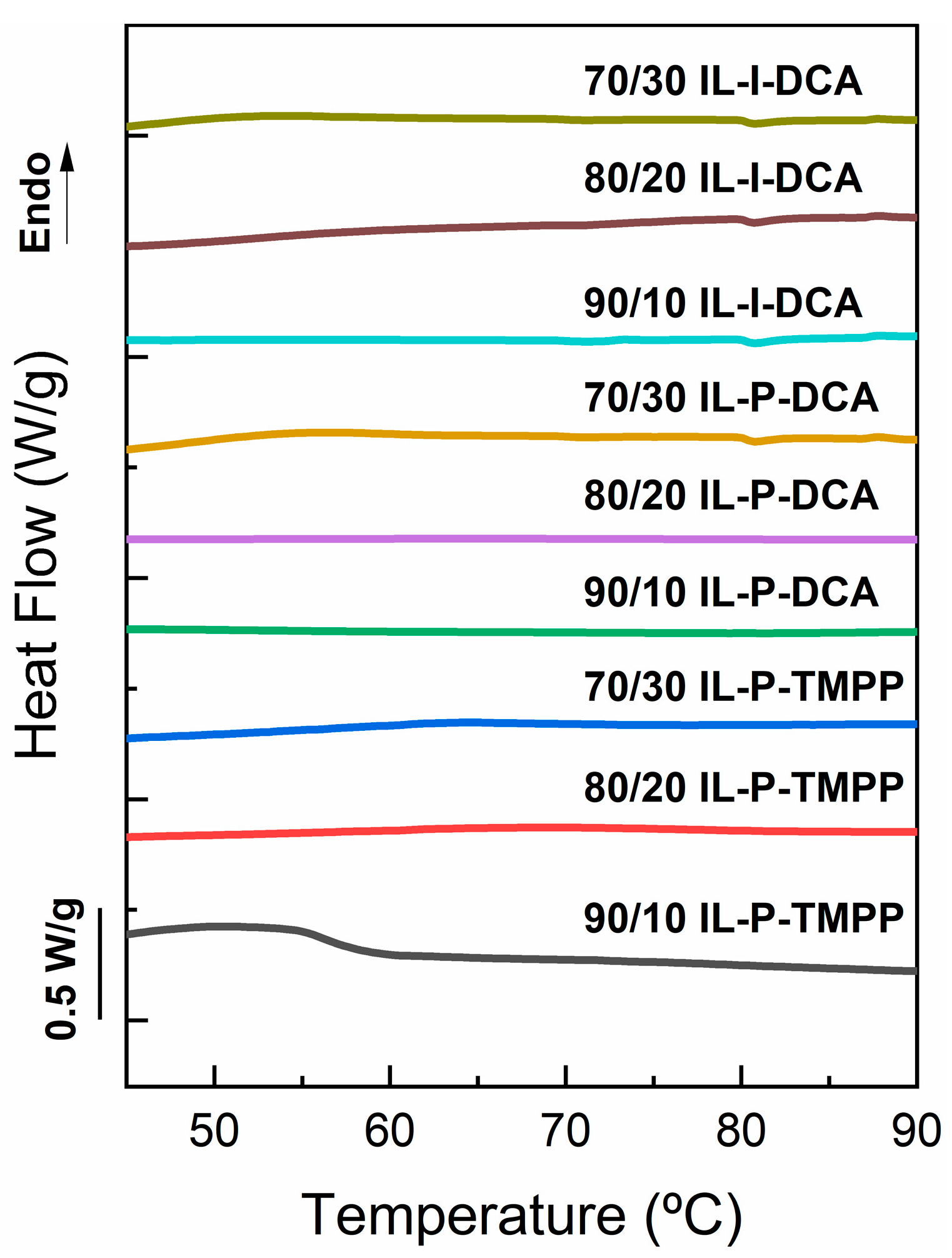
3.1. Phase Behaviour
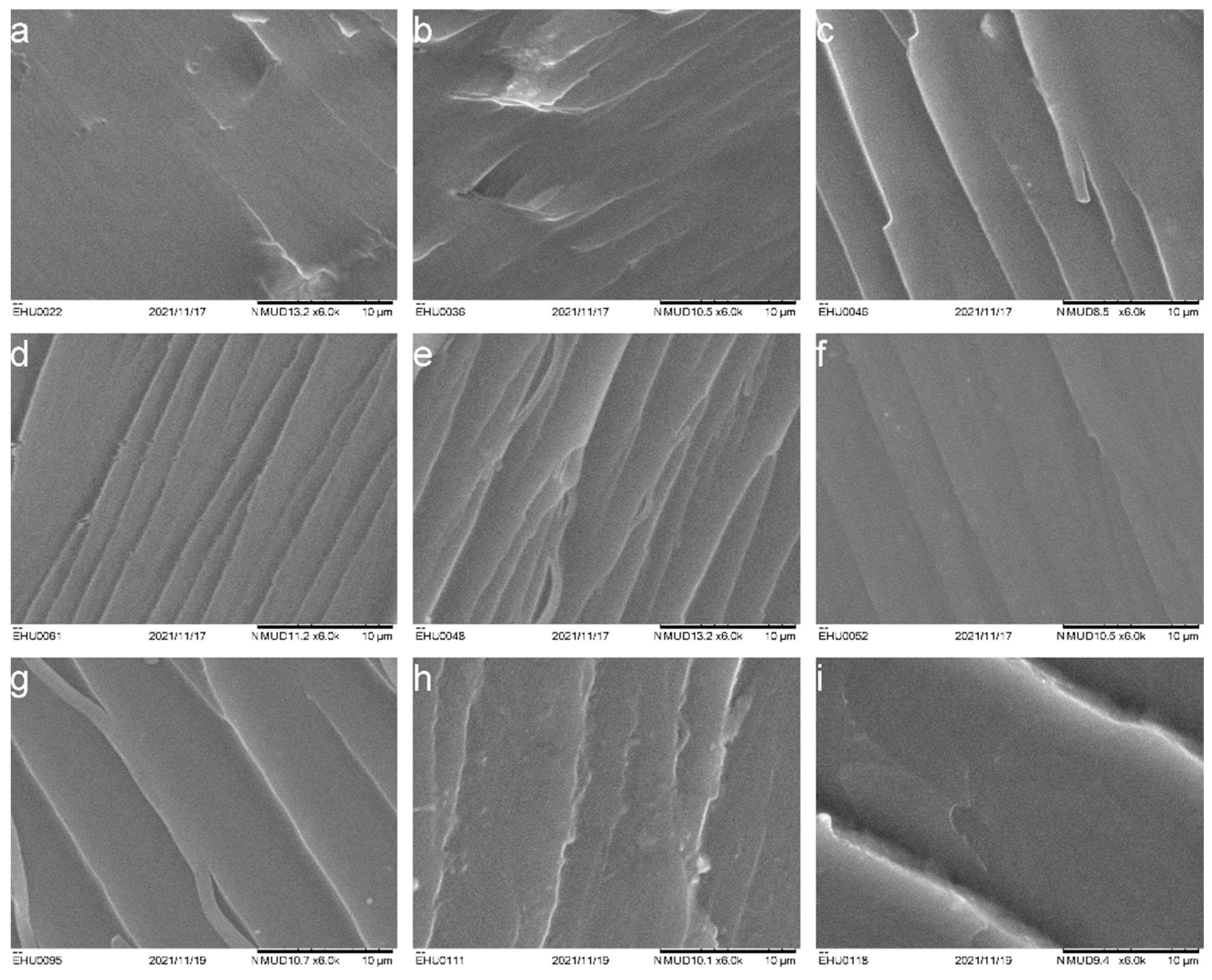
3.2. Microstructure
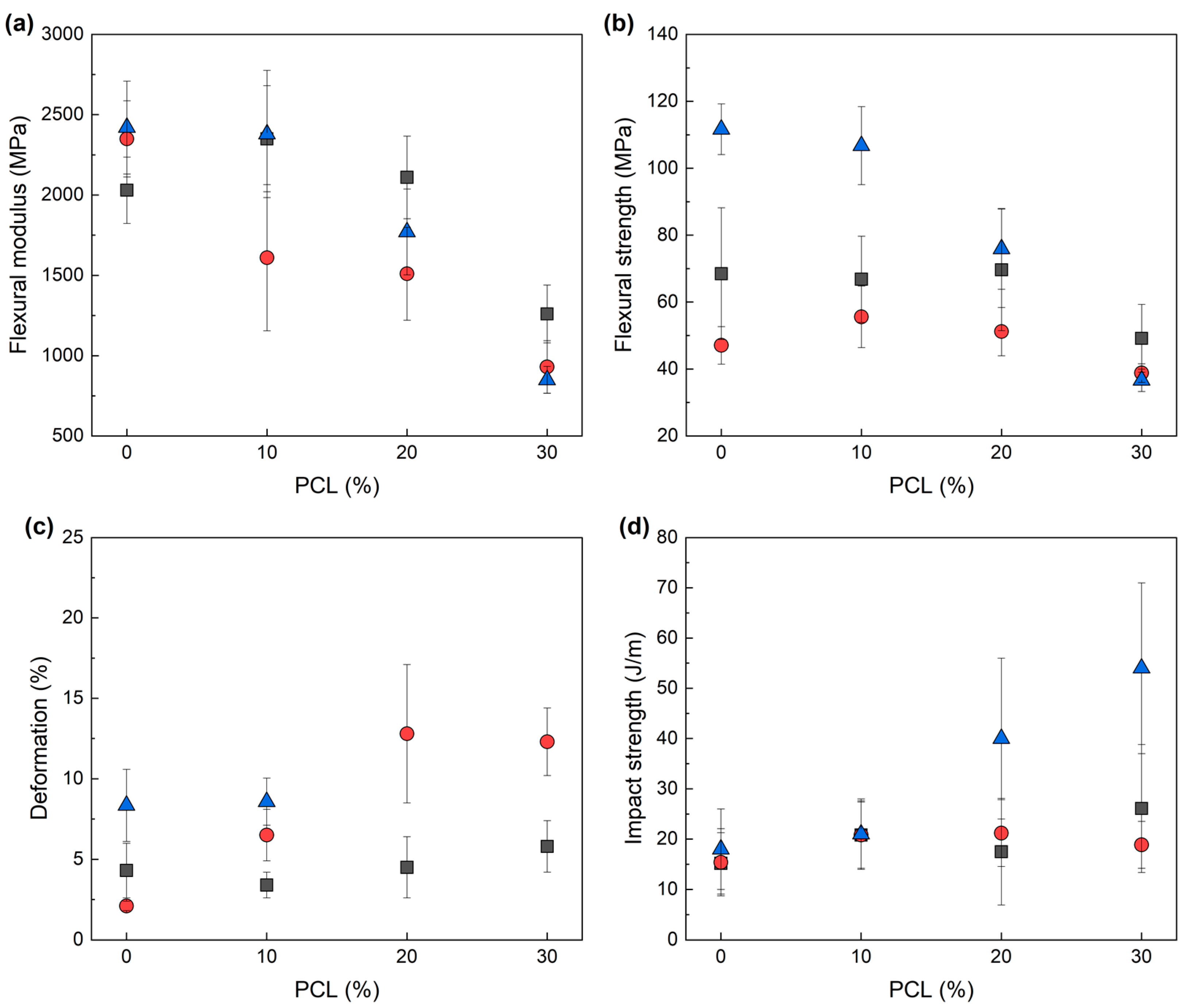
3.3. Mechanical Properties
3.4. Adhesive Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Ahmadi, Z. Nanostructured epoxy adhesives: A review. Prog. Org. Coat. 2019, 135, 449–453. [Google Scholar] [CrossRef]
- Maggiore, S.; Banea, M.D.; Stagnaro, P.; Luciano, G. A review of structural adhesive joints in hybrid joining processes. Polymers 2021, 13, 3961. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Kausar, A. Performance of corrosion protective epoxy blend-based nanocomposite coatings: A review. Polym. Plast. Technol. Mater. 2020, 59, 658–673. [Google Scholar] [CrossRef]
- Wazalwar, R.; Sahu, M.; Raichur, A.M. Mechanical properties of aerospace epoxy composites reinforced with 2D nano-fillers: Current status and road to industrialization. Nanoscale Adv. 2021, 3, 2741–2776. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, H.; Tzounis, L.; Santagiuliana, G.; Bilotti, E.; Papageorgiou, D.G. Multifunctional epoxy nanocomposites reinforced by two-dimensional materials: A review. Carbon 2021, 185, 57–81. [Google Scholar] [CrossRef]
- Jayan, J.S.; Saritha, A.; Joseph, K. Innovative materials of this era for toughening the epoxy matrix: A review. Polym. Compos. 2018, 39, E1959–E1986. [Google Scholar] [CrossRef]
- Ratna, D.; Banthia, A.K. Toughened epoxy adhesive modified with acrylate based liquid rubber. Polym. Int. 2000, 49, 281–287. [Google Scholar] [CrossRef]
- Ratna, D.; Banthia, A.K. Rubber toughened epoxy. Macromol. Res. 2004, 12, 11–21. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.T.; Wu, J.H.; Ma, L.J.; Finlow, D.E.; Lin, S.Q.; Song, K.M. Thermal and mechanical properties of epoxy resin toughened with epoxidized soybean oil. J. Therm. Anal. Calorim. 2013, 113, 939–945. [Google Scholar] [CrossRef]
- Ratna, D. Mechanical properties and morphology of epoxidized soyabean-oil-modified epoxy resin. Polym. Int. 2001, 50, 179–184. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L.; Lee, J.R. Thermal and mechanical properties of tetrafunctional epoxy resin toughened with epoxidized soybean oil. Mater. Sci. Eng. 2004, 374, 109–114. [Google Scholar] [CrossRef]
