Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Model Solutions
2.3. Pharmaceutical Pretreatment
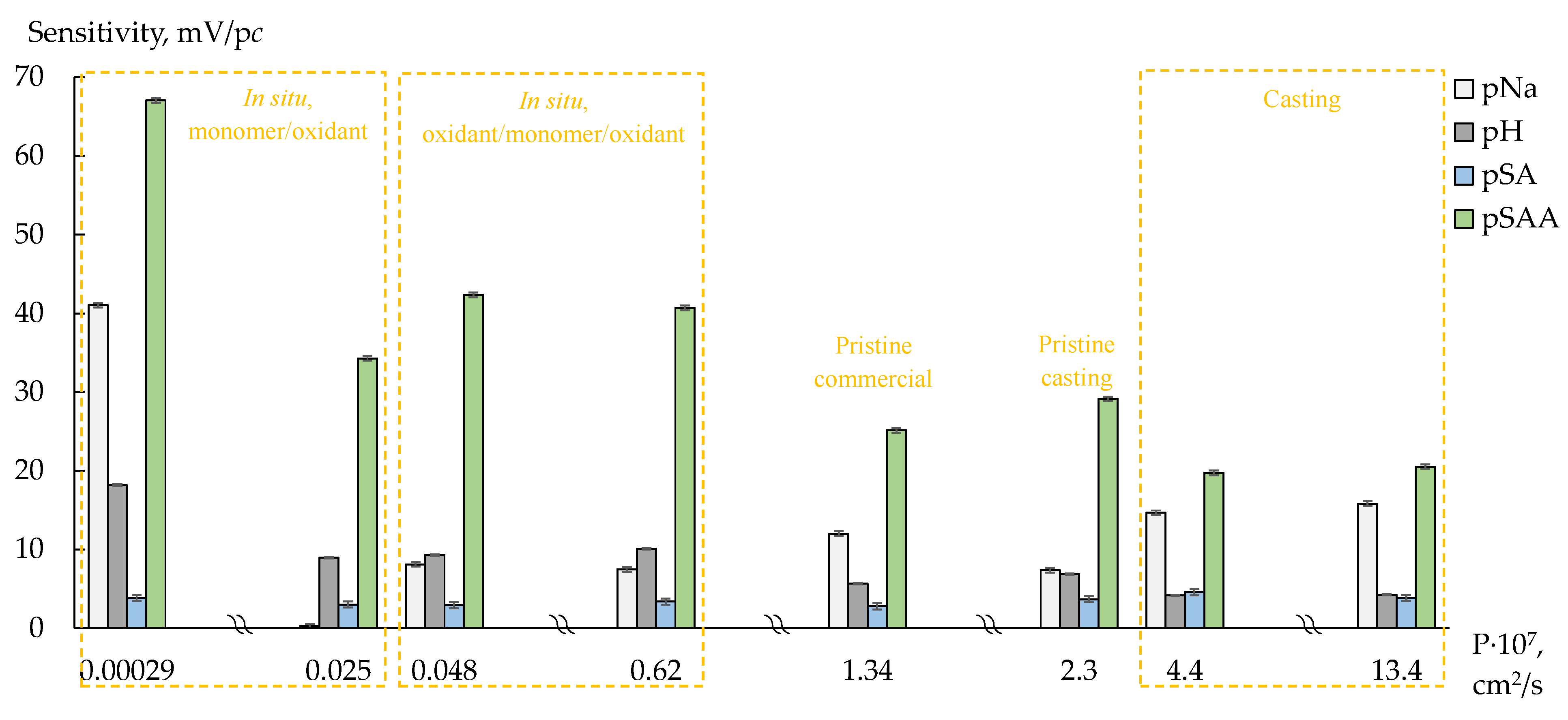
2.4. Membrane Preparation
2.5. Apparatus and Experiment Procedure
2.6. Data Processing Procedure
3. Results and Discussion
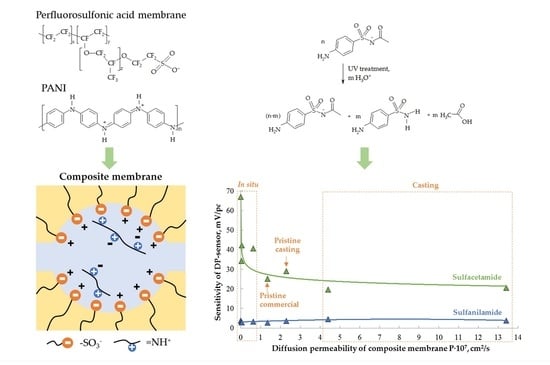
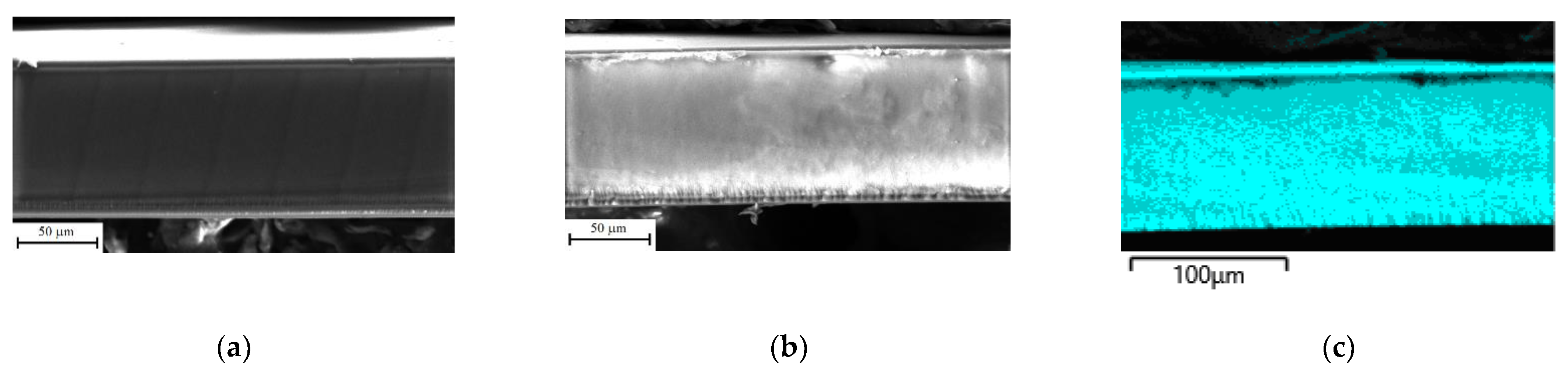
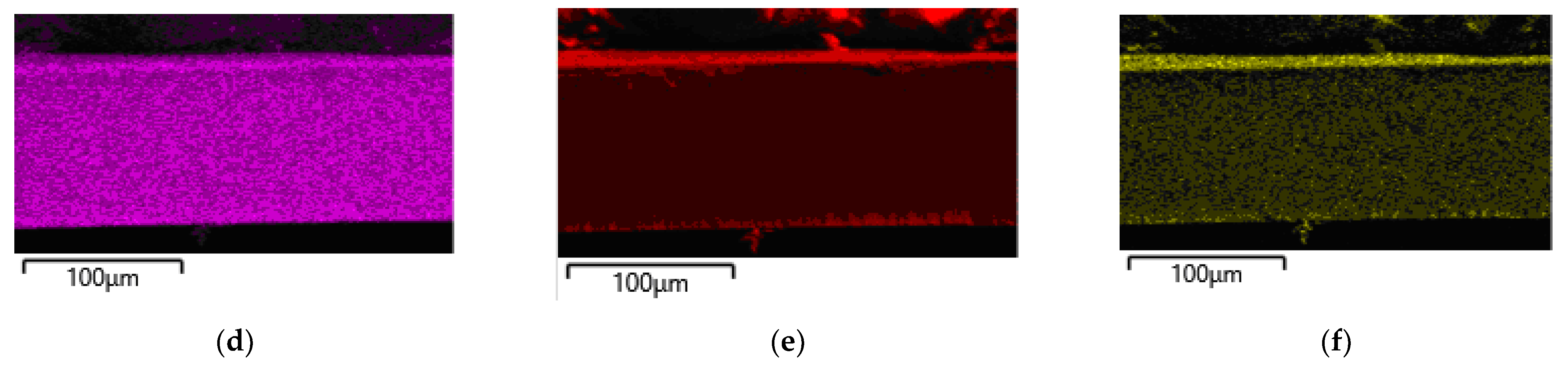
3.1. Properties of Membranes
3.2. Characteristics of the DP-Sensors
3.2.1. Stability and Reproducibility of the DP-Sensors
3.2.2. Cross-Sensitivity of the DP-Sensors
3.2.3. Analysis of Model Solutions and Pharmaceuticals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sengupta, P.; Chatterjee, B.; Tekade, R.K. Current Regulatory Requirements and Practical Approaches for Stability Analysis of Pharmaceutical Products: A Comprehensive Review. Int. J. Pharm. 2018, 543, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa e Silva, J.P. Photostabilization Strategies of Photosensitive Drugs. Int. J. Pharm. 2018, 541, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Khajouei, G.; Finklea, H.O.; Lin, L.-S. UV/Chlorine Advanced Oxidation Processes for Degradation of Contaminants in Water and Wastewater: A Comprehensive Review. J. Environ. Chem. Eng. 2022, 10, 107508. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater. Polymers 2020, 12, 2648. [Google Scholar] [CrossRef]
- El-Ragehy, N.A.; Hegazy, M.A.; AbdElHamid, G.; Tawfik, S.A. Validated Chromatographic Methods for the Simultaneous Determination of Sulfacetamide Sodium and Prednisolone Acetate in Their Ophthalmic Suspension. J. Chromatogr. Sci. 2017, 55, 1000–1005. [Google Scholar] [CrossRef]
- Cai, Z.; Hu, X.; Zong, R.; Wu, H.; Jin, X.; Yin, H.; Huang, C.; Xiang, Y.; Ye, N. A Graphene Oxide-Molybdenum Disulfide Composite Used as Stationary Phase for Determination of Sulfonamides in Open-Tubular Capillary Electrochromatography. J. Chromatogr. A 2020, 1629, 461487. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Miao, J.; Yin, Z.; Zhai, Y.; Shi, H.; Li, Z. Determination of Sulfa Antibiotic Residues in River and Particulate Matter by Field-amplified Sample Injection-capillary Zone Electrophoresis. Electrophoresis 2020, 41, 1584–1591. [Google Scholar] [CrossRef]
