Active Flexible Films for Food Packaging: A Review
Abstract
:1. Introduction
2. Active Flexible Packaging
2.1. Scavenging Systems
2.2. Releasing Systems
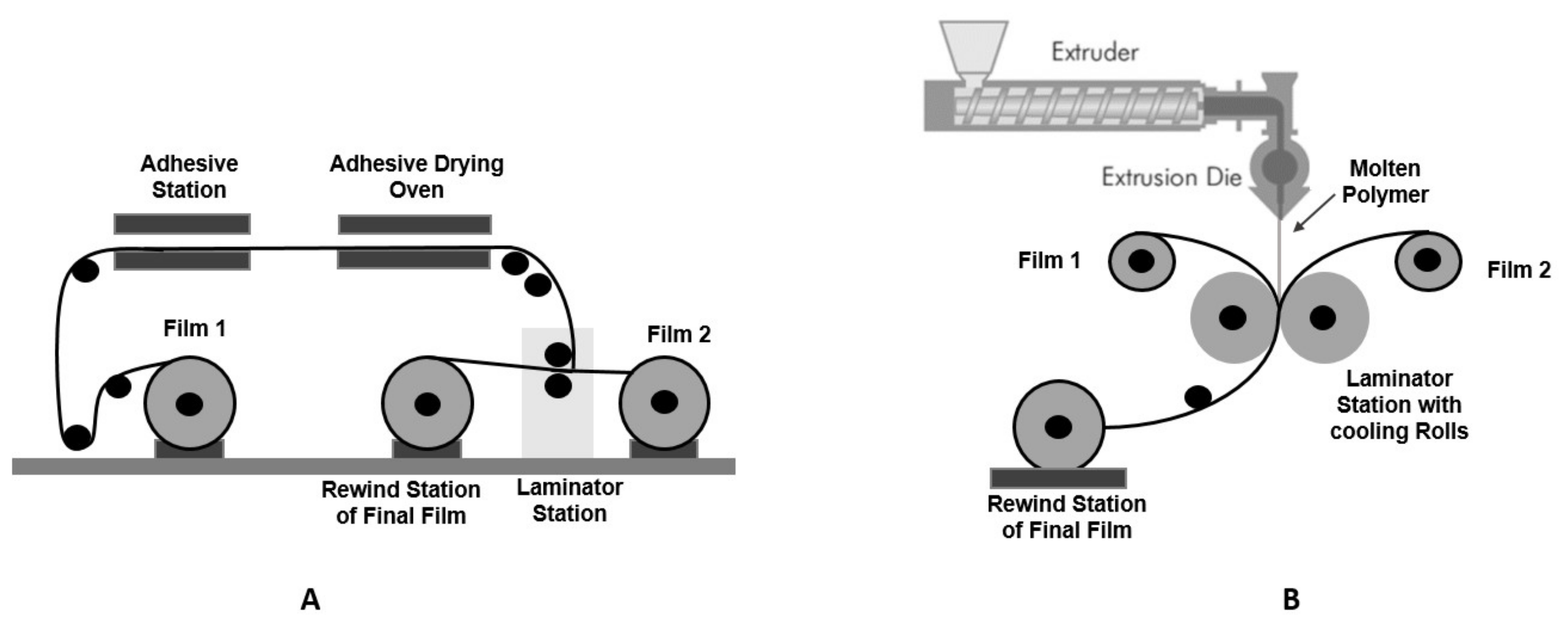
3. Methodologies for the Production of Active Flexible Packaging
3.1. Direct Incorporation of Active Agents in the Polymer Film Matrix
| Active Agent (AA) | Material/Matrix | Packaging Function | Processes Used | Food Product Tested/Packaging Type | Active Agent Amount | Main Effects Compared Control Film | Amount of AA Migrated * | References |
|---|---|---|---|---|---|---|---|---|
| Amosorb DFC 4020 | PET/PET—containing AA/PET | Oxygen scavenger | Cast film co-extrusion (Temperature profile: 285–280 °C) | Fresh apple slices | 10 g/100 g polymer | The multilayer films with higher thickness in internal active layer reduced the browning of fresh apple slices packaged after 15 days storage at 8 °C. This packaging also allowed preserving the initial values of the acidity and sugar content of apples. | nd | [23] |
| Iron | PET/Adhesive/Al/Adhesive/PE—containing AA/PE | Oxygen scavenger | Film extrusion and lamination (temperatures not specified) | Salami in a baked bread roll | - | The food samples stored 30 days at 23 °C with active film and with sealing defects of 10 mm, showed that the presence of OS was advantageous in the permanence of color of product, when compared to the packaging without OS. | nd | [25] |
| α-TOC and synthetic materials (BHA and BHT) | PE-HD—containing TiO2/EVOH/PE-LD—containing antioxidant | Antioxidant activity | Blown film co-extrusion (temperatures not specified) | Whole milk powder/direct contact | 4 g of α-TOC, 4 g of α-TOC mix with 1.5 g of BHA, 1.5 g of BHT and 1.5 g of BHA (all by 100 g polymer) | The multilayer film with α-TOC in contact with whole the milk powder showed a more gradual release of α-TOC during the 30 days storage (26.8% at 30 days). In addition, this film contributed to protect vitamin A degradation presents in whole milk powder. | α-TOC–63 ± 2 µg/g α-TOC mix with BHA—64 ± 0.6 µg/g (Product stored during 30 days at 30 °C) Regulation (EU) allows a maximum of 60 mg/kg of α-TOC | [52] |
| Nis., Chit., PSorbate or AgZeo | PE-LD/PA/PE-LD -containing AA | Antimicrobial activity | Blown film extrusion (temperatures not specified) | Chicken drumsticks/direct contact | 2 g/100 g polymer | The results indicated that the use of active bags with nisin and chitosan reduced the levels of total aerobic mesophilic bacteria (APC) and total coliform in chicken drumsticks storage during 6 days at 5 °C. | nd | [8] |
| NPs Ag, CuO and ZnO | PE-LD film | Antimicrobial activity | Film extrusion (Temperature profile: 180–239 °C) | Cheese/ns | 1 g metal nanoparticles/100 g polymer | All active films with metal NPs showed a decline of the number of coliform bacteria of 4.21 log cfu/g after 4 weeks of storage at 4 ± 0.5 °C. The effect of each individual NPs on decreasing coliform load had the following order: CuO > ZnO > Ag. | CuO—0.23 ± 0.005 mg/kg (it was used the simulant B at 40 °C for 10 days) EFSA1 legislation allows a maximum of 10 mg of Cu/kg of food | [47] |
| Ag/TiO2 NPs | PE-LD film | Antimicrobial activity | Blown film extrusion (temperatures not specified) | Rice/ns | 9 g/100 g polymer | Reduction from 7.15 to 5.48 log CFU/g in rice stored with active packaging after one month. | Ag+—0.0035 mg/kg (product stored 35 days at 37 °C and relative humidity of 70%) EFSA1 legislation allows a maximum of silver migration of 0.05 mg of Ag+/kg of food. | [49] |
| P105 powder (TiO2 + Ag NPs) and ZnO NPs | PE-LD film | Antimicrobial activity | Film extrusion (Temperature profile: 60–160 °C) | Fresh orange juice/direct contact | 1.5 and 5 g of P105 powder (TiO2 + Ag NPs) and 0.25 and 1 g of ZnO NPs (all by 100 g polymer) | Nanocomposite film containing nano-Ag showed higher antimicrobial activity than films with nano-ZnO when they are used to pack orange juice. | 5 g of P105 (Ag)–0.15 ± 0.002 µg/L 0.25 g ZnO–0.68 ± 0.002 µg/L 1 g ZnO–0.54 ± 0.005 µg/L (product stored at 40 °C for 112 days) EFSA1 legislation allows a maximum of 10 ppm of Ag Regulation (EU) allows a maximum of 25 mg of Zn/kg of food | [53] |
| α-TOC | PE-LD/PP blend film | Antioxidant activity | Film extrusion (Temperature profile: 221 °C) | - | 3000 mg/kg | The PE-LD/PP blend films with higher PP ratio showed a longer induction period of oxidation against linoleic acid oxidation (6 days) due to the low releasing of TOC in LDE/PP blend films, allowing an antioxidant effect for more time. | nd | [55] |
| α-TOC | PE-LD film | Antioxidant activity | Film extrusion (Temperature profile: 165 °C) | Corn oil/direct contact | 20 and 40 mg/g | Increase of shelf life of corn oil from 12 to 16 weeks stored at 30 °C. | nd | [56] |
| Proallium | PLA film | Antioxidant and antimicrobial activity | Film extrusion (Temperature profile: 200–205 °C) | Salad/ns | 2, 5 and 6.5 g/100 g polymer | The films developed showed no significant antioxidant activity; however, they showed effectiveness during the storage time (7 days) against all microorganisms studied, except for aerobic bacteria. | nd | [40] |
| α-TOC | PLA film | Antioxidant activity | Blown film extrusion (Temperature profile: 165–170 °C) | - | 3 g/100 g polymer | Diffusion of α-TOC to fractioned coconut oil was slower than to ethanol with 5.1–12.9% of release. Diffusion of α-TOC to soybean oil was able to decrease the induction of the oxidation at 20 and 30 °C, but not at 40 ºC. | nd | [57] |