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Fabrication and evaluation of acrylated epoxidized castor oil-toughened diglycidyl ether of bisphenol A nanocomposites. Can. J. Chem. Eng. 2015, 93, 2107–2116. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L.; Lee, J.R. Effect of biodegradable epoxidized castor oil on physicochemical and mechanical properties of epoxy resins. Macromol. Chem. Phys. 2004, 205, 2048–2054. [Google Scholar] [CrossRef]
- Chen, J.L.; Chang, F.C. Temperature-dependent phase behavior in poly(ε-caprolactone)-epoxy blends. Polymer 2001, 42, 2193–2199. [Google Scholar] [CrossRef]
- Luo, X.; Liu, X.-F.; Ding, X.-M.; Chen, L.; Chen, S.-C.; Wang, Y.-Z. Effects of curing temperature on the structure and properties of epoxy resin-poly(ε-caprolactam) blends. Polymer 2021, 228, 123940. [Google Scholar] [CrossRef]
- Remiro, P.M.; Marieta, C.; Riccardi, C.; Mondragon, I. Influence of curing conditions on the morphologies of a PMMA-modified epoxy matrix. Polymer 2001, 42, 9909–9914. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Sidhardhan, S.K.; Jose, S.; Hameed, N.; Salim, N.V.; Siengchin, S.; Pionteck, J.; Magueresse, A.; Grohens, Y. Miscibility, phase morphology, thermomechanical, viscoelastic and surface properties of poly(ε-caprolactone) modified epoxy systems: Effect of curing agents. Ind. Eng. Chem. Res. 2016, 55, 10055–10064. [Google Scholar] [CrossRef]
- Raghava, R.S. Development and characterization of thermosetting thermoplastic polymer blends for applications in damage-tolerant composites. J. Polym. Sci. B Polym. Phys. 1988, 26, 65–81. [Google Scholar] [CrossRef]
- Raghava, R.S. Role of matrix-particle interface adhesion on fracture-toughness of dual phase epoxy-polyethersulfone blend. J. Polym. Sci. B Polym. Phys. 1987, 25, 1017–1031. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Partridge, I.K. Phase-separation in cross-linked resins containing polymeric modifiers. Polym. Eng. Sci. 1986, 26, 54–62. [Google Scholar] [CrossRef]
- Chen, J.L.; Chang, F.C. Phase separation process in poly(ε-caprolactone)-epoxy blends. Macromolecules 1999, 32, 5348–5356. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Partridge, I.K. Phase-separation in epoxy-resins containing polyethersulfone. Polymer 1983, 24, 639–644. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Gilbert, A.H. Toughening tetrafunctional epoxy-resins using polyetherimide. Polymer 1989, 30, 213–217. [Google Scholar] [CrossRef]