- Semail, N.-F.; Abdul Keyon, A.S.; Saad, B.; Kamaruzaman, S.; Mohamad Zain, N.N.; Lim, V.; Miskam, M.; Wan Abdullah, W.N.; Yahaya, N.; Chen, D.D.Y. Simultaneous Preconcentration and Determination of Sulfonamide Antibiotics in Milk and Yoghurt by Dynamic PH Junction Focusing Coupled with Capillary Electrophoresis. Talanta 2022, 236, 122833. [Google Scholar] [CrossRef]
- Givianrad, M.H.; Saber-Tehrani, M.; Aberoomand-Azar, P.; Mohagheghian, M. H-Point Standard Additions Method for Simultaneous Determination of Sulfamethoxazole and Trimethoprim in Pharmaceutical Formulations and Biological Fluids with Simultaneous Addition of Two Analytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1196–1200. [Google Scholar] [CrossRef]
- Dinali, L.A.F.; de Oliveira, H.L.; Teixeira, L.S.; da Silva, A.T.M.; D’Oliveira, K.A.; Cuin, A.; Borges, K.B. Efficient Development of a Magnetic Molecularly Imprinted Polymer for Selective Determination of Trimethoprim and Sulfamethoxazole in Milk. Microchem. J. 2020, 154, 104648. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Wang, X.; Wang, J.; Qian, F.; Gu, H.; Zhang, Z. Magnetic Solid Phase Extraction of Sulfonamides Based on Carboxylated Magnetic Graphene Oxide Nanoparticles in Environmental Waters. J. Chromatogr. A 2018, 1575, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Liu, L.; Wang, X.; Wang, M.-L.; Lin, J.-M.; Zhao, R.-S. Spherical Mesoporous Covalent Organic Framework as a Solid-Phase Extraction Adsorbent for the Ultrasensitive Determination of Sulfonamides in Food and Water Samples by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1625, 461275. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Meng, Z.; Zhao, J.; Zhao, H.; Zhao, L. Eco-Friendly Ultrasonic Assisted Liquid–Liquid Microextraction Method Based on Hydrophobic Deep Eutectic Solvent for the Determination of Sulfonamides in Fruit Juices. J. Chromatogr. A 2020, 1609, 460520. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ma, S.; Zhu, K.; Wang, M.; Li, J.; Arabi, M.; Liu, H.; Li, Y.; Chen, L. Simultaneous Enrichment/Determination of Six Sulfonamides in Animal Husbandry Products and Environmental Waters by Pressure-Assisted Electrokinetic Injection Coupled with Capillary Zone Electrophoresis. J. Food Compos. Anal. 2020, 88, 103462. [Google Scholar] [CrossRef]