| PSorbate or/and OEO | TPS/PBAT-Ecoflex® blend film | Antioxidant and antibacterial activity | Blown film extrusion (Temperature profile: 90–120 °C) | Chicken steaks frozen/ns | 0.5 and 1 g/100 g polymer | Active film showed a reduction of 50% in TBARS values and a delay in microbial development when using the film with OEO and PS. | nd | [58] |
| HNTs | PE-LD film | Ethylene scavenger | Blown film extrusion (Temperature profile: 165–185 °C) | Bananas and tomatoes/ns | 1, 3 and 5 g/100 g polymer | The results showed that the presence of 5% w/w HNTs improved the ethylene adsorption capacity of PE films by 20%. Active films slowed down the ripening process of bananas during 8 days and tomatoes only decreased their firmness 16% after 10 days of storage. | nd | [32] |
| NaCl crystals | PP film | Moisture absorber | Cast film extrusion (Temperature profile: 180–250 °C) | - | 0.03 g or 0.06 g per 1 g of film | The PP film developed with NaCl crystals showed an absorption capacity of water vapor around 0.8 g water/g film at 97% relative humidity. | nd | [31] |
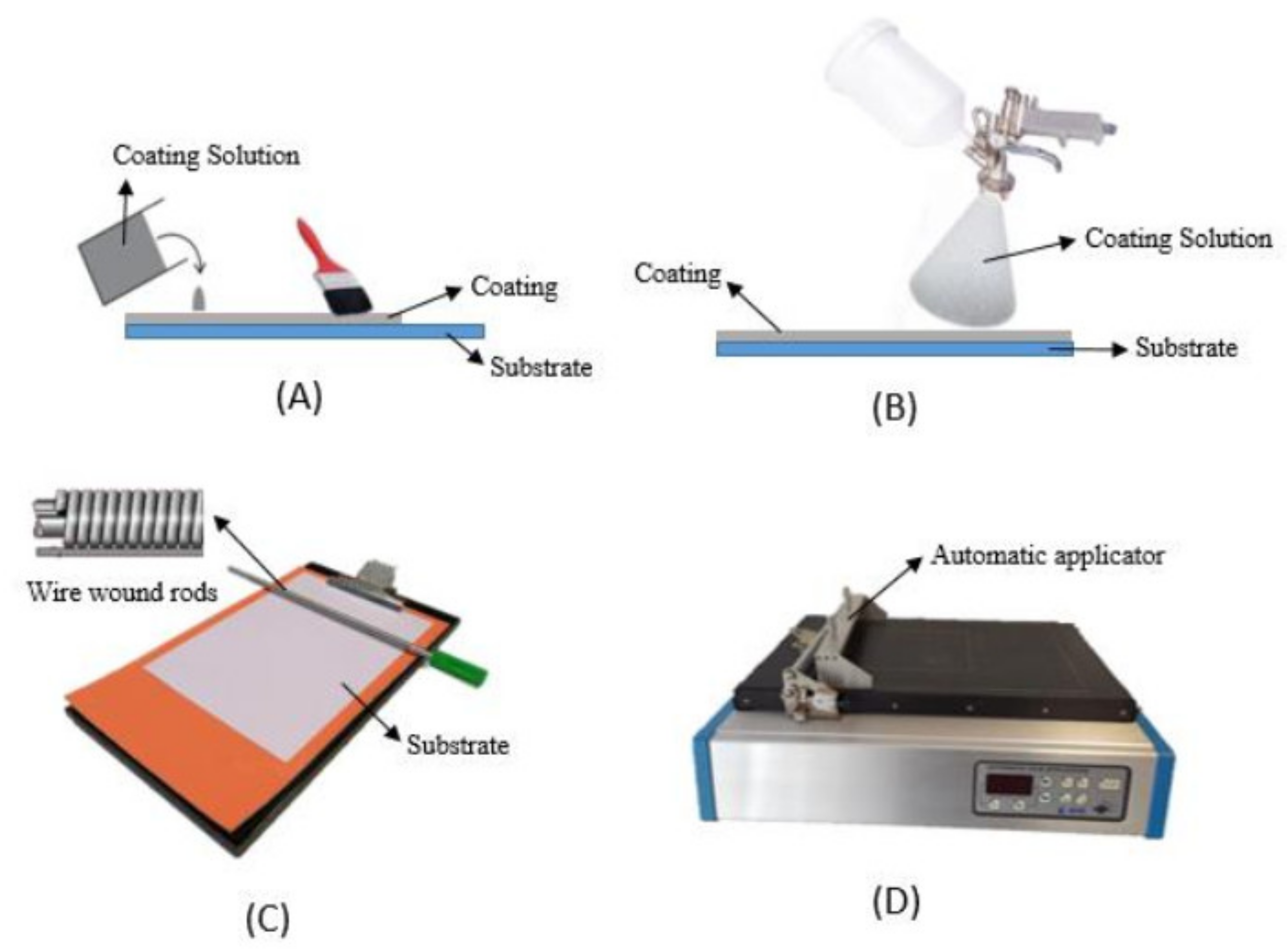
3.2. Incorporation of the Active Agents by Coating
| Active Agent | Packaging Material | Packaging Function | Processes Used | Food Product Tested/Packaging Type | Active Agent Amount | Main Effects Compared Control Film | References |
|---|---|---|---|---|---|---|---|
| Rosemary extract | PE-LD films | Antioxidant activity | Brushing | Pork patties/direct contact | 0.45 mg/cm2 | The results demonstrated that PE-LD film coated with rosemary was the most effective active packaging to protect pork patties storage during 60 days at 5 °C. | [37] |
| Natural extract obtained from a brewery residual | PE-LD films | Antioxidant activity | Plate coater | Beef/headspace | 3.2 g/m2 | The results showed that active antioxidant films coated with natural extracts decreased lipid oxidation by up to 90% during 17 days stored at 4 °C. | [63] |
| Pyrogallol (PG) (a natural phenolic compound) | PE-LD films modified with sodium carbonate | Oxygen scavengers | Plate stripe coater | Soybean oil/headspace | Thickness 60—62 µm | The soybean oil samples packed with PE-LD/PG films coated with 10 and 20% PG and storage at 23 °C and 60 °C showed a better stabilizing effect during 30 days than oil packaged with pure PE-LD. | [64] |
| Chitosan, lauric arginate ester (LAE), sodium lactate (NaL), and sorbic acid (SA) | PLA films | Antimicrobial activity | Brushing or spraying | Ready-to-eat meat (RTE)/direct contact | 0.39 and 1.92 mg/cm2 of Chitosan; 1.94 and 3.89 µg/cm2 of LAE; 0.78, 1.56, 3.89 and 7.78 mg/cm2 of NaL and 0.12 and 0.23 mg/cm2 of SA | The results showed that PLA films containing LAE were those that most significantly inhibited the growth of the tested microorganisms. The PLA films coated with NaL, and SA showed to reduce significantly the growth of L. innocua but were less effective against Salmonella. | [65] |
| Chitosan and ZnO/chitosan | PE-LD films | Antimicrobial activity | Spraying (and plasma treatment) | Okra/ns | Not specified | The results showed that total bacterial concentrations in films coated with chitosan/ZnO coatings were reduced by 63%. | [72] |
| Chitosan and Ag/chitosan | Ethylene copolymer (EVA) film | Antimicrobial activity | Plate coater (and corona treatment) | Beef and chicken meat exudates/ns | Not specified | The film coated with chitosan reduced colony counts of E. coli 25922 and of L. monocytogenes Scott A by 5 and 2–3 log10, respectively, after 24 h exposure. However, this activity was increased when silver ions were incorporated into the films that, for example, originated the complete killing of E. coli O157:H7 DD3795. | [67] |
| Oregano essential oil and green tea extract | Multilayer film: PET/PE/EVOH/PE | Antioxidant activity | Rollers, tampograph, serigraphy or spraying systems | Foal steaks/ns | 1.5and 2.0 g/m2 | The active films with essential oregano oil were significantly more efficient than those with green tea extract in case of extended fresh odor and color from 7 to 14 days, compared to the control. | [10] |
| Oregano extract | PP film | Antioxidant activity | Rollers, tampograph, serigraphy or spraying systems | Fresh beef//ns | Not specified | The results showed to be efficient in extending the fresh odor and color from 14 to 23 days. However, the addition of oregano should be around 1% due to the unacceptable oregano odor when the concentration is higher. | [62] |
| Star anise essential oil (SAEO) and thymol (TH) | Multilayer film PP/SAEO/PET/TH/PE-LD | Insect repellent and antimicrobial activity | Automatic control coater | Slices bread/ns | Thickness of active coating was 13.20 ± 1.72 µm | The developed film showed a strong and sustained insect repellent activity, lower microbial counts and better visual appearance of bread after 14 days of storage. | [69] |
| Green tea extract | Multilayer film: OPP/OPP | Antioxidant activity | Lamination | Dark chocolate peanuts and milk chocolatecereals/headspace | Not specified | The results demonstrated that it is possible to increase the shelf life of these products from 9 to 18 months without active agent migration from packaging. | [42] |