- Das, B.; Chakraborty, D.; Hajra, A.K.; Sinha, S. Epoxy poly(methyl methacrylate) interpenetrating polymer networks morphology, mechanical and thermal-properties. J. Appl. Polym. Sci. 1994, 53, 1491–1496. [Google Scholar] [CrossRef]
- Guo, Q.P.; Peng, X.S.; Wang, Z.J. The miscibility and morphology of epoxy resin poly(ethylene oxide) blends. Polymer 1991, 32, 53–57. [Google Scholar] [CrossRef]
- Chen, M.C.; Hourston, D.J.; Sun, W.B. Miscibility and fracture-behavior of an epoxy-resin bisphenol-a polycarbonate blend. Eur. Polym. J. 1992, 28, 1471–1475. [Google Scholar] [CrossRef]
- Barone, L.; Carciotto, S.; Cicala, G.; Recca, A. Thermomechanical properties of epoxy/poly(ε-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1576–1582. [Google Scholar] [CrossRef]
- Clark, J.N.; Daly, J.H.; Garton, A. Hydrogen-bonding in epoxy-resin poly(ε-caprolactone) blends. J. Appl. Polym. Sci. 1984, 29, 3381–3390. [Google Scholar] [CrossRef]
- Cohades, A.; Manfredi, E.; Plummer, C.J.G.; Michaud, V. Thermal mending in immiscible poly(ε-caprolactone)/epoxy blends. Eur. Polym. J. 2016, 81, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Siddhamalli, S.K. Toughening of epoxy/polycaprolactone composites via reaction induced phase separation. Polym. Compos. 2000, 21, 846–855. [Google Scholar] [CrossRef]
- Noshay, A.; Robeson, L.M. Epoxy/modifier block copolymers. J. Polym. Sci. A Polym. Chem. 1974, 12, 689–705. [Google Scholar] [CrossRef]
- Guo, Q.; Harrats, C.; Groeninckx, G.; Reynaers, H.; Koch, M.H.J. Miscibility, crystallization and real-time small-angle X-ray scattering investigation of the semicrystalline morphology in thermosetting polymer blends. Polymer 2001, 42, 6031–6041. [Google Scholar] [CrossRef]
- Chen, J.L.; Huang, H.M.; Li, M.S.; Chang, F.C. Transesterification in homogeneous poly(ε-caprolactone)-epoxy blends. J. Appl. Polym. Sci. 1999, 71, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Zheng, S.X. Influence of intramolecular specific interactions on phase behavior of epoxy resin and poly(ε-caprolactone) blends cured with aromatic amines. Polymer 2005, 46, 5828–5839. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, H.; Guo, Q. Epoxy resin/poly(ε-caprolactone) blends cured with 2,2-bis[4-(4-aminophenoxy)phenyl]propane. I. Miscibility and crystallization kinetics. J. Polym. Sci. B Polym. Phys. 2003, 41, 1085–1098. [Google Scholar] [CrossRef]
