On the Use of Oxidation Induction Time as a Kinetic Parameter for Condition Monitoring and Lifetime Evaluation under Ionizing Radiation Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Irradiation
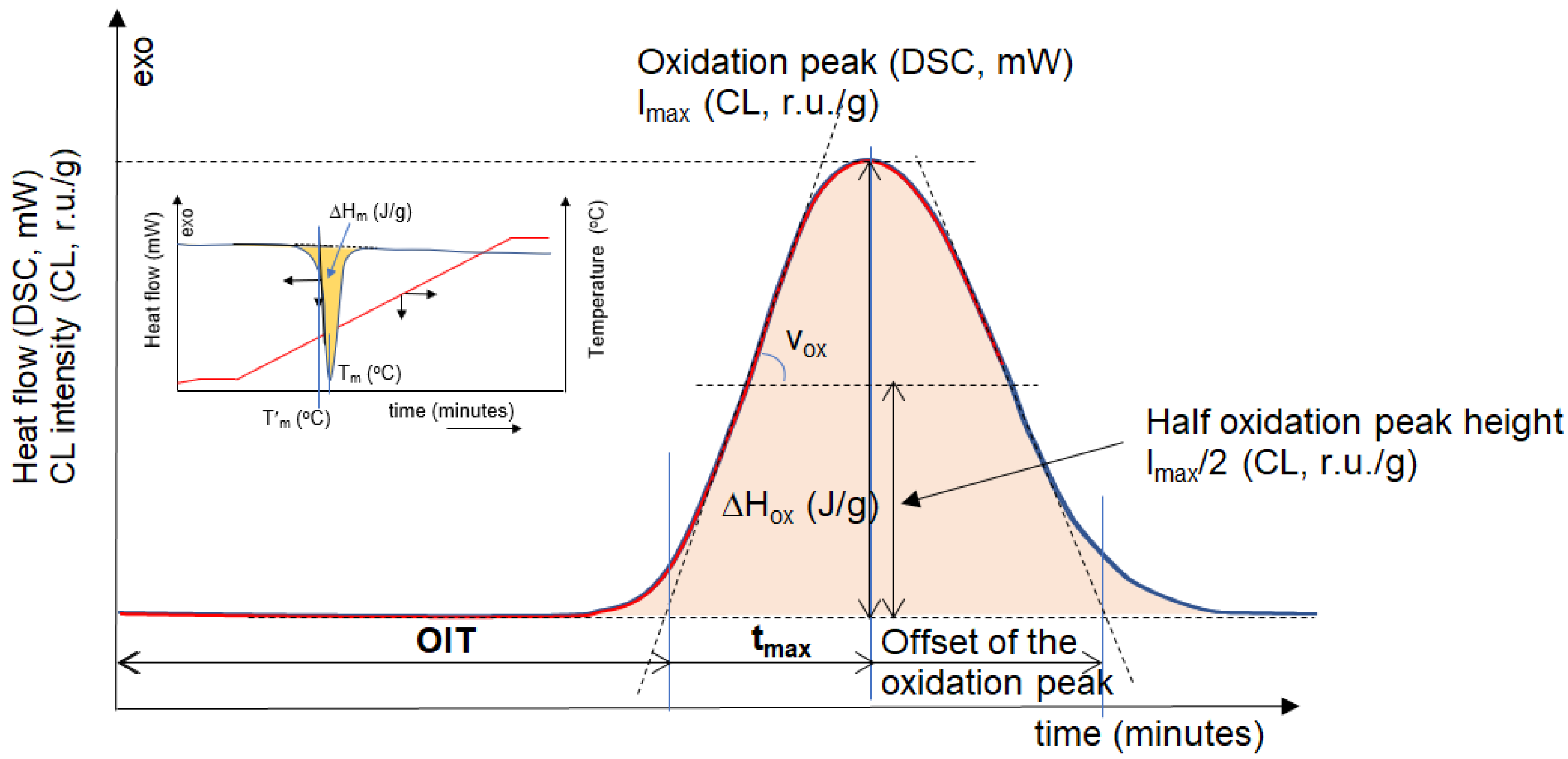
2.3. Characterization Methods
3. Results and Discussion
3.1. Kinetic Model: Methodology and Premises
- (a)
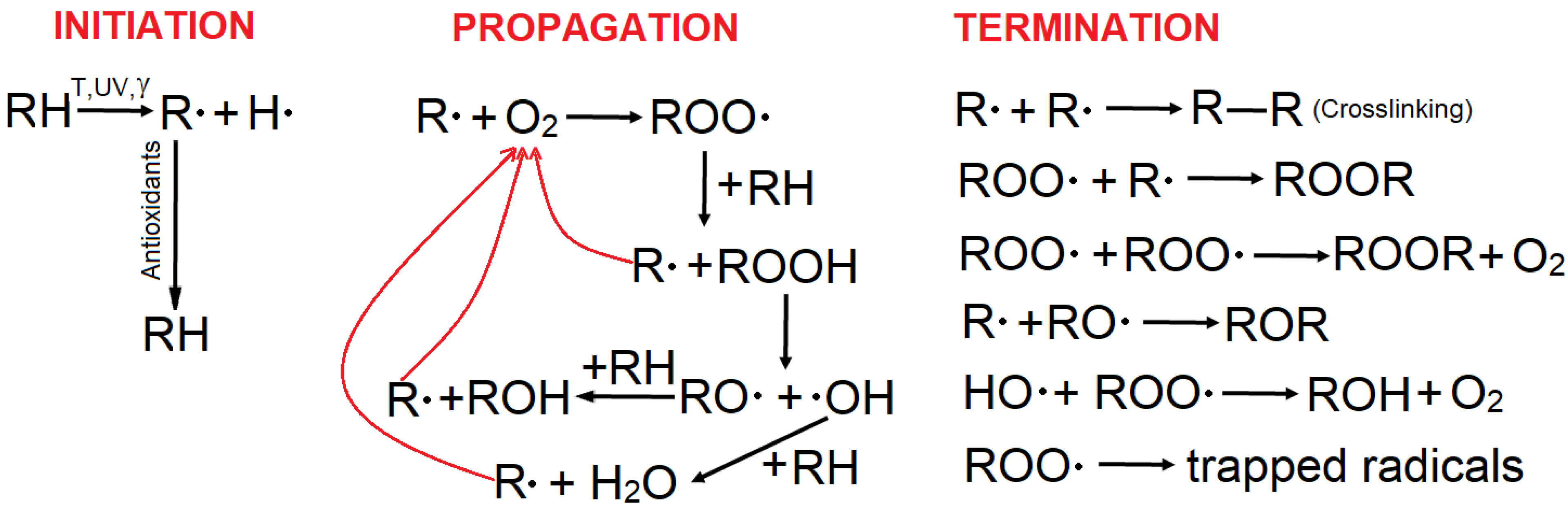
- The effect of the stress factors, irrespective of their nature, consists of molecular scissions, leading to free radicals. These species react rapidly with the surrounding oxygen in air and trigger oxidation chain reactions (Scheme 1), leading to material deterioration and implicitly shortening their lifetime. In the case of ethylene polymers, lifetime diminution is caused by the progressive consumption of antioxidants.The initiation of degradation (produced by breaking of labile chemical bonds of the polymer) occurs by the action of different stress factors (high temperatures, light, radiation, electric field, etc.). The formed free radicals are subsequently trapped by oxygen, and the degradation propagates as chain reactions of free radicals thus formed. On average, each free radical formed subsequently involves the reaction of five oxygen molecules [46]. The second component of propagation consists of reactions of hydroperoxides.Even in the absence of oxygen, stress factors can induce free radicals, producing polymer degradation by scission and eventual crosslinking. The subsequent air exposure of such material results in its oxidation due to the reactions of the trapped free radicals [47].
- (b)
- Antioxidants (together with other additives) are chemically consumed in the reactions with the free radicals. Hence, a continuous depletion of the antioxidant content initially introduced in the polymer occurs during the service time of the material. During this time, no material deterioration occurs (or it is negligible), with the antioxidants acting as chemical barriers against the spreading of the oxidation process.