- Errayess, S.A.; Lahcen, A.A.; Idrissi, L.; Marcoaldi, C.; Chiavarini, S.; Amine, A. A Sensitive Method for the Determination of Sulfonamides in Seawater Samples by Solid Phase Extraction and UV–Visible Spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 181, 276–285. [Google Scholar] [CrossRef]
- Peixoto, P.S.; Tóth, I.V.; Machado, S.; Barreiros, L.; Machado, A.; Bordalo, A.A.; Lima, J.L.F.C.; Segundo, M.A. Screening of Sulfonamides in Waters Based on Miniaturized Solid Phase Extraction and Microplate Spectrophotometric Detection. Anal. Methods 2018, 10, 690–696. [Google Scholar] [CrossRef]
- Khataei, M.M.; Epi, S.B.H.; Lood, R.; Spégel, P.; Yamini, Y.; Turner, C. A Review of Green Solvent Extraction Techniques and Their Use in Antibiotic Residue Analysis. J. Pharm. Biomed. Anal. 2022, 209, 114487. [Google Scholar] [CrossRef]
- Yari, A.; Shams, A. Silver-Filled MWCNT Nanocomposite as a Sensing Element for Voltammetric Determination of Sulfamethoxazole. Anal. Chim. Acta 2018, 1039, 51–58. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Sreeja, B.S.; Krishna Kumar, K.; Padmalaya, G. Static and Dynamic Analysis of Sulfamethoxazole Using GO/ZnO Modified Glassy Carbon Electrode by Differential Pulse Voltammetry and Amperometry Techniques. Chemosphere 2022, 302, 134926. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Kanchi, S.; Redhi, G.G. A Novel IL-f-ZnONPs@MWCNTs Nanocomposite Fabricated Glassy Carbon Electrode for the Determination of Sulfamethoxazole. J. Mol. Liq. 2022, 359, 119232. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Settu, R.; Chen, S.-M.; Chen, T.-W. Voltammetric Sensing of Sulfamethoxazole Using a Glassy Carbon Electrode Modified with a Graphitic Carbon Nitride and Zinc Oxide Nanocomposite. Microchim Acta 2018, 185, 396. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Li, Z.; Zhao, S. A New Electrochemical Sensor for Simultaneous Detection of Sulfamethoxazole and Trimethoprim Antibiotics Based on Graphene and ZnO Nanorods Modified Glassy Carbon Electrode. Microchem. J. 2020, 159, 105440. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Modification of Carbon Paste Electrode with NiO/Graphene Oxide Nanocomposite and Ionic Liquids for Fabrication of High Sensitive Voltammetric Sensor on Sulfamethoxazole Analysis. J. Mol. Liq. 2016, 220, 329–333. [Google Scholar] [CrossRef]
- Melo Henrique, J.; Rocha Camargo, J.; Gabriel de Oliveira, G.; Santos Stefano, J.; Campos Janegitz, B. Disposable Electrochemical Sensor Based on Shellac and Graphite for Sulfamethoxazole Detection. Microchem. J. 2021, 170, 106701. [Google Scholar] [CrossRef]