- Zheng, S.; Guo, Q.; Chan, C.-M. Epoxy resin/poly(ε-caprolactone) blends cured with 2,2-bis[4-(4-aminophenoxy)phenyl]propane. II. Studies by Fourier transform infrared and carbon-13 cross-polarization/magic-angle spinning nuclear magnetic resonance spectroscopy. J. Polym. Sci. B Polym. Phys. 2003, 41, 1099–1111. [Google Scholar] [CrossRef]
- Kishi, H.; Uesawa, K.; Matsuda, S.; Murakami, A. Adhesive strength and mechanisms of epoxy resins toughened with pre-formed thermoplastic polymer particles. J. Adhes. Sci. Technol. 2005, 19, 1277–1290. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Robert, T.M.; Mathew, D.; Suma, D.D.; Thomas, D. Novel epoxy resin adhesives toughened by functionalized poly (ether ether ketone) s. Int. J. Adhes. Adhes. 2021, 106, 102816. [Google Scholar] [CrossRef]
- Ekrem, M.; Avci, A. Effects of polyvinyl alcohol nanofiber mats on the adhesion strength and fracture toughness of epoxy adhesive joints. Compos. Part B Eng. 2018, 138, 256–264. [Google Scholar] [CrossRef]
- Buonocore, G.G.; Schiavo, L.; Attianese, I.; Borriello, A. Hyperbranched polymers as modifiers of epoxy adhesives. Compos. Part B Eng. 2013, 53, 187–192. [Google Scholar] [CrossRef]
- Arnebold, A.; Wellmann, S.; Hartwig, A. Partially crystalline epoxy networks with superior mechanical and adhesion properties. J. Adhes. Sci. Technol. 2016, 30, 960–971. [Google Scholar] [CrossRef]
- Luo, X.F.; Ou, R.Q.; Eberly, D.E.; Singhal, A.; Viratyaporn, W.; Mather, P.T. A thermoplastic/thermoset blend exhibiting thermal mending and reversible adhesion. ACS Appl. Mater. Interfaces 2009, 1, 612–620. [Google Scholar] [CrossRef]
- Luo, X.F.; Lauber, K.E.; Mather, P.T. A thermally responsive, rigid, and reversible adhesive. Polymer 2010, 51, 1169–1175. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Zenker, M. High performance epoxy composites cured with ionic liquids. J. Ind. Eng. Chem. 2015, 31, 192–198. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Sikorski, W. Deep eutectic ionic liquids as epoxy resin curing agents. Int. J. Polym. Anal. Charact. 2014, 19, 682–692. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T. Epoxy resin crosslinked with conventional and deep eutectic ionic liquids. Polimery 2012, 57, 456–462. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy resin/ionic liquid systems: The influence of imidazolium cation size and anion type on reactivity and thermomechanical properties. Ind. Eng. Chem. Res. 2012, 51, 5197–5206. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Spychaj, T. Ionic liquids as convenient latent hardeners of epoxy resins. Polimery 2003, 48, 833–835. [Google Scholar] [CrossRef]