- (c)
- The OIT values, which are determined at high temperatures specific to the test, correspond to the lifetime of the material under the conditions of exposure. The OIT value is directly proportional to the concentration of antioxidants in the polymeric material [2,35,45]. Therefore, OIT measurement can be used to “titrate” the antioxidant content of the sample. Thus, decreasing kinetics of OIT can be used to describe the kinetics of antioxidant depletion. Given the direct connection between OIT and material lifetime, decreasing OIT kinetics can be used to evaluate the lifetime at moderate temperatures specific to service conditions. As previously mentioned, the main problems for extrapolations are (i) the existence of a transition point (e.g., melting point of crystallinity), which can cause changes in the degradation mechanism; and (ii) the influence of temperature on the rates of different reactions. Hence, the degradation mechanism is expected to be different at higher temperatures as compared to moderate temperatures (due to the activation or deactivation of specific reactions).
- (d)
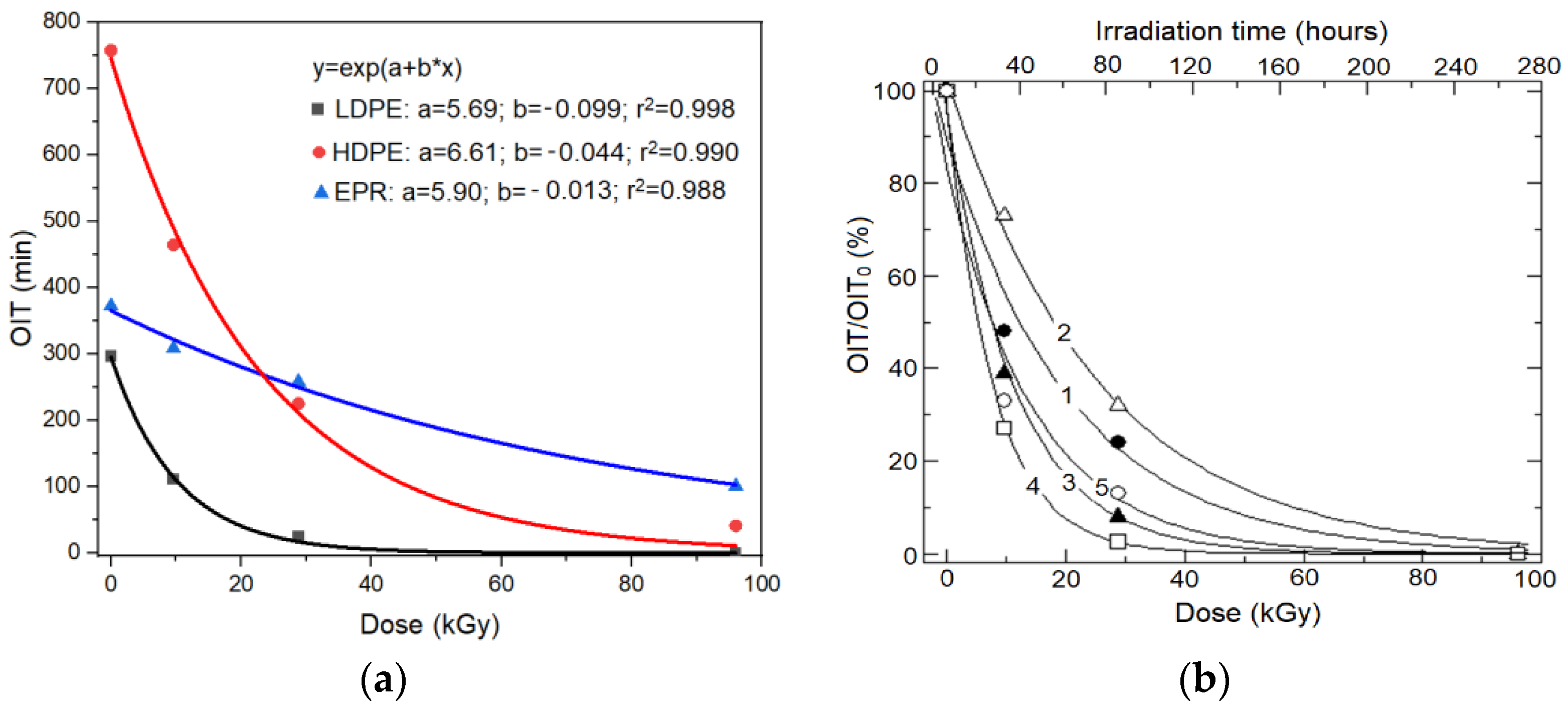
- We hypothesize that under similar (quasi-constant) exposure conditions of a material, the resulting OIT reduction is proportional to the time spent in mild conditions specific to aging in real conditions. Thus, OIT can be used as a revealing agent of the degradation effect produced under mild conditions and not as an accelerated aging test. In this case, the lifetime of the material under real conditions is equal to the exposure time resulting in a zero OIT value.The above hypothesis was confirmed by experimental data (see Figure 2), enabling a “bypass” of the problem of extrapolation, with the OIT used as a “marker” of the remaining lifetime of the material.
3.2. Kinetic Model for Evaluating the Lifetime of Polymeric Materials
3.2.1. Evaluation of the Remaining Service Life (tx) When the Elapsed Aging/Service Time Is Known