- Sgobbi, L.F.; Razzino, C.A.; Machado, S.A.S. A Disposable Electrochemical Sensor for Simultaneous Detection of Sulfamethoxazole and Trimethoprim Antibiotics in Urine Based on Multiwalled Nanotubes Decorated with Prussian Blue Nanocubes Modified Screen-Printed Electrode. Electrochim. Acta 2016, 191, 1010–1017. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N., Jr.; Machado, S.A.S. Paper-Based Electrochemical Sensors with Reduced Graphene Nanoribbons for Simultaneous Detection of Sulfamethoxazole and Trimethoprim in Water Samples. J. Electroanal. Chem. 2021, 882, 114985. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Heitor, A.M.; Sá, L.C.; Barbosa, J.; da Conceição, M.; Montenegro, B.S.M.; Sales, M.G.F. Solid Contact PVC Membrane Electrodes Based on Neutral or Charged Carriers for the Selective Reading of Anionic Sulfamethoxazole and Their Application to the Analysis of Aquaculture Water. Int. J. Environ. Anal. Chem. 2012, 92, 479–495. [Google Scholar] [CrossRef][Green Version]
- El-Ragehy, N.A.; Hegazy, M.A.; AbdElHamid, G.; Tawfik, S.A. Validated Potentiometric Method for the Determination of Sulfacetamide Sodium; Application to Its Pharmaceutical Formulations and Spiked Rabbit Aqueous Humor. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 207–212. [Google Scholar] [CrossRef]
- Kamel, A.H.; Almeida, S.A.; Sales, M.G.F.; Moreira, F.T. Sulfadiazine-Potentiometric Sensors for Flow and Batch Determinations of Sulfadiazine in Drugs and Biological Fluids. Anal. Sci. 2009, 25, 365–371. [Google Scholar] [CrossRef]
- Soleymanpour, A.; Rezvani, S.A. Development of a Novel Carbon Paste Sensor for Determination of Micromolar Amounts of Sulfaquinoxaline in Pharmaceutical and Biological Samples. Mater. Sci. Eng. C 2016, 58, 504–509. [Google Scholar] [CrossRef]
- Arvand, M.; Alirezanejad, F. Sulfamethoxazole-Imprinted Polymeric Receptor as Ionophore for Potentiometric Transduction. Electroanalysis 2011, 23, 1948–1957. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Truta, L.A.A.N.A.; Queirós, R.B.; Montenegro, M.C.B.S.M.; Cunha, A.L.; Sales, M.G.F. Optimizing Potentiometric Ionophore and Electrode Design for Environmental On-Site Control of Antibiotic Drugs: Application to Sulfamethoxazole. Biosens. Bioelectron. 