- Rahmathullah, M.A.M.; Jeyarajasingam, A.; Merritt, B.; VanLandingham, M.; McKnight, S.H.; Palmese, G.R. Room temperature ionic liquids as thermally latent initiators for polymerization of epoxy resins. Macromolecules 2009, 42, 3219–3221. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, M.H.; Wei, W.; Zheng, C.M.; Gao, J.; Zhang, W.X.; Zheng, C.B.; Deng, P.Y.; Xing, Y. DGEBA/imidazolium ionic liquid systems: The influence of anions on the reactivity and properties of epoxy systems. J. Adhes. Sci. Technol. 2018, 32, 1114–1127. [Google Scholar] [CrossRef]
- Maksym, P.; Tarnacka, M.; Dzienia, A.; Matuszek, K.; Chrobok, A.; Kaminski, K.; Paluch, M. Enhanced polymerization rate and conductivity of ionic liquid-based epoxy resin. Macromolecules 2017, 50, 3262–3272. [Google Scholar] [CrossRef]
- Leclere, M.; Livi, S.; Marechal, M.; Picard, L.; Duchet-Rumeau, J. The properties of new epoxy networks swollen with ionic liquids. RSC Adv. 2016, 6, 56193–56204. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Livi, S.; Soares, B.G.; Pruvost, S.; Duchet-Rumeau, J.; Gerard, J.-F. Ionic liquids: A new route for the design of epoxy networks. ACS Sustain. Chem. Eng. 2016, 4, 481–490. [Google Scholar] [CrossRef]
- Livi, S.; Silva, A.A.; Thimont, Y.; Nguyen, T.K.L.; Soares, B.G.; Gerard, J.F.; Duchet-Rumeau, J. Nanostructured thermosets from ionic liquid building block-epoxy prepolymer mixtures. RSC Adv. 2014, 4, 28099–28106. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.L.; Livi, S.; Pruvost, S.; Soares, B.G.; Duchet-Rumeau, J. Ionic liquids as reactive additives for the preparation and modification of epoxy networks. J. Polym. Sci. A Polym. Chem. 2014, 52, 3463–3471. [Google Scholar] [CrossRef]
- Silva, A.A.; Livi, S.; Netto, D.B.; Soares, B.G.; Duchet, J.; Gerard, J.F. New epoxy systems based on ionic liquid. Polymer 2013, 54, 2123–2129. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Investigating the mechanism through which ionic liquids initiate the polymerisation of epoxy resins. Polymer 2018, 139, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.L.; Livi, S.; Soares, B.G.; Benes, H.; Geerard, J.F.; Duchet-Rumeau, J. Toughening of epoxy/ionic liquid networks with thermoplastics pased on Poly(2,6-dimethy1-1,4-phenylene ether) (PPE). ACS Sustain. Chem. Eng. 2017, 5, 1153–1164. [Google Scholar] [CrossRef]