| Sage leaf (SL) and Bay leaf (BL) extracts | Multilayer film: PET/PE-LD | Antioxidant activity | Coating machine (KK coater, RK print). | Fried potatoes/headspace | 0.025 and 0.03 g/m2 | The results showed a strong antioxidant activity of SL and BL, either evaluated alone or as food packaging for fried potatoes. For example, in case of the malondialdehyde (MDA) the SL extract was more efficient, showing a reduction of 40% of MDA compared to the control, while BL showed a reduction of 31%. | [43] |
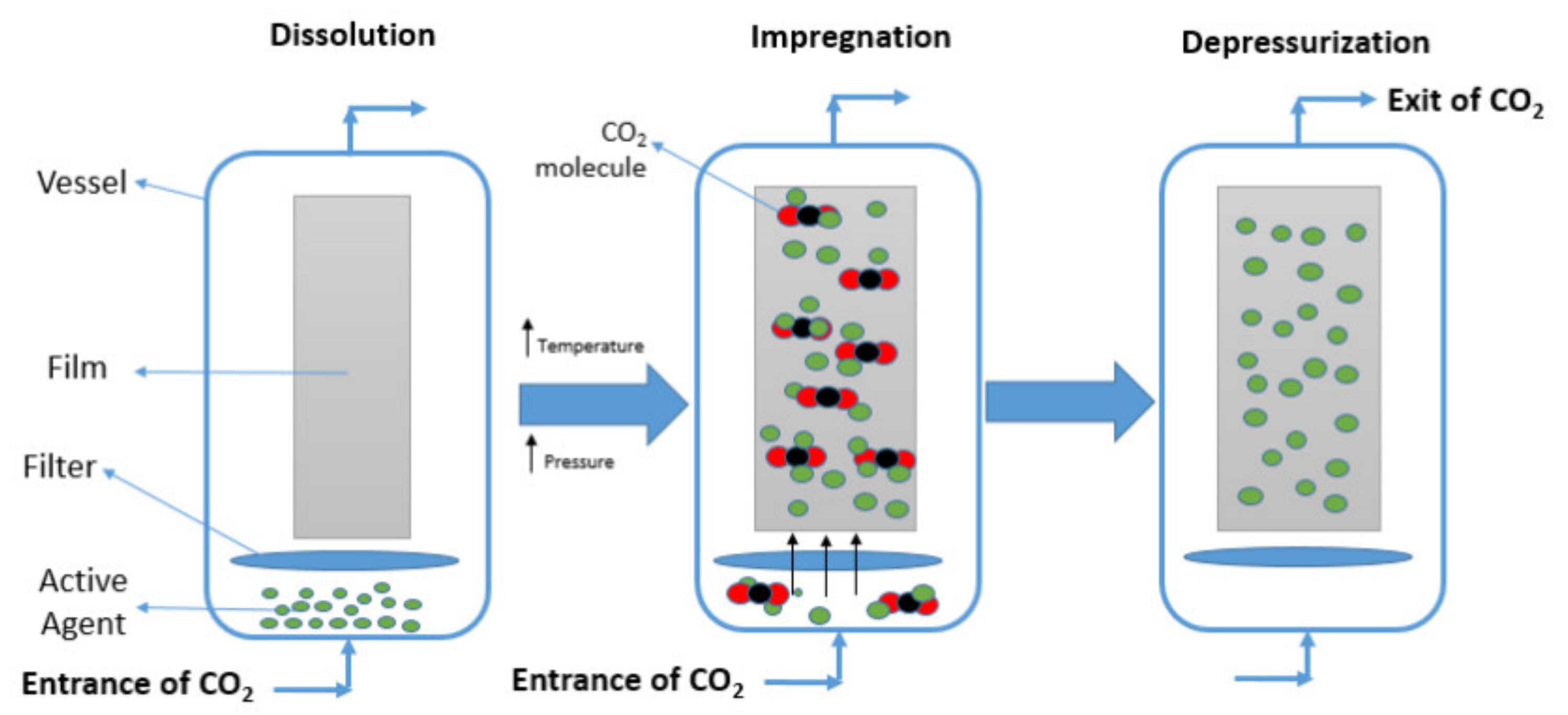
3.3. Incorporation of Active Agents by Supercritical Impregnation
| Active Agent | Material/Matrix | Packaging Function | Tested Product/Packaging Type | Active Agent Amount | Main effects Compared Control Film | References |
|---|---|---|---|---|---|---|
| Eugenol | PE-LLD film | Antioxidant activity | - | 0.5 and 6 wt.% | The results of antioxidant activity showed approximately 80% inhibition after 96 h, regardless the eugenol loading. | [76] |
| Olive leaf extract (OLE) | PET/PP multilayer film | Antioxidant and antimicrobial activity | Cherry tomatoes/ns | 2–5.5 mg/g film | The results showed that the tomatoes packed with the impregnated film did not show any physical change for the first 30 days and their appearance remained the same as at the initial moment of the experiment. | [77] |
| Polyphenols extracted of mango leaf extract | PET/PP multilayer film | Antioxidant activity | Lettuce and tangerine/ns | 36–40 mg of total polyphenols (TP)/100 g film | The results showed that the films increase the shelf-life of lettuces for 14 days and tangerines until 39 days by preventing microbial infections and organoleptic deterioration. | [78] |
| α-TOC | PET film, PP film and PET/PP multilayer film | Antioxidant activity | - | 2.66—3.20 mg/cm2 film | The results showed that it is possible to produce a multilayer film with controlled releasing of α-TOC until 14 h. | [20] |
| Thymol | PE-LD film/Cloisite 20A nanocomposite film | Antimicrobial activity | - | 0.3—1 wt.% | They reported that the presence of nanoclays makes the release of thymol from PE-LD film difficult allowing a sustained release over time of the active compound. | [81] |
| Cinnamaldehyde essential oil (Ci) | PLA film | Antibacterial activity | - | 72–162 mg/g film | The films impregnated with 13% of Ci showed strong antibacterial activity against E. coli and S. aureus, where no microorganism was detected. | [80] |
4. Processes to Incorporate the Active Agents into Carriers
| Active Agent | Materials of Carrier | Material/Matrix | Packaging Function | Tested Product/Packaging Type | Loading of AA | Active Agent Amount | Main Effects Compared Control Film | References |
|---|---|---|---|---|---|---|---|---|
| Green tea | Crystallinemicroporous aluminosilicates | PE film | Antioxidant activity | Fresh minced meat/direct contact | 6.4–12.8 mg/g of carrier material | 20 and 40 wt.% | The results showed that active packaging developed extended the shelf life of fresh minced meat for 3 days when compared to a control sample. | [82] |
| α-TOC | Mesoporous silica | PE-LD film | Oxygen scavengers | - | α-TOC/silica weight ratio was 0.42—0.73 | 3 wt.% | The results demonstrated a slower release of α-TOC into a silica substrate (decrease about 60%) when compared to films samples with free tocopherol. | [83] |
| α-TOC | Mesoporous silica | PE-LD film | Antioxidant activity | - | Not specified | 1 wt.% | The results exhibited radical scavenging activity of the active film, which increased from 28.45% to 46.50% during 24 h of DPPH test. | [84] |
| Eugenol | Mesoporous silica (MCM–41) | PHBV films | Antimicrobial activity | - | 500 mg/g of MCM—41 | 2.5, 5, 7.5 and 10 wt.% | The electrospun PHBV films incorporated mesoporous silica nanoparticles with eugenol showed antimicrobial activity after 15 days. | [85] |
| Carvacrol | Halloysite nanotubes | PE film | Antimicrobial activity | Chicken meat/ns | - | 15 wt.% | The results showed that the samples packaged with films developed with HNTs loaded with carvacrol decreased 85% in the viability of cells, demonstrating a strong bactericidal effect against A. hydrophila. | [86] |
| Sorbic acid (SA) | beta-cyclodextrin (β-CD) | PBAT film | Antimicrobial activity | - | 100 mg SA/1 g β-CD | 1 wt.% | The results showed that active films developed were not efficient in the control microorganisms, due to the low concentration (1% w/w) of active agent used in the film formulation. | [87] |
| Gallic acid | PLA fibers | PLA film | Antioxidant activity | - | 40% based on the PLA weight | Not specified | The results showed that the PLA films containing the electrospun GA-loaded interlayer have a sustained release of the active agent for 10 weeks. | [38] |
| Oregano essential oil (OEO) and ZnO NPs | PHBV fibers | PHA film | Antimicrobial activity | - | Not specified | 2.5 wt.% of OEO and 2.25 wt.% of ZnO NPs | The multilayer films developed showed a high antimicrobial and antioxidant activities in both open and closed systems for up to 15 days. | [88] |
| Carvacrol | PCL fibers | Starch film | Antimicrobial activity | - | 12 g/100 g fibers | 15 wt.% | The active film developed showed the antimicrobial effect against E. coli, but was not effective at controlling the growth of Listeria innocua. | [89] |