2012, 35, 319–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeida, S.A.A.; Moreira, F.T.C.; Heitor, A.M.; Montenegro, M.C.B.S.M.; Aguilar, G.G.; Sales, M.G.F. Sulphonamide-Imprinted Sol–Gel Materials as Ionophores in Potentiometric Transduction. Mater. Sci. Eng. C 2011, 31, 1784–1790. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid Molecularly Imprinted Polymer for Amoxicillin Detection. Biosens. Bioelectron. 2018, 118, 102–107. [Google Scholar] [CrossRef]
- Ahmed, S.; Anwar, N.; Sheraz, M.A.; Ahmad, I. Validation of a Stability-Indicating Spectrometric Method for the Determination of Sulfacetamide Sodium in Pure Form and Ophthalmic Preparations. J. Pharm. Bioallied. Sci. 2017, 9, 126–134. [Google Scholar] [CrossRef]
- Sayed, R.A.; Mohamed, A.R.; Hassan, W.S.; Elmasry, M.S. Earth-Friendly-Assessed Chromatographic Platforms for Rapid Analysis of Sulfacetamide Sodium and Prednisolone Acetate in Presence of Sulfanilamide Impurity: Application on Ophthalmic Formulation and Aqueous Humor. Sustain. Chem. Pharm. 2022, 27, 100694. [Google Scholar] [CrossRef]
- Yaroshenko, I.S.; Alyapyshev, M.Y.; Babain, V.A.; Legin, A.V.; Kirsanov, D.O. Potentiometric Sensors and Multisensor Systems for the Determination of Lanthanides. J. Anal. Chem. 2019, 74, 1003–1018. [Google Scholar] [CrossRef]
- Ashina, J.; Babain, V.; Kirsanov, D.; Legin, A. A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures. Chemosensors 2021, 9, 23. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Parshina, A.; Kolganova, T.; Safronova, E.; Osipov, A.; Lapshina, E.; Yelnikova, A.; Bobreshova, O.; Yaroslavtsev, A. Perfluorosulfonic Acid Membranes Thermally Treated and Modified by Dopants with Proton-Acceptor Properties for Asparaginate and Potassium Ions Determination in Pharmaceuticals. Membranes 2019, 9, 142. [Google Scholar] [CrossRef]
- Safronova, E.; Parshina, A.; Kolganova, T.; Yelnikova, A.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Potentiometric Multisensory System Based on Perfluorosulfonic Acid Membranes and Carbon Nanotubes for Sulfacetamide Determination in Pharmaceuticals. J. Electroanal. Chem. 2020, 873, 114435. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yurova, P.A.; Titova, T.S.; Polovkova, M.A.; Korchagin, O.V.; Bogdanovskaya, V.A.; Yaroslavtsev, A.B. The Influence of Poly(3,4-ethylenedioxythiophene) Modification on the Transport Properties and Fuel Cell Performance of Nafion-117 Membranes. J. Appl. Polym. Sci. 2021, 138, 50644. [Google Scholar] [CrossRef]