- Halawani, N.; Donato, R.; Benes, H.; Brus, J.; Kobera, L.; Pruvost, S.; Duchet-Rumeau, J.; Gerard, J.-F.; Livi, S. Thermoset-thermoplastic-ionic liquid ternary hybrids as novel functional polymer materials. Polymer 2021, 218, 123507. [Google Scholar] [CrossRef]
- Orduna, L.; Razquin, I.; Aranburu, N.; Guerrica-Echevarría, G. Are ionic liquids effective curing agents for preparing epoxy adhesives? Int. J. Adhes. Adhes. 2022; under revision for publication. [Google Scholar]
- Heux, L.; Halary, J.L.; Laupretre, F.; Monnerie, L. Dynamic mechanical and C-13 nmr investigations of molecular motions involved in the beta relaxation of epoxy networks based on DGEBA and aliphatic amines. Polymer 1997, 38, 1767–1778. [Google Scholar] [CrossRef]
- Smirnov, Y.N.; Glavina, T.A.; Efremova, A.I. Effect of the molecular mobility of epoxy amine crosslinked polymers on their relaxation and physicomechanical characteristics. Polym. Sci. Ser. A 2011, 53, 30–36. [Google Scholar] [CrossRef]
- Lutzen, H.; Gesing, T.M.; Kim, B.K.; Hartwig, A. Novel cationically polymerized epoxy/poly(ε-caprolactone) polymers showing a shape memory effect. Polymer 2012, 53, 6089–6095. [Google Scholar] [CrossRef]
- Iregui, A.; Irusta, L.; Martin, L.; Gonzalez, A. Analysis of the process parameters for obtaining a stable electrospun process in different composition epoxy/Poly ε-caprolactone blends with shape memory properties. Polymers 2019, 11, 475. [Google Scholar] [CrossRef] [Green Version]
- Arnebold, A.; Wellmann, S.; Hartwig, A. Network dynamics in cationically polymerized, crosslinked epoxy resins and its influence on crystallinity and toughness. Polymer 2016, 91, 14–23. [Google Scholar] [CrossRef]
- Remiro, P.M.; Cortazar, M.M.; Calahorra, M.E.; Calafel, M.M. Miscibility and crystallization of an amine-cured epoxy resin modified with crystalline poly(ε-caprolactone). Macromol. Chem. Phys. 2001, 202, 1077–1088. [Google Scholar] [CrossRef]
- Chen, J.-L.; Chang, F.-C. Phase separation and melting behavior in poly(ε-caprolactone)-epoxy blends cured by 3,3′-dimethylmethylene-di(cyclohexylamine). J. Appl. Polym. Sci. 2003, 89, 3107–3114. [Google Scholar] [CrossRef] [Green Version]