3.2.2. Evaluation of the Service Life (tx) of Polymeric Insulation When the OIT0 Value Is Not Known
3.2.3. Evaluation of the Maximum Supported Dose (Dx) for a Cable in Use
3.3. Examples of Application of the Kinetic Model on Various Ethylene-Based Electrical Insulation Materials
3.3.1. LDPE/XLPE Insulation Materials
3.3.2. EPR Copolymer Insulation Materials
3.3.3. EVA Copolymer Insulation Materials
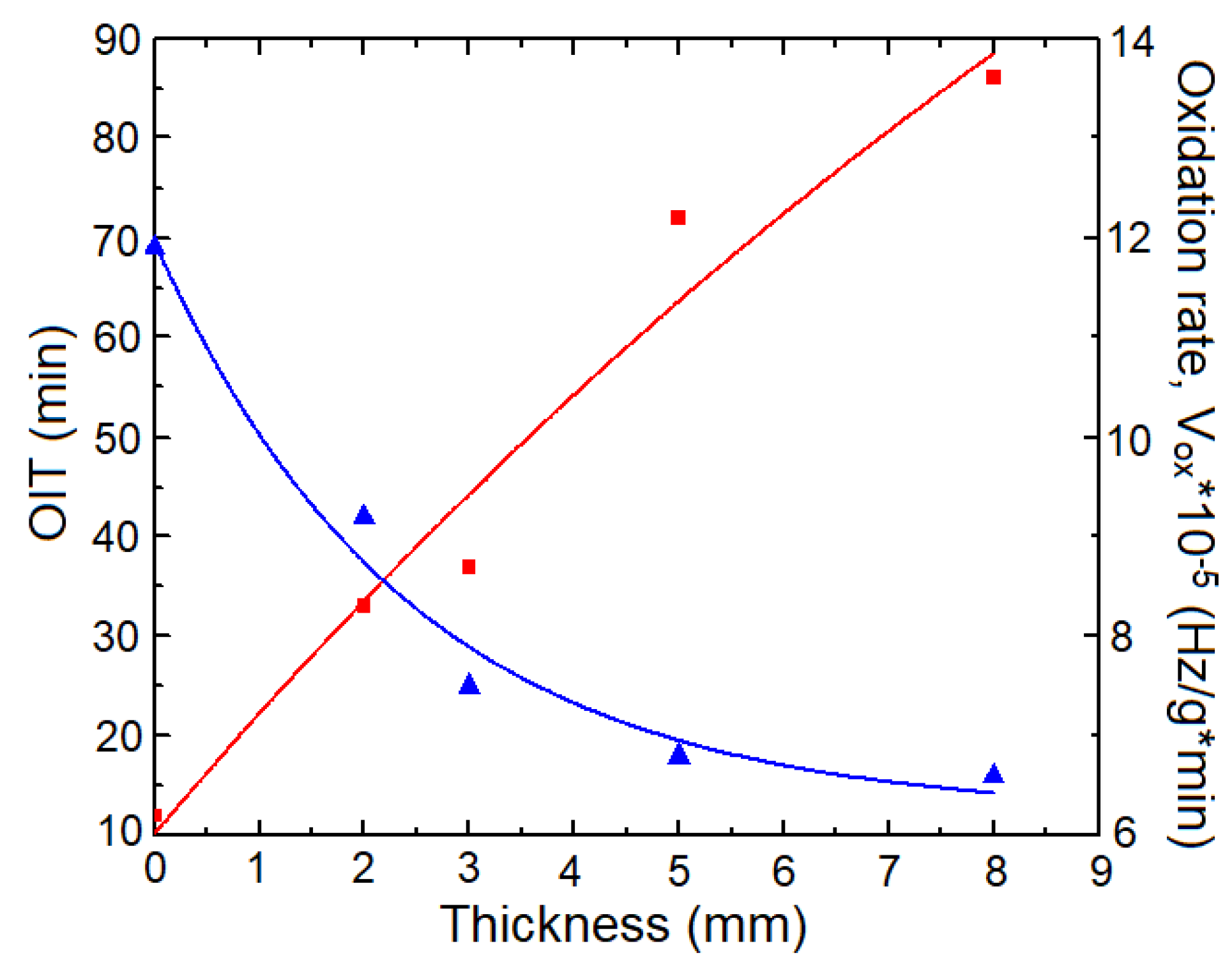
3.4. Radiation-Induced Oxidation Profiles in Cable Depth by OIT Measurements
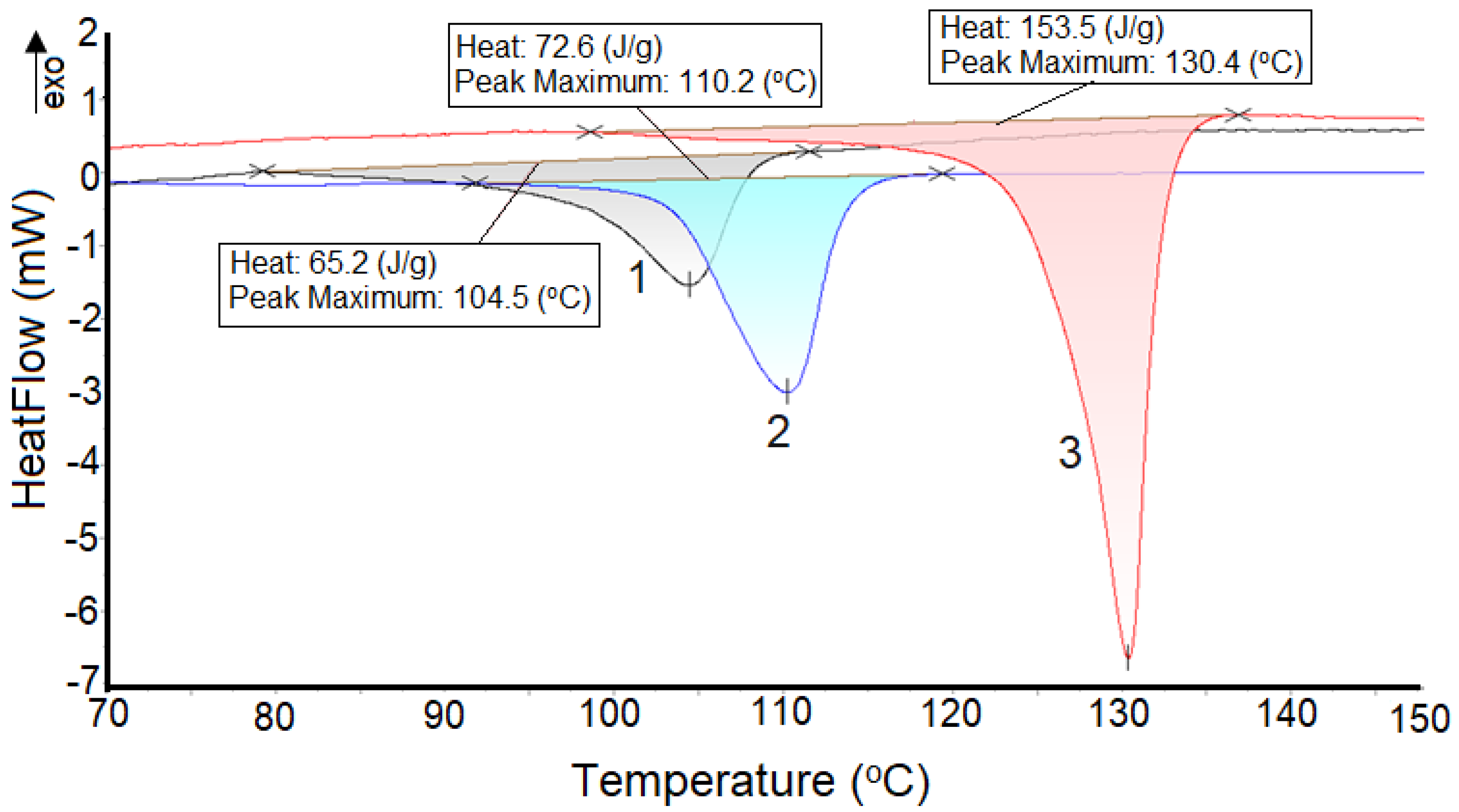
3.5. DSC-CL Correlation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maléchaux, A.; Colombani, J.; Amat, S.; Marque, S.R.A.; Dupuy, N. Influence of Gamma Irradiation on Electric Cables Models: Study of Additive Effects by Mid-Infrared Spectroscopy. Polymers 2021, 13, 1451. [Google Scholar] [CrossRef] [PubMed]
- Ilie, S.; Setnescu, R.; Lungulescu, E.M.; Marinescu, V.; Ilie, D.; Setnescu, T.; Mares, G. Investigations of a mechanically failed cable insulation used in indoor conditions. Polym. Test 2011, 30, 173–182. [Google Scholar] [CrossRef]
- Suraci, S.V.; Fabiani, D.; Colin, X.; Roland, S. Chemical and electrical characterization of XLPE cables exposed to radio-thermal aging. IEEE 2020, 2020, 57–60. [Google Scholar]
- Thue, W.A. Electrical Power Cable Engineering; Crc Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Athawale, A.A.; Joshi, A.M. Electronic Applications of Ethylene Propylene Diene Monomer Rubber and Its Composites. In Flexible and Stretchable Electronic Composites; Ponnamma, D., Sadasivuni, K.K., Wan, C., Thomas, S., Al-Ali AlMa’adeed, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 305–333. [Google Scholar]