| Oregano essential oil (OEO) | alfa-and gamma-cyclodextrin (α- and ɣ-CD) | PHBV | Antioxidant and antimicrobial activity | - | Weight ratios of α-CD:OEO and γ-CD:OEO were 80:20 wt/wt and 85:15 wt/wt, respectively | 10, 15, 20, 25, and 30 wt.% | The activity of films was evaluated during storage and it was observed that they are stable up to 15 days, which was explained by the protection offered by the developed system. | [90] |
| Resveratrol | Chitosan | PE and PP film | Antimicrobial and antioxidant activity | - | Not specified | 2 wt.% | The active films showed over 90% reduction of S. aureus and over 77% reduction of E. coli as compared to untreated samples and increase antioxidant activity for over a factor of 10. | [91] |
| Carvacrol and thymol | Oil-in-water emulsion | BO-PP film | Antimicrobial activity | - | Not specified | 1, 2, 5 and 10 wt.% | The results demonstrated that thymol and carvacrol microencapsulated and added on surface film were able to act for fresh food preservation against microorganisms, such as E. coli O157:H7, S. aureus, L. innocua, Saccharomyces cerevisiae, and Aspergillus niger. | [92] |
5. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Atomic layer deposition |
| Al2O3 | Aluminum oxide |
| Ag | Silver |
| Ag NPs | Silver nanoparticles |
| AgZeo | Silver substituted zeolite |
| Al | Aluminum |
| BHT | Butylated hydroxytoluene |
| BHA | Butylated hydroxyanisole |
| BioPE | Biobased linear low-density polyethylene |
| BO-PLA | Biaxially oriented polylactic acid |
| BO-PP | Biaxially oriented polypropylene |
| CA | Citric acid |
| CaO | Calcium oxide |
| Chit | Chitosan |
| CuO | Copper oxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| EC | European Commission |
| EU | European Union |
| EVA | Ethylene vinyl acetate copolymer |
| EVOH | Ethylene vinyl alcohol |
| PE-HD | High-density polyethylene |
| HNTs | Halloysite nanotubes |
| KMnO4 | Potassium permanganate |
| LA | Lactic acid |
| LbL | Layer-by-layer |
| PE-LD | Low-density polyethylene |
| LevA | Levulinic acid |
| PE-LLD | Linear low-density polyethylene |
| MAP | Modified atmosphere packaging |
| NPs | Nanoparticles |
| Nis | Nisin |
| OEO | Oregano essential oil |
| OPP | Bioriented polypropylene |
| OS | Oxygen scavenger |
| PA | Polyamide |
| PAA | Poly(acrylic acid) |
| PBAT | Poly (butylene adipate-coterephthalate) |
| PCL | Poly-(ε-caprolactone) |
| PE | Polyethylene |
| PEI | Polyethyleneimine |
| PEO | Poly(ethylene oxide) |
| PE-g-MA | Polyethylene-grafted maleic anhydride |
| PP | Polypropylene |
| PET | Polyethylene terephthalate |
| PHAs | Polyhydroxyalkanoates |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Poly(lactic acid) |
| PS | Potassium sorbate |
| PSS | Poly (sodium-4-styrene sulfonate) |
| PVA | Poly(vinyl alcohol) |
| PVdC | Poly(vinylidene chloride) |
| R | Value of the antimicrobial activity |
| RH | Relative humidity |
| RTE | Ready-to-eat meat |
| SC-CO2 | Supercritical impregnation process by carbon dioxide |
| SFCs | Supercritical fluids |
| TBARS | Thiobarbituric acid reactive substance |
| TBHQ | Tert-butylhydroquinone |
| TPP | Sodium tripolyphosphate |
| TPS | Thermoplastic starch |
| TiO2 | Titanium dioxide |
| UV | Ultraviolet |
| ZnO | Zinc oxide |
| α-TOC | Alfa-tocopherol |
References
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.; Kochhar, A. Active Packaging in Food Industry: A Review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataei, S.; Azari, P.; Hassan, A.; Pingguan-Murphy, B.; Yahya, R.; Muhamad, F. Essential Oils-Loaded Electrospun Biopolymers: A Future Perspective for Active Food Packaging. Adv. Polym. Technol. 2020, 2020, 9040535. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Angulo, I.; Lagarón, J.M.; Paseiro-Losada, P.; Cruz, J.M. Development of new active packaging films containing bioactive nanocomposites. Innov. Food Sci. Emerg. Technol. 2014, 26, 310–318. [Google Scholar] [CrossRef]
- Pereira De Abreu, D.A.; Paseiro Losada, P.; Maroto, J.; Cruz, J.M. Natural antioxidant active packaging film and its effect on lipid damage in frozen blue shark (Prionace glauca). Innov. Food Sci. Emerg. Technol. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Singh, P.; Wani, A.A.; Saengerlaub, S. Active packaging of food products: Recent trends. Nutr. Food Sci. 2011, 41, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Soysal, Ç.; Bozkurt, H.; Dirican, E.; Güçlü, M.; Bozhüyük, E.D.; Uslu, A.E.; Kaya, S. Effect of antimicrobial packaging on physicochemical and microbial quality of chicken drumsticks. Food Control 2015, 54, 294–299. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [Green Version]
- Covas, J.; Hilliou, L. Chapter 5—Production and Processing of Polymer-Based Nanocomposites. In Nanomaterials for Food Packaging; Cerqueira, M.Â.P.R., Lagaron, J.M., Castro, L.M.P., Vicente, A.A.M.O.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–146. ISBN 9780323512718. [Google Scholar]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Water Sorption Properties of Extruded Zein Films. J. Agric. Food Chem. 2004, 52, 3100–3105. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; He, Y.; Yang, J.; Wang, X.; Lan, T.; Peng, L. Fabrication of food-safe superhydrophobic cellulose paper with improved moisture and air barrier properties. Carbohydr. Polym. 2019, 211, 22–30. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging applications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533. [Google Scholar] [CrossRef]