- Ratna, D. Modification of epoxy resins for improvement of adhesion: A critical review. J. Adhes. Sci. Technol. 2003, 17, 1655–1668. [Google Scholar] [CrossRef]
- Ratna, D.; Banthia, A.K. Epoxidized soybean oil toughened epoxy adhesive. J. Adhes. Sci. Technol. 2000, 14, 15–25. [Google Scholar] [CrossRef]
- Quan, D.; Murphy, N.; Ivankovic, A. Fracture behaviour of a rubber nano-modified structural epoxy adhesive: Bond gap effects and fracture damage zone. Int. J. Adhes. Adhes. 2017, 77, 138–150. [Google Scholar] [CrossRef]
- Quan, D.; Murphy, N.; Ivankovic, A. Fracture behaviour of epoxy adhesive joints modified with core-shell rubber nanoparticles. Eng. Fract. Mech. 2017, 182, 566–576. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Impact-strength improvement of epoxy resins reinforced with a biodegradable polymer. Mater. Sci. Eng. 2008, 478, 402–405. [Google Scholar] [CrossRef]
- Zhou, H.Z.; Liu, H.Y.; Zhou, H.M.; Zhang, Y.; Gao, X.P.; Mai, Y.W. On adhesive properties of nano-silica/epoxy bonded single-lap joints. Mater. Des. 2016, 95, 212–218. [Google Scholar] [CrossRef]
- Quan, D.; Carolan, D.; Rouge, C.; Murphy, N.; Ivankovic, A. Mechanical and fracture properties of epoxy adhesives modified with graphene nanoplatelets and rubber particles. Int. J. Adhes. Adhes. 2018, 81, 21–29. [Google Scholar] [CrossRef]
- Quan, D.; Carolan, D.; Rouge, C.; Murphy, N.; Lyankoyic, A. Carbon nanotubes and core-shell rubber nanoparticles modified structural epoxy adhesives. J. Mater. Sci. 2017, 52, 4493–4508. [Google Scholar] [CrossRef]
- Bascom, W.D.; Cottington, R.L. Effect of temperature on adhesive fracture behaviour of an elastomer epoxy resin. J. Adhes. 1976, 7, 333–346. [Google Scholar] [CrossRef]
- Achary, P.S.; Latha, P.B.; Ramaswamy, R. Room temperature curing of CTBN-toughened epoxy adhesive with elevated-temperature service capability. J. Appl. Polym. Sci. 1990, 41, 151–162. [Google Scholar] [CrossRef]


| IL Type | Initiation Mechanism | Reference |
|---|---|---|
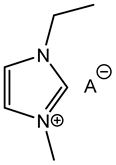 |  | [59] |
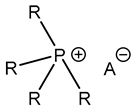
 | [49,59] | |
 | [59] | |
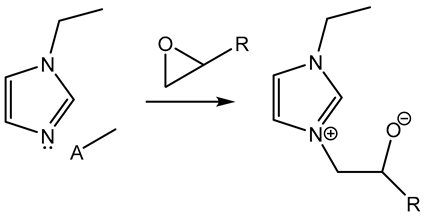
 |  | [56,57,58] |
| Material | Structure |
|---|---|
| DGEBA | 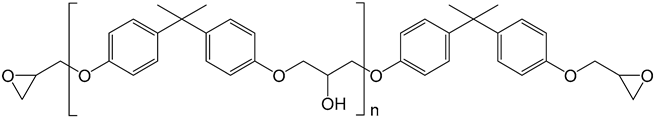 |
| PCL | 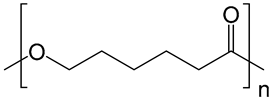 |
| IL-P-TMPP | 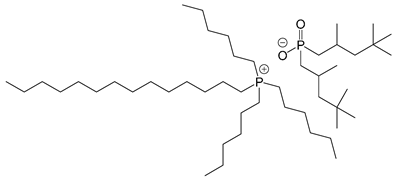 |
| IL-P-DCA |  |
| IL-I-DCA | 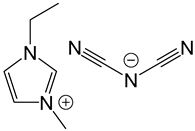 |
| Curing Agent | Concentration | Curing Protocol |