- Küchler, A. High Voltage Engineering: Fundamentals-Technology-Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Li, J.; Zhou, C.; Cao, D.; Liu, H. Synergistic Effects of Amine-Containing Antioxidants on the Aging Performances of Ethylene Propylene Diene Rubber. ChemistrySelect 2020, 5, 4961–4966. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Su, H.; Li, C.; Fan, W.; Jing, X. Enhanced thermal resistance and ablation properties of ethylene-propylene-diene monomer rubber with boron-containing phenolic resins. React. Funct. Polym. 2022, 170, 105136. [Google Scholar] [CrossRef]
- Latocha, C.; Uhniat, M. The kinetics of oxidative induction of LDPE stabilized with commercial antioxidants. Polym. Degrad. Stab. 1992, 35, 17–22. [Google Scholar] [CrossRef]
- Lungulescu, E.-M. Contributions to the Study and Characterization of Degradation Processes of the Insulating Polymeric Materials in High-Energy Radiation Fields. Ph.D. Thesis, University of Bucharest, Bucharest, Romania, 2014. [Google Scholar]
- Focke, W.W.; Van Der Westhuizen, I. Oxidation induction time and oxidation onset temperature of polyethylene in air. J. Therm. Anal. Calorim. 2010, 99, 285–293. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M.; Mariatti, M. Oxidative induction and performance of oil palm fiber reinforced polypropylene composites—Effects of coupling agent and UV stabilizer. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105577. [Google Scholar] [CrossRef]
- Richaud, E. Kinetic modelling of phenols consumption during polyethylene thermal oxidation. Eur. Polym. J. 2013, 49, 2223–2232. [Google Scholar] [CrossRef] [Green Version]
- IEC TS 61244-3:2005—Long-Term Radiation Ageing in Polymers—Part 3: Procedures for in-Service Monitoring of Low-Voltage Cable Materials. 2005. Available online: https://webstore.iec.ch/p-preview/info_iec61244-3%7Bed2.0%7Den.pdf (accessed on 5 May 2022).
- Pertin, T.; Minatchy, G.; Adoue, M.; Flory, A.; Romana, L. Investigation of nanoindentation as a fast characterization tool for polymer degradation study. Polym. Test 2020, 81, 106194. [Google Scholar] [CrossRef]
- Eder, G.C.; Lin, Y.; Voronko, Y.; Spoljaric-Lukacic, L. On-Site Identification of the Material Composition of PV Modules with Mobile Spectroscopic Devices. Energies 2020, 13, 1903. [Google Scholar] [CrossRef]