- Stenina, I.A.; Il’ina, A.A.; Pinus, I.Y.; Sergeev, V.G.; Yaroslavtsev, A.B. Cation Mobility in Systems Based on High-Molecular-Weight Sulfonic Acids and Polyaniline. Russ. Chem. Bull. 2008, 57, 2261–2264. [Google Scholar] [CrossRef]
- Kumar, A.; Ibraheem, S.; Ali, S.; Maiyalagan, T.; Javed, M.S.; Gupta, R.K.; Saad, A.; Yasin, G. Polypyrrole and Polyaniline-Based Membranes for Fuel Cell Devices: A Review. Surf. Interfaces 2022, 29, 101738. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.; Norby, T.; Chatzitakis, A. Composite Membranes for High Temperature PEM Fuel Cells and Electrolysers: A Critical Review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of Cation Exchange Membranes Modified by Polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, R.F.; Atcherley, C.W.; Russell, W.S.; Xie, J.Y.; Lu, D.; Laude, N.D.; Porreca, F.; Heien, M.L. Biocompatible PEDOT:Nafion Composite Electrode Coatings for Selective Detection of Neurotransmitters in Vivo. Anal. Chem. 2015, 87, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-S.; Chang, Y.-H.; Tsai, M.-L. Highly Sensitive Amperometric Creatinine Biosensor Based on Creatinine Deiminase/Nafion®-Nanostructured Polyaniline Composite Sensing Film Prepared with Cyclic Voltammetry. Mater. Chem. Phys. 2018, 219, 1–12. [Google Scholar] [CrossRef]
- Yuan, Y.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. Nafion/Polyaniline/Zeolitic Imidazolate Framework-8 Nanocomposite Sensor for the Electrochemical Determination of Dopamine. J. Electroanal. Chem. 2018, 824, 147–152. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, Y.M.; Galal, A. Layered-Designed Composite Sensor Based on Crown Ether/Nafion®/Polymer/Carbon Nanotubes for Determination of Norepinephrine, Paracetamol, Tyrosine and Ascorbic Acid in Biological Fluids. J. Electroanal. Chem. 2018, 828, 11–23. [Google Scholar] [CrossRef]
- Loza, N.V.; Falina, I.V.; Kononenko, N.A.; Kudashova, D.S. Some Aspects of Polyaniline Template Synthesis within and on the Surface of Perfluorinated Cation Exchange Membrane. Synth. Met. 2020, 261, 116292. [Google Scholar] [CrossRef]