|---|---|---|
| IL-P-TMPP | 10 phr | 2 h 80 °C/2 h 120 °C/1 h 150 °C/1 h 170 °C |
| IL-P-DCA | 10 phr | 2 h 120 °C/2 h 140 °C/1 h 170 °C |
| IL-I-DCA | 10 phr | 2 h 110 °C/1 h 140 °C/1 h 170 °C |
| IL | PCL (%) | Tg (°C) | νe (mol/m3) |
|---|---|---|---|
| IL-P-TMPP | 0 | 168 | 11,509 |
| 10 | 141 | 6756 | |
| 20 | 112 | 4487 | |
| 30 | 83 | 3462 | |
| IL-P-DCA | 0 | 172 | 12,616 |
| 10 | 144 | 5338 | |
| 20 | 113 | 3117 | |
| 30 | 86 | 2252 | |
| IL-I-DCA | 0 | 160 | 4625 |
| 10 | 120 | 1912 | |
| 20 | 96 | 1197 | |
| 30 | 72 | 302 |
| IL | PCL (%) | Flexural Modulus (MPa) | Flexural Strength (MPa) | Deformation at Break (%) | Impact Strength (J/m) |
|---|---|---|---|---|---|
| IL-P-TMPP | 0 | 2030 ± 210 | 68.5 ± 19.7 | 4.3 ± 1.7 | 15 ± 6 |
| 10 | 2350 ± 330 | 66.8 ± 2.0 | 3.4 ± 0.8 | 21 ± 7 | |
| 20 | 2110 ± 260 | 69.7 ± 11.3 | 4.5 ± 1.9 | 18 ± 11 | |
| 30 | 1260 ± 180 | 49.2 ± 2.9 | 5.8 ± 1.6 | 26 ± 13 | |
| IL-P-DCA | 0 | 2350 ± 240 | 47.1 ± 5.6 | 2.1 ± 0.3 | 15 ± 7 |
| 10 | 1610 ± 450 | 55.6 ± 9.2 | 6.5 ± 1.6 | 21 ± 7 | |
| 20 | 1510 ± 290 | 51.2 ± 7.2 | 12.8 ± 4.4 | 21 ± 7 | |
| 30 | 930 ± 160 | 38.8 ± 2.8 | 12.3 ± 2.1 | 19 ± 5 | |
| IL-I-DCA | 0 | 2420 ± 290 | 111.6 ± 7.6 | 8.3 ± 2.2 | 18 ± 8 |
| 10 | 2380 ± 400 | 106.8 ± 11.7 | 8.6 ± 1.5 | 21 ± 7 | |
| 20 | 1770 ± 270 | 75.9 ± 12.1 | * | 40 ± 16 | |
| 30 | 850 ± 80 | 36.6 ± 3.3 | * | 54 ± 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orduna, L.; Razquin, I.; Otaegi, I.; Aranburu, N.; Guerrica-Echevarría, G. Ionic Liquid-Cured Epoxy/PCL Blends with Improved Toughness and Adhesive Properties. Polymers 2022, 14, 2679. https://doi.org/10.3390/polym14132679
Orduna L, Razquin I, Otaegi I, Aranburu N, Guerrica-Echevarría G. Ionic Liquid-Cured Epoxy/PCL Blends with Improved Toughness and Adhesive Properties. Polymers. 2022; 14(13):2679. https://doi.org/10.3390/polym14132679
Chicago/Turabian StyleOrduna, Lidia, Iker Razquin, Itziar Otaegi, Nora Aranburu, and Gonzalo Guerrica-Echevarría. 2022. "Ionic Liquid-Cured Epoxy/PCL Blends with Improved Toughness and Adhesive Properties" Polymers 14, no. 13: 2679. https://doi.org/10.3390/polym14132679
APA StyleOrduna, L., Razquin, I., Otaegi, I., Aranburu, N., & Guerrica-Echevarría, G. (2022). Ionic Liquid-Cured Epoxy/PCL Blends with Improved Toughness and Adhesive Properties. Polymers, 14(13), 2679. https://doi.org/10.3390/polym14132679