- Fischer, J.; Metzsch-Zilligen, E.; Zou, M.; Pfaendner, R. A novel class of high molecular weight multifunctional antioxidants for polymers based on thiol-ene click reaction. Polym. Degrad. Stab. 2020, 173, 109099. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Suraci, S.V.; Fabiani, D.; Mazzocchetti, L.; Giorgini, L. Degradation Assessment of Polyethylene-Based Material Through Electrical and Chemical-Physical Analyses. Energies 2020, 13, 650. [Google Scholar] [CrossRef] [Green Version]
- Boukezzi, L.; Boubakeur, A.; Laurent, C.; Lallouani, M. DSC Study of Artificial Thermal Aging of XLPE Insulation Cables. In Proceedings of the IEEE International Conference on Solid Dielectrics, Winchester, UK, 8–13 July 2007; pp. 146–149. [Google Scholar]
- Jingdong, L.; Li, C. Research on the thermal aging life prediction of XLPE cable. J. Phys. Conf. Ser. 2020, 1570, 012050. [Google Scholar] [CrossRef]
- Shwehdi, M.H.; Morsy, M.A.; Abugurain, A. Thermal aging tests on XLPE and PVC cable insulation materials of Saudi Arabia. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Albuquerque, NM, USA, 19–22 October 2003; pp. 176–180. [Google Scholar]
- Dakin, T.W. Electrical Insulation Deterioration Treated as a Chemical Rate Phenomenon. Trans. Am. Inst. Electr. Eng. 1948, 67, 113–122. [Google Scholar] [CrossRef]
- Lin, L.; Lin, C.; Geng, P.; Lei, Z.; Song, J.; Tian, M. Aging Life Evaluation of Coal Mining Flexible EPR Cables Under Multi-Stresses. IEEE Access 2020, 8, 53539–53546. [Google Scholar] [CrossRef]
- IEC 60216-1; 2013 Electrical Insulating Materials—Thermal Endurance Properties—Part 1: Ageing Procedures and Evaluation of Test Results. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- TEAM CABLES. European Tools and Methodologies for an Efficient Ageing Management of Nuclear Power Plant Cables. Available online: https://www.team-cables.eu/ (accessed on 6 April 2022).