- Franco, P.; Incarnato, L.; De Marco, I. Supercritical CO2 impregnation of α-tocopherol into PET/PP films for active packaging applications. J. CO2 Util. 2019, 34, 266–273. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Ahn, B.J.; Gaikwad, K.K.; Lee, Y.S. Characterization and properties of LDPE film with gallic-acid-based oxygen scavenging system useful as a functional packaging material. J. Appl. Polym. Sci. 2016, 133, 44138. [Google Scholar] [CrossRef]
- Di Maio, L.; Scarfato, P.; Galdi, M.R.; Incarnato, L. Development and oxygen scavenging performance of three-layer active PET films for food packaging. J. Appl. Polym. Sci. 2015, 132, 41465. [Google Scholar] [CrossRef]
- Matche, R.S.; Sreekumar, R.K.; Raj, B. Modification of linear low-density polyethylene film using oxygen scavengers for its application in storage of bun and bread. J. Appl. Polym. Sci. 2011, 122, 55–63. [Google Scholar] [CrossRef]
- Sängerlaub, S.; Gibis, D.; Kirchhoff, E.; Tittjung, M.; Schmid, M.; Müller, K. Compensation of Pinhole Defects in Food Packages by Application of Iron-based Oxygen Scavenging Multilayer Films. Packag. Technol. Sci. 2013, 26, 17–30. [Google Scholar] [CrossRef]
- Shin, Y.; Shin, J.; Lee, Y.S. Preparation and characterization of multilayer film incorporating oxygen scavenger. Macromol. Res. 2011, 19, 869–875. [Google Scholar] [CrossRef]
- Chen, C.W.; Xie, J.; Yang, F.X.; Zhang, H.L.; Xu, Z.W.; Liu, J.L.; Chen, Y.J. Development of moisture-absorbing and antioxidant active packaging film based on poly(vinyl alcohol) incorporated with green tea extract and its effect on the quality of dried eel. J. Food Process. Preserv. 2018, 42, e13374. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, S.G. Preparation and swelling behavior of moisture-absorbing polyurethane films impregnated with superabsorbent sodium polyacrylate particles. J. Appl. Polym. Sci. 2016, 133, 43973. [Google Scholar] [CrossRef]
- Chand, K.; Kumar, S. Effect of Active Packaging and Coating Materials on Quality Parameters of Jaggery Cubes. Int. J. Eng. Res. 2018, 7, 4–9. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.S. Characteristics of moisture-absorbing film impregnated with synthesized attapulgite with acrylamide and its effect on the quality of seasoned laver during storage. J. Food Eng. 2013, 116, 829–839. [Google Scholar] [CrossRef]
- Sängerlaub, S.; Seibel, K.; Miesbauer, O.; Pant, A.; Kiese, S.; Rodler, N.; Schmid, M.; Müller, K. Functional properties of foamed and/or stretched polypropylene-films containing sodium chloride particles for humidity regulation. Polym. Test. 2018, 65, 339–351. [Google Scholar] [CrossRef]
- Tas, C.E.; Hendessi, S.; Baysal, M.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Halloysite Nanotubes/Polyethylene Nanocomposites for Active Food Packaging Materials with Ethylene Scavenging and Gas Barrier Properties. Food Bioprocess Technol. 2017, 10, 789–798. [Google Scholar] [CrossRef]
- Srithammaraj, K.; Magaraphan, R.; Manuspiya, H. Modified Porous Clay Heterostructures by Organic–Inorganic Hybrids for Nanocomposite Ethylene Scavenging/Sensor Packaging Film. Packag. Technol. Sci. 2012, 25, 63–72. [Google Scholar] [CrossRef]
- Boonruang, K.; Chonhenchob, V.; Singh, S.P.; Chinsirikul, W.; Fuongfuchat, A. Comparison of Various Packaging Films for Mango Export. Packag. Technol. Sci. 2012, 25, 107–118. [Google Scholar] [CrossRef]
- Esturk, O.; Ayhan, Z.; Gokkurt, T. Production and Application of Active Packaging Film with Ethylene Adsorber to Increase the Shelf Life of Broccoli (Brassica oleracea L. var. Italica). Packag. Technol. Sci. 2014, 27, 179–191. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antioxidant Polyethylene Films Based On A Resveratrol Containing Clay Of Interest In Food Packaging Applications|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2214289415300119?token=90922F3D2440CAF6A65DC36ED590788F26E271C821005623F69B89F780919755B7EBF0E8195E246085D9FA6EEB1C8DA6&originRegion=eu-west-1&originCreation=20210729160631 (accessed on 29 July 2021).
- Bolumar, T.; LaPeña, D.; Skibsted, L.H.; Orlien, V. Rosemary and oxygen scavenger in active packaging for prevention of high-pressure induced lipid oxidation in pork patties. Food Packag. Shelf Life 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Appl. Sci. 2019, 9, 30533. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.Y.; EL-Sayed, S.M.; EL-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian Soft White Cheese Shelf Life Using A Novel Chitosan/Carboxymethyl Cellulose/Zinc Oxide Bionanocomposite Film|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0144861716305367?token=548191AFA89F297C72DE875E19C4823F9D1F0D6F6193FC787E6A2A30480AC67AC044A4FE7407DE22019A5BECCFB81005&originRegion=eu-west-1&originCreation=20210729135351 (accessed on 29 July 2021).
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bãnos, A.; Núñez, C.; Bermúdez, J.M.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Characterisation and evaluation of PLA films containing an extract of Allium spp. to be used in the packaging of ready-to-eat salads under controlled atmospheres. LWT Food Sci. Technol. 2015, 64, 1354–1361. [Google Scholar] [CrossRef]
- Syamsu, K.; Warsiki, E.; Yuliani, S.; Widayanti, S.M. Nano Zeolite-kmno4 as Ethylene Adsorber in Active Packaging of Horticulture Products (Musa Paradisiaca). Int. J. Sci. Basic Appl. Res. 2016, 30, 93–103. [Google Scholar]