- Safronova, E.; Safronov, D.; Lysova, A.; Parshina, A.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Sensitivity of Potentiometric Sensors Based on Nafion®-Type Membranes and Effect of the Membranes Mechanical, Thermal, and Hydrothermal Treatments on the on Their Properties. Sens. Actuators B Chem. 2017, 240, 1016–1023. [Google Scholar] [CrossRef]
- Gicevicius, M.; Kucinski, J.; Ramanaviciene, A.; Ramanavicius, A. Tuning the Optical PH Sensing Properties of Polyaniline-Based Layer by Electrochemical Copolymerization of Aniline with o-Phenylenediamine. Dye. Pigment. 2019, 170, 107457. [Google Scholar] [CrossRef]
- Wijsboom, Y.H.; Sheynin, Y.; Patra, A.; Zamoshchik, N.; Vardimon, R.; Leitus, G.; Bendikov, M. Tuning of Electronic Properties and Rigidity in PEDOT Analogs. J. Mater. Chem. 2011, 21, 1368–1372. [Google Scholar] [CrossRef]
- Singh, R.K.; Kunimatsu, K.; Miyatake, K.; Tsuneda, T. Experimental and Theoretical Infrared Spectroscopic Study on Hydrated Nafion Membrane. Macromolecules 2016, 49, 6621–6629. [Google Scholar] [CrossRef]
- Ping, Z. In Situ FTIR–Attenuated Total Reflection Spectroscopic Investigations on the Base–Acid Transitions of Polyaniline. Base–Acid Transition in the Emeraldine Form of Polyaniline. J. Chem. Soc. Faraday Trans. 1996, 92, 3063–3067. [Google Scholar] [CrossRef]
- Titova, T.S.; Yurova, P.A.; Kolganova, T.S.; Stenina, I.A.; Parshina, A.V.; Bobreshova, O.V.; Yaroslavtsev, A.B. Potentiometric Sensors Based on Nafion Membranes Modified by PEDOT for Determining Procaine, Lidocaine, and Bupivacaine in Aqueous Solutions and Pharmaceuticals. J. Anal. Chem. 2020, 75, 1072–1079. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic Mobility in Ion-Exchange Membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Karavanova, Y.A.; Safronova, E.Y. Ionic Conductivity of Hybrid Membranes. Pet. Chem. 2011, 51, 473–479. [Google Scholar] [CrossRef]
- Novikova, S.A.; Safronova, E.Y.; Lysova, A.A.; Yaroslavtsev, A.B. Influence of Incorporated Nanoparticles on the Ionic Conductivity of MF-4SC Membrane. Mendeleev Commun. 2010, 20, 156–157. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef] [PubMed]




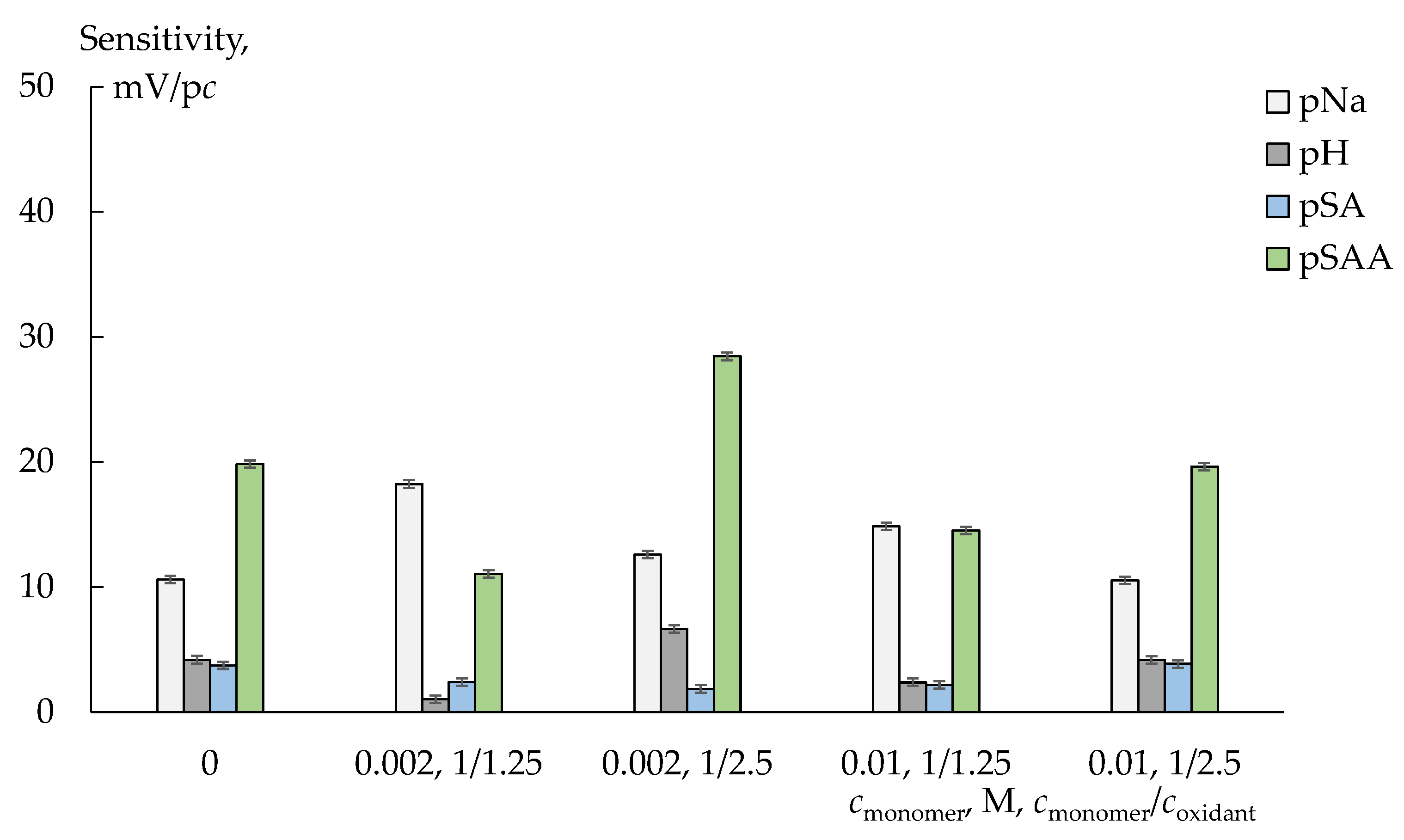
| r12 | r13 | r14 | r23 | r24 | r34 | det r |
|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.2 | 0.4 | 0.5 | 0.5 | 0.5 |
| Array | Calibration Equations Systems | ε, mV | D, mV2 | Relative Error, % | RSD,% (n = 4, p = 0.95) | ||||