- Gillen, K.T.; Clough, R.L. Predictive aging results in radiation environments. Radiat. Phys. Chem. 1993, 41, 803–815. [Google Scholar] [CrossRef]
- Tavlet, M.; Schönbacher, H. Compilation of Radiation Damage Test Data, Pt.1 1: Halogen-Free Cable Insulating Materials, CERN-89-12, Technical Inspection and Safety Commission CERN; CERN: Geneva, Switzerland, 1989. [Google Scholar]
- Tavlet, M. Aging of organic materials around high-energy particle accelerators. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1997, 131, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yves Le Gac, P.; Richaud, E. Thermal oxidation of poly(dicyclopentadiene)—Effect of phenolic and hindered amine stabilizers. Polym. Degrad. Stab. 2021, 183, 109267. [Google Scholar] [CrossRef]
- Mareş, G.; Ciutacu, S.; Budrugeac, P.; Chiparǎ, M. Determination of the lifetime of ethylene-propylene rubber under the simultaneous action of heat and ionizing radiation. Polym. Degrad. Stab. 1991, 32, 31–38. [Google Scholar] [CrossRef]
- ASTM D3895-19; Standard Test Method for Oxidative-Induction Time of Polyolefins by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Taddei, P.; Affatato, S.; Fagnano, C.; Toni, A. Oxidation in ultrahigh molecular weight polyethylene and cross-linked polyethylene acetabular cups tested against roughened femoral heads in a hip joint simulator. Biomacromolecules 2006, 7, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Billingham, N.C.; Grigg, M.N. The kinetic order of decomposition of polymer hydroperoxides assessed by chemiluminescence. Polym. Degrad. Stab. 2004, 83, 441–451. [Google Scholar] [CrossRef]
- Setnescu, R.; Jipa, S.; Osawa, Z. Chemiluminescence study on the oxidation of several polyolefins—I. Thermal-induced degradation of additive-free polyolefins. Polym. Degrad. Stab. 1998, 60, 377–383. [Google Scholar] [CrossRef]
- Celina, M.; Linde, E.; Brunson, D.; Quintana, A.; Giron, N. Overview of accelerated aging and polymer degradation kinetics for combined radiation-thermal environments. Polym. Degrad. Stab. 2019, 166, 353–378. [Google Scholar] [CrossRef]
- Niki, E. Chapter 1—Lipid Peroxidation and Its Inhibition. In Atmospheric Oxidation and Antioxidants; Scott, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 1–31. [Google Scholar]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–92. [Google Scholar]
- Allen, N.S.; Chirinis-Padron, A.; Henman, T.J. The photo-stabilisation of polypropylene: A review. Polym. Degrad. Stab. 1985, 13, 31–76. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Bolland, J.L. Kinetics of olefin oxidation. Q. Rev. Chem. Soc. 1949, 3, 1–21. [Google Scholar] [CrossRef]
- Xu, A.; Roland, S.; Colin, X. Thermal ageing of a silane-crosslinked polyethylene stabilised with a thiodipropionate antioxidant. Polym. Degrad. Stab. 2020, 181, 109276. [Google Scholar] [CrossRef]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating Antioxidant Functionality into Polymer Materials: Fundamentals, Strategies, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef] [PubMed]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent Advances in Antioxidant Polymers: From Sustainable and Natural Monomers to Synthesis and Applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef] [PubMed]
- Clough, R.; Gillen, K. Gamma-radiation induced oxidation and mechanisms of its inhibition. Oxid. Inhib. Org. Mater. 1990, 2, 191–218. [Google Scholar]
- Kornacka, E.M. Radiation-induced oxidation of polymers. Appl. Ioniz. Radiat. Mater. Processing 2017, 1, 183–192. [Google Scholar]
- Audouin, L.; Langlois, V.; Verdu, J.; de Bruijn, J.C.M. Role of oxygen diffusion in polymer ageing: Kinetic and mechanical aspects. J. Mater. Sci. 1994, 29, 569–583. [Google Scholar] [CrossRef]
- IEC 60544-4:2003; Electrical Insulating Materials—Determination of the Effects of Ionizing Radiation—Part 4: Classification System for Service in Radiation Environments. International Electrotechnical Commission: Geneva, Switzerland, 2003.
- Auriemma, F.; Scoti, M.; De Stefano, F.; Talarico, G.; Ruiz de Ballesteros, O.; Di Girolamo, R.; Malafronte, A.; De Rosa, C.; Cipullo, R.; Van Duin, M. Microstructural insight on strain-induced crystallization of ethylene/propylene(/diene) random copolymers. Polymer 2021, 227, 123848. [Google Scholar] [CrossRef]
- Tefferi, M.; Li, Z.; Uehara, H.; Chen, Q.; Cao, Y. Characterization of space charge and DC field distribution in XLPE and EPR during voltage polarity reversal with thermal gradient. IEEE 2017, 2017, 617–620. [Google Scholar]
- Abdullah, R.H.; Essa, A.F. Measuring the peeling strength between polymers (low density polyethylene, ethylene propylene diene monomer (EPDM) rubber) and brass and evaluating the effect of thermal and chemical oxidation on the metal. Mater. Today Proc. 2021, 47, 5992–5998. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C. Advances in high-field insulating polymeric materials over the past 50 years. IEEE Electr. Insul. Mag. 2013, 29, 26–36. [Google Scholar] [CrossRef]
- Tefferi, M.; Li, Z.; Cao, Y.; Uehara, H.; Chen, Q. Novel EPR-insulated DC cables for future multi-terminal MVDC integration. IEEE Electr. Insul. Mag. 2019, 35, 20–27. [Google Scholar] [CrossRef]