- Carrizo, D.; Taborda, G.; Nerín, C.; Bosetti, O. Extension of shelf life of two fatty foods using a new antioxidant multilayer packaging containing green tea extract. Innov. Food Sci. Emerg. Technol. 2016, 33, 534–541. [Google Scholar] [CrossRef]
- Oudjedi, K.; Manso, S.; Nerin, C.; Hassissen, N.; Zaidi, F. New active antioxidant multilayer food packaging films containing Algerian Sage and Bay leaves extracts and their application for oxidative stability of fried potatoes. Food Control 2019, 98, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extendthe shelf life of fish. Food Control. 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef]
- Ousji, O.; Sleno, L. Identification of in vitro metabolites of synthetic phenolic antioxidants BHT, BHA, and TBHQ by LC-HRMS/MS. Int. J. Mol. Sci. 2020, 21, 49525. [Google Scholar] [CrossRef]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial properties of LDPE nanocomposite films in packaging of UF cheese. LWT Food Sci. Technol. 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Cutter, C.N.; Willett, J.L.; Siragusa, G.R. Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett. Appl. Microbiol. 2001, 33, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, C.; Zhang, Y.; Yao, J.; Yang, W.; Hu, Q.; Wang, C.; Cao, C. Effect of stable antimicrobial nano-silver packaging on inhibiting mildew and in storage of rice. Food Chem. 2017, 215, 477–482. [Google Scholar] [CrossRef]
- Rollini, M.; Nielsen, T.; Musatti, A.; Limbo, S.; Piergiovanni, L.; Munoz, P.H.; Gavara, R.; Barringer, S. Antimicrobial Performance of Two Different Packaging Materials on the Microbiological Quality of Fresh Salmon. Coatings 2016, 6, 6. [Google Scholar] [CrossRef]
- Pang, Y.-H.; Sheen, S.; Zhou, S.; Liu, L.; Yam, K.L. Antimicrobial Effects of Allyl Isothiocyanate and Modified Atmosphere on Pseduomonas Aeruginosa in Fresh Catfish Fillet under Abuse Temperatures. J. Food Sci. 2013, 78, M555–M559. [Google Scholar] [CrossRef]
- Granda-Restrepo, D.; Peralta, E.; Troncoso-Rojas, R.; Soto-Valdez, H. Release of antioxidants from co-extruded active packaging developed for whole milk powder. Int. Dairy J. 2009, 19, 481–488. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Emamifar, A.; Mohammadizadeh, M. Preparation and Application of LDPE/ZnO Nanocomposites for Extending Shelf Life of Fresh Strawberries. Food Technol. Biotechnol. 2015, 53, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, D.S.; Yam, K.L. Release property and antioxidant effectiveness of tocopherol-incorporated LDPE/PP blend films. Food Addit. Contam. Part A 2012, 29, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Graciano-Verdugo, A.Z.; Soto-Valdez, H.; Peralta, E.; Cruz-Zárate, P.; Islas-Rubio, A.R.; Sánchez-Valdes, S.; Sánchez-Escalante, A.; González-Méndez, N.; González-Ríos, H. Migration of α-tocopherol from LDPE films to corn oil and its effect on the oxidative stability. Food Res. Int. 2010, 43, 1073–1078. [Google Scholar] [CrossRef]
- Manzanarez-López, F.; Soto-Valdez, H.; Auras, R.; Peralta, E. Release of α-Tocopherol from Poly(lactic acid) films, and its effect on the oxidative stability of soybean oil. J. Food Eng. 2011, 104, 508–517. [Google Scholar] [CrossRef]
- Cestari, L.A.; Gaiotto, R.C.; Antigo, J.L.; Scapim, M.R.S.; Madrona, G.S.; Yamashita, F.; Pozza, M.S.S.; Prado, I.N. Effect of active packaging on low-sodium restructured chicken steaks. J. Food Sci. Technol. 2015, 52, 3376–3382. [Google Scholar] [CrossRef] [Green Version]
- Pant, A.F.; Sangerlaub, S.; Muller, K. Gallic acid as an oxygen scavenger in bio-based multilayer packaging films. Materials 2017, 10, 489. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Review of surface treatment methods for polyamide fi lms for potential application as smart packaging materials: Surface structure, antimicrobial and spectral properties. Food Packag. Shelf Life 2020, 24, 100475. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Camo, J.; Lorés, A.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Display life of beef packaged with an antioxidant active film as a function of the concentration of oregano extract. Meat Sci. 2011, 88, 174–178. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of new active packaging films coated with natural phenolic compounds to improve the oxidative stability of beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. A new pyrogallol coated oxygen scavenging film and their effect on oxidative stability of soybean oil under different storage conditions. Food Sci. Biotechnol. 2017, 26, 1535–1543. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Yang, R. Antimicrobial Polylactic Acid Packaging Films against Listeria and Salmonella in Culture Medium and on Ready-to-Eat Meat. Food Bioprocess Technol. 2014, 7, 3293–3307. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Joerger, R.D.; Sabesan, S.; Visioli, D.; Urian, D.; Joerger, M.C. Antimicrobial activity of chitosan attached to ethylene copolymer films. Packag. Technol. Sci. 2009, 22, 125–138. [Google Scholar] [CrossRef]
- Lardiés, O.G.; Nerin de la Puerta, C.; Garcia, J.A.B.; Rabinal, P.R. Antioxidant Active Varnish. European Patent EP1477519A1, 17 November 2004. pp. 1–8. Available online: https://data.epo.org/gpi/EP1477519A1-Antioxidant-active-varnish (accessed on 29 July 2021).