|---|---|---|---|---|---|---|---|---|---|
| SAA− | SA | Na+ | SAA− | SA | Na+ | ||||
| 1 | 3 | 20 | 1.7–4 | 3–11 | 1.2–17 | 8–16 | 8–10 | 7–16 | |
| 4 | 10 | ||||||||
| 3 | 12 | ||||||||
| 2 | 3 | 20 | 0.5–21 | 3–15 | 1.8–9 | 10–20 | 5–16 | 7–20 | |
| 4 | 10 | ||||||||
| 4 | 27 | ||||||||
| Method | Spectrophotometry | The DP-Sensor Array 1 | The DP-Sensor Array 2 | |
|---|---|---|---|---|
| Pharmaceutical pretreatment | Dilution 1/25,000 | UV treatment, dilution 1/25,000 | UV treatment, dilution 1/500 | UV treatment, dilution 1/500 |
| c(SAA−), M (pharmaceutical solution) | (3.40 ± 0.02) × 10−5 | (3.11 ± 0.04) × 10−5 | (1.58 ± 0.15) × 10−3 | (1.57 ± 0.13) × 10−3 |
| c(SA), M (pharmaceutical solution) | - | insignificant | (1.35 ± 0.17) × 10−4 | (1.33 ± 0.15) × 10−4 |
| c(Na+), M (pharmaceutical solution) | - | - | (1.75 ± 0.16) × 10−3 | (1.76 ± 0.15) × 10−3 |
| RSD(SAA−), % (n = 4, p = 0.95) | 0.3 | 1.5 | 6 | 7 |
| RSD(SA), % (n = 4, p = 0.95) | - | - | 8 | 9 |
| RSD(Na+), % (n = 4, p = 0.95) | - | - | 6 | 7 |
| c(SAANa), mg/mL (preparation) | 201 ± 2 | 185 ± 2 | 185 ± 17 | 185 ± 16 |
| c(SA), mg/mL (preparation) | - | - | 11.6 ± 1.5 | 11.4 ± 1.3 |
| Relative error(SAANa), % | - | - | 1.4 | 1.2 |
| Relative error(SA), % | - | - | 1.7 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshina, A.; Yelnikova, A.; Titova, T.; Kolganova, T.; Yurova, P.; Stenina, I.; Bobreshova, O.; Yaroslavtsev, A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals. Polymers 2022, 14, 2545. https://doi.org/10.3390/polym14132545
Parshina A, Yelnikova A, Titova T, Kolganova T, Yurova P, Stenina I, Bobreshova O, Yaroslavtsev A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals. Polymers. 2022; 14(13):2545. https://doi.org/10.3390/polym14132545
Chicago/Turabian StyleParshina, Anna, Anastasia Yelnikova, Tatyana Titova, Tatyana Kolganova, Polina Yurova, Irina Stenina, Olga Bobreshova, and Andrey Yaroslavtsev. 2022. "Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals" Polymers 14, no. 13: 2545. https://doi.org/10.3390/polym14132545
APA StyleParshina, A., Yelnikova, A., Titova, T., Kolganova, T., Yurova, P., Stenina, I., Bobreshova, O., & Yaroslavtsev, A. (2022). Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals. Polymers, 14(13), 2545. https://doi.org/10.3390/polym14132545