- Ji Yeon, K.; Dae Hee, P. Thermal analysis and statistical evaluation of EPR used in nuclear power plants. In Proceedings of the IEEE Electrical Insulation Conference (EIC), Seattle, WA, USA, 7–10 June 2015; pp. 5–8. [Google Scholar] [CrossRef]
- Alshaketheep, T.; Murakami, K.; Sekimura, N. Effect of insulator environment on thermal ageing estimations for ethylene propylene rubber insulated nuclear power plant cables. J. Nucl. Sci. Technol. 2016, 53, 1366–1370. [Google Scholar] [CrossRef]
- Henderson, A.M. Ethylene-vinyl acetate (EVA) copolymers: A general review. IEEE Electr. Insul. Mag. 1993, 9, 30–38. [Google Scholar] [CrossRef]
- Son, C.E.; Choi, S.-S. Characterization of Poly (ethylene-co-vinyl acetate)(EVA) Using Thermal Analytical Techniques. Elastomers Compos. 2019, 54, 61–69. [Google Scholar]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. Dyna 2019, 86, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, K.; Eriksson, P.; Reitberger, T.; Stenberg, B. Chemiluminescence as a Tool for Polyolefin Oxidation Studies. In Long Term Properties of Polyolefins; Albertsson, A.-C., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 151–176. [Google Scholar]
- Fearon, P.K.; Bigger, S.W.; Billingham, N.C. DSC combined with chemiluminescence for studying polymer oxidation. J. Therm. Anal. Calorim. 2004, 76, 75–83. [Google Scholar] [CrossRef]
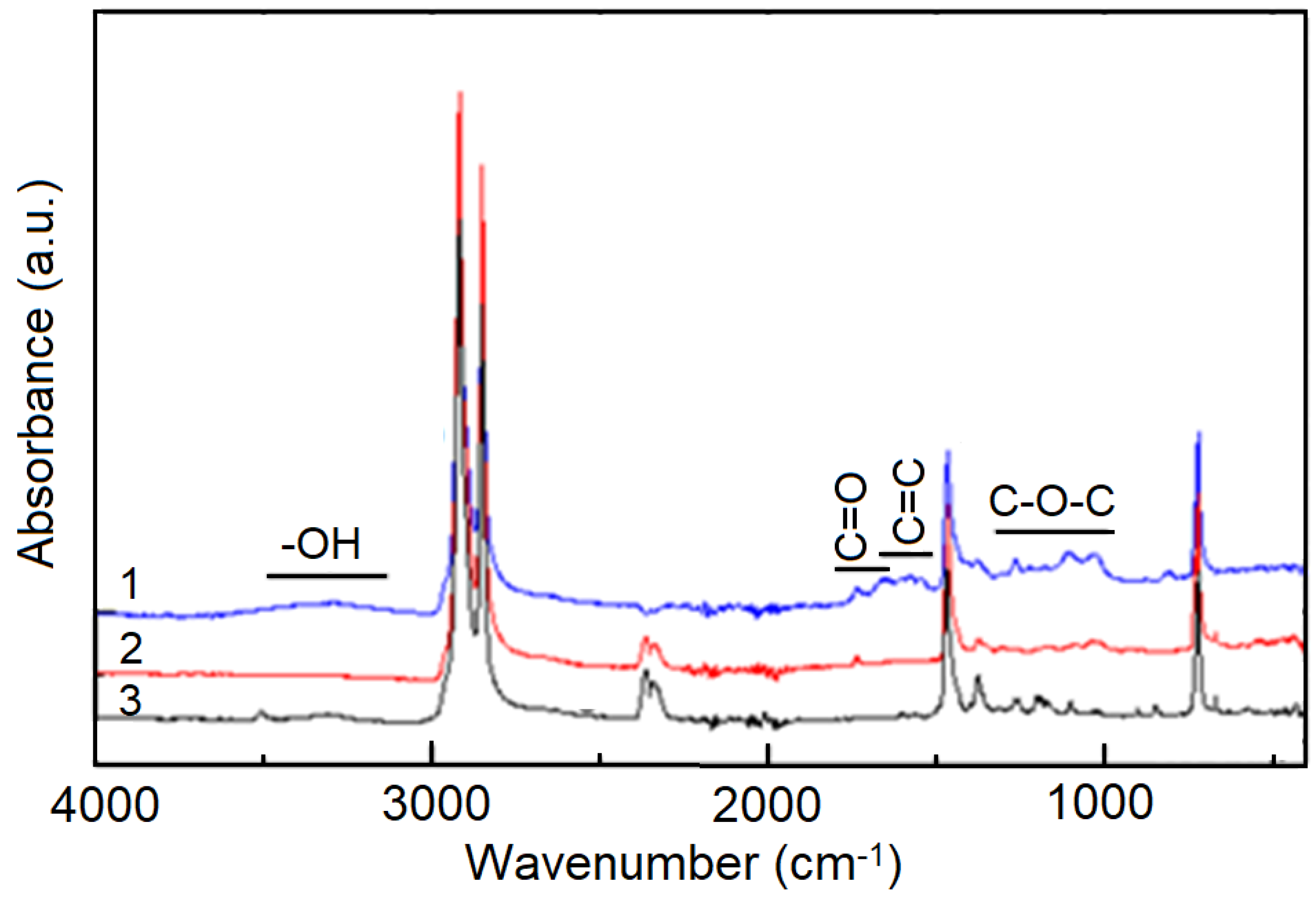


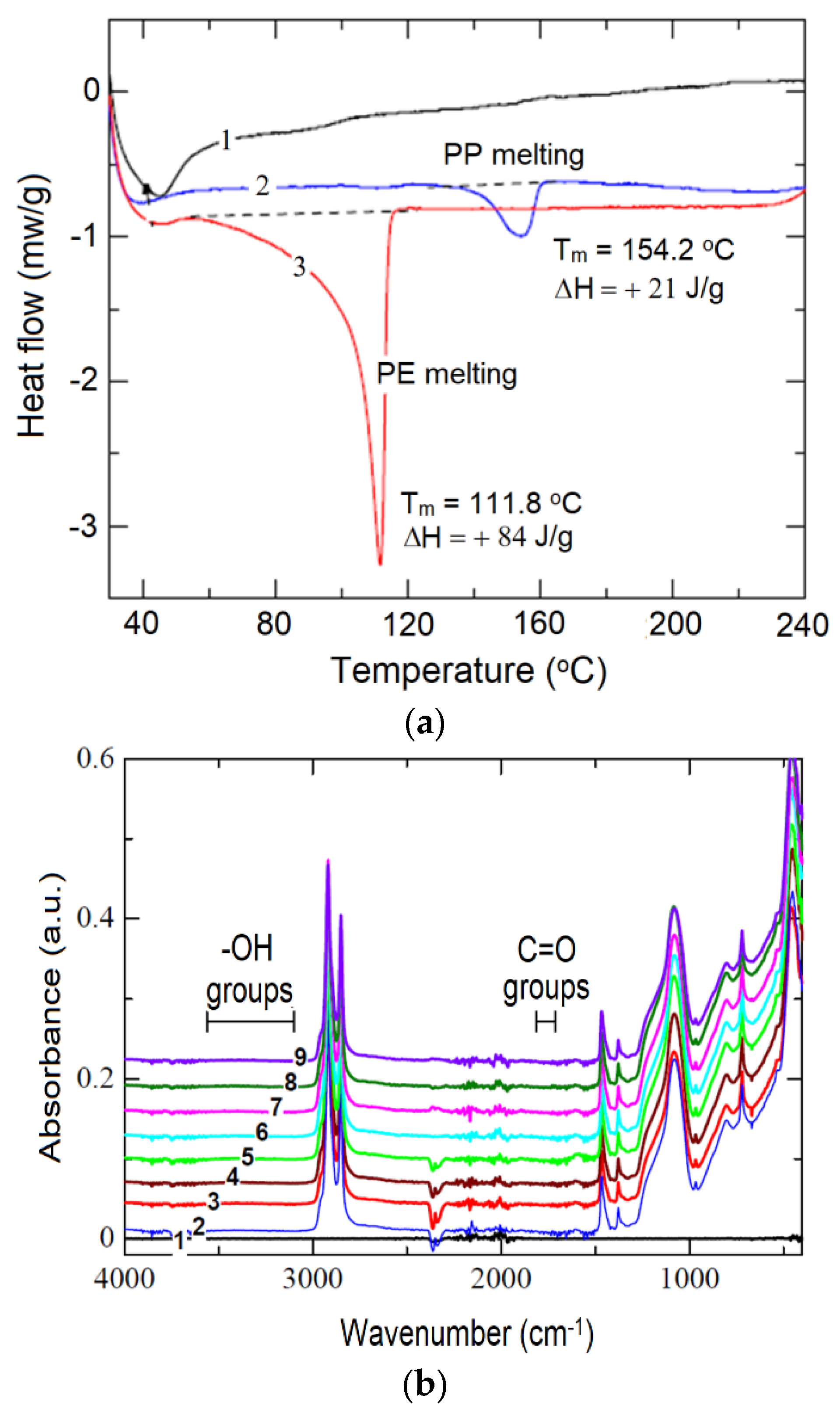