- Lee, J.S.; Park, M.A.; Yoon, C.S.; Na, J.H.; Han, J. Characterization and Preservation Performance of Multilayer Film with Insect Repellent and Antimicrobial Activities for Sliced Wheat Bread Packaging. J. Food Sci. 2019, 84, 3194–3203. [Google Scholar] [CrossRef]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Cummins, E.; Kerry, J.P.; Morris, M.A. The potential use of a layer-by-layer strategy to develop LDPE antimicrobial films coated with silver nanoparticles for packaging applications. J. Colloid Interface Sci. 2016, 461, 239–248. [Google Scholar] [CrossRef]
- Vähä-Nissi, M.; Pitkänen, M.; Salo, E.; Kenttä, E.; Tanskanen, A.; Sajavaara, T.; Putkonen, M.; Sievänen, J.; Sneck, A.; Rättö, M.; et al. Antibacterial and barrier properties of oriented polymer films with ZnO thin films applied with atomic layer deposition at low temperatures. Thin Solid Film. 2014, 562, 331–337. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite zinc oxide-chitosan coatings on polyethylene films for extending storage life of okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef] [Green Version]
- Tadesse Abate, M.; Ferri, A.; Guan, J.; Chen, G.; Nierstrasz, V. Impregnation of materials in supercritical CO2 to impart various functionalities. In Advanced Supercritical Fluids Technologies; InTechOpen: London, UK, 2020; pp. 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rojas, A.; Torres, A.; José Galotto, M.; Guarda, A.; Julio, R. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of olive leaf extract to obtain bioactive films effective in cherry tomato preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez De La Ossa-Fernández, E.J. Supercritical impregnation of antioxidant mango polyphenols into a multilayer PET/PP food-grade film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Añazco, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Effect of pressure and time on scCO2-assisted incorporation of thymol into LDPE-based nanocomposites for active food packaging. J. CO2 Util. 2018, 26, 434–444. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Torres, A.; Martínez, F.; Salazar, L.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Assessment of kinetic release of thymol from LDPE nanocomposites obtained by supercritical impregnation: Effect of depressurization rate and nanoclay content. Eur. Polym. J. 2017, 93, 294–306. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant packaging with encapsulated green tea for fresh minced meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, N.; Attianese, I.; Giuliana, G.; Caputo, D.; Lavorgna, M.; Mensitieri, G.; Lavorgna, M. Microporous and Mesoporous Materials a -Tocopherol release from active polymer films loaded with functionalized SBA-15 mesoporous silica. Microporous Mesoporous Mater. 2013, 167, 10–15. [Google Scholar] [CrossRef]
- Sun, L.; Lu, L.; Qiu, X.; Tang, Y. Development of low-density polyethylene antioxidant active fi lms containing a -tocopherol loaded with MCM-41 (Mobil Composition of Matter No. 41). Food Control 2017, 71, 193–199. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Cabedo, L.; Torres-Giner, S. Electrospun Antimicrobial Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica. Nanoparticles 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Alkan, B.; Sehit, E.; Tas, C.E.; Unal, S.; Cebeci, F.C. Carvacrol loaded halloysite coatings for antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 20, 100300. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; de Gomes, B.O.; Batista, A.F.P.; Mikcha, J.M.G.; Yamashita, F.; Scapim, M.R.S.; de Bergamasco, R.C. Development of sorbic acid microcapsules and application in starch-poly (butylene adipate co-terephthalate) films. J. Food Process. Preserv. 2021, 45, e15459. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Escuin, J.M.; Bourbon, A.I.; Cabedo, L.; Nevo, Y.; Cerqueira, M.A.; Lagaron, J.M. Development of active barrier multilayer films based on electrospun antimicrobial hot-tack food waste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and cellulose nanocrystal interlayers. Nanomaterials 2020, 10, 2356. [Google Scholar] [CrossRef]
- Chiralt, A.; Tampau, A.; Gonz, C. Food Hydrocolloids Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone)nano fibres. Application in starch multilayer films. Food Hydrocoll. 2018, 79, 158–169. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Enescu, D.; Torres-Giner, S.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.; Fuciños, P.; Lagaron, J.M. Development of electrospun active films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by the incorporation of cyclodextrin inclusion complexes containing oregano essential oil. Food Hydrocoll. 2020, 108, 106013. [Google Scholar] [CrossRef]
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Zemljič, L.F. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef] [Green Version]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Jose, M. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
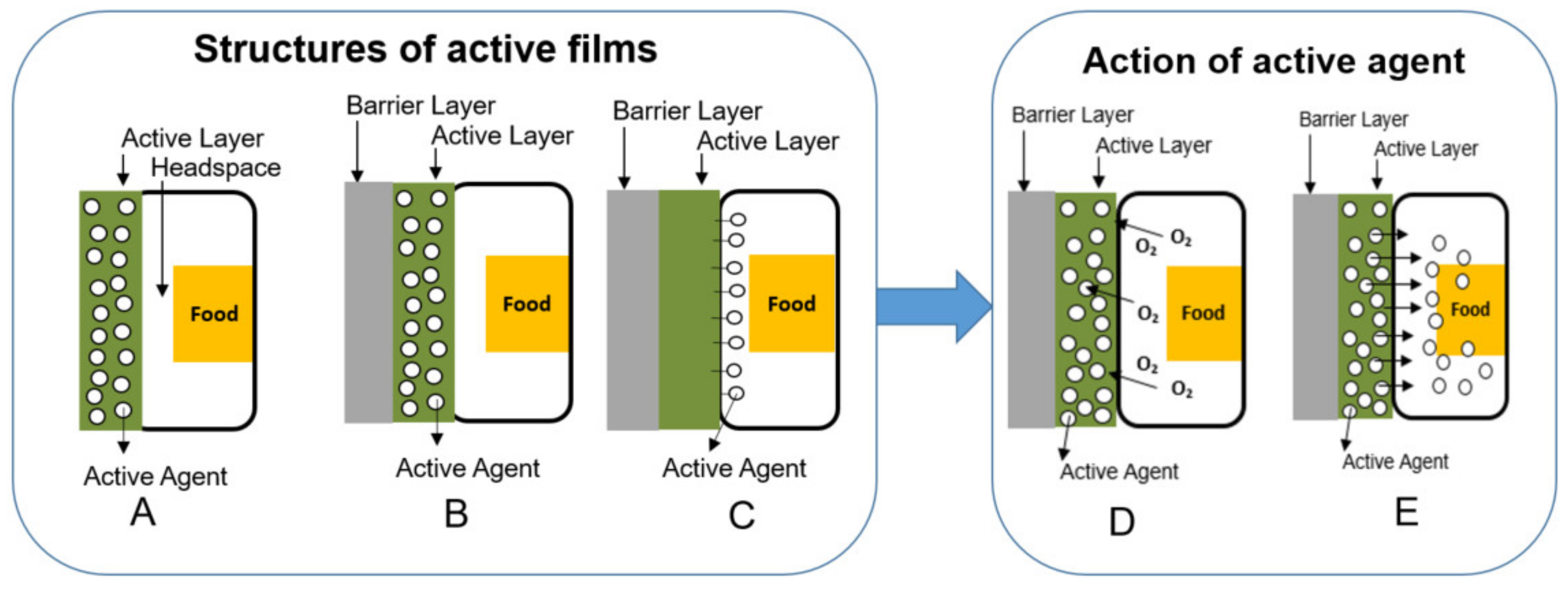


| Active Scavenger System (or Absorber) | Classification | Materials | Mechanism | Potential Benefits | References |
| Oxygen scavenger | Metallic and metallic oxides | Iron, ferrous oxide, cobalt, zinc, copper, magnesium, aluminum, titanium | Oxidation of metals with the supply of moisture and action of an optional catalyst. | Prevention of discoloration; prevention of mold growth; retention of vitamin C content, prevention of browning; prevention of rancidity. | [22,23,24,25,26] |
| Inorganic | Sulfite, thiosulfate, dithionite, hydrogen sulfite, titanium dioxide | Oxidation of inorganic substrate by UV light. | |||
| Organic | Ascorbic acid, tocopherol, gallic acid, hydroquinone, catechol, rongalit, sorbose, lignin, pyrogallol, glucose oxidase, laccase | Oxidation of organic substrate with metallic catalyst or alkaline substance. | |||
| Polymer-based | Polymer metallic complex | Oxidation of polymer components with metallic catalyst (mostly cobalt). | |||
| Moisture absorber | Inorganic | Silica gel (SiO2), potassium chloride (KCl), calcium chloride (CaCl2), sodium chloride (NaCl), calcium sulfate (CaSO4) | The common process is adsorption and absorption. | To control the moisture content in headspace of packaging and absorber of liquids. | [27,28,29,30,31] |
| Organic | Sorbitol, fructose, cellulose and their derivatives (e.g., carboxymethylcellulose (CMC)) | ||||
| Polymer-based | Polyvinyl alcohol (PVOH) and sodium polyacrylate | ||||
| Other synthesized | Synthesized attapulgite with acrylamide | ||||
| Ethylene scavenger | Minerals | Clays modified (e.g., MMT, organoclays, halloysite nanotubes (HNTs)) and zeolites, titanium dioxide (TiO2) | Adsorption process and cation exchange. | Reduction in ripening and senescence of fruits and vegetables. | [32,33,34,35] |
| Metallic and metallic oxides | Silver (Ag) and zinc oxide (ZnO) | Activated by either UV light, visible light or both. | |||
| Active Releaser system (or emitter) | Classification | Materials | Mechanism | Potential benefits | References |
| Antioxidants | Organic | Tocopherol, carvacrol, quercetin, catechin, thymol, gallic acid, ascorbic acid, rosemary, green tea, oregano, cinnamon, sage leaf and bay leaf extracts, eugenol, olive leaf, mango leaf | Free radicals and peroxides react to retard or block the actual oxidation reactions. | Prevention of fat oxidation and food deterioration maintenance of nutritional quality, texture and functionality. | [36,37,38] |
| Metallic and metallic oxides, and inorganic | Silver (Ag), copper (Cu), titanium dioxide (TiO2) and zinc oxide (ZnO) | Catalytic function that reduces the rate oxidation. | |||
| Antimicrobials | Organic | Allyl- isothiocyanate, cinnamaldehyde, carvacrol, thymol, eugenol, oregano, basil leaf, extract of allium, lauric arginate ester, sodium lactate, sorbic acid, citric acid | Metabolic and reproductive processes of microorganisms are blocked or inhibited. Cell wall conformation modification. | Inhibition of spoilage and retardation of pathogenic microorganism’s growth. | [8,39,40] |
| Polymers | Chitosan and ε-Polylysine | ||||
| Enzymes, bacteriocins and antibiotics | Lysozyme, lactoferrin, nisin, lactocins, pediocin, enterocins | ||||
| Metallic and metallic oxides, and inorganic | Silver (Ag), copper (Cu), titanium dioxide (TiO2) and zinc oxide (ZnO) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. https://doi.org/10.3390/polym14122442
Azevedo AG, Barros C, Miranda S, Machado AV, Castro O, Silva B, Saraiva M, Silva AS, Pastrana L, Carneiro OS, et al. Active Flexible Films for Food Packaging: A Review. Polymers. 2022; 14(12):2442. https://doi.org/10.3390/polym14122442
Chicago/Turabian StyleAzevedo, Ana G., Carolina Barros, Sónia Miranda, Ana Vera Machado, Olga Castro, Bruno Silva, Margarida Saraiva, Ana Sanches Silva, Lorenzo Pastrana, Olga Sousa Carneiro, and et al. 2022. "Active Flexible Films for Food Packaging: A Review" Polymers 14, no. 12: 2442. https://doi.org/10.3390/polym14122442
APA StyleAzevedo, A. G., Barros, C., Miranda, S., Machado, A. V., Castro, O., Silva, B., Saraiva, M., Silva, A. S., Pastrana, L., Carneiro, O. S., & Cerqueira, M. A. (2022). Active Flexible Films for Food Packaging: A Review. Polymers, 14(12), 2442. https://doi.org/10.3390/polym14122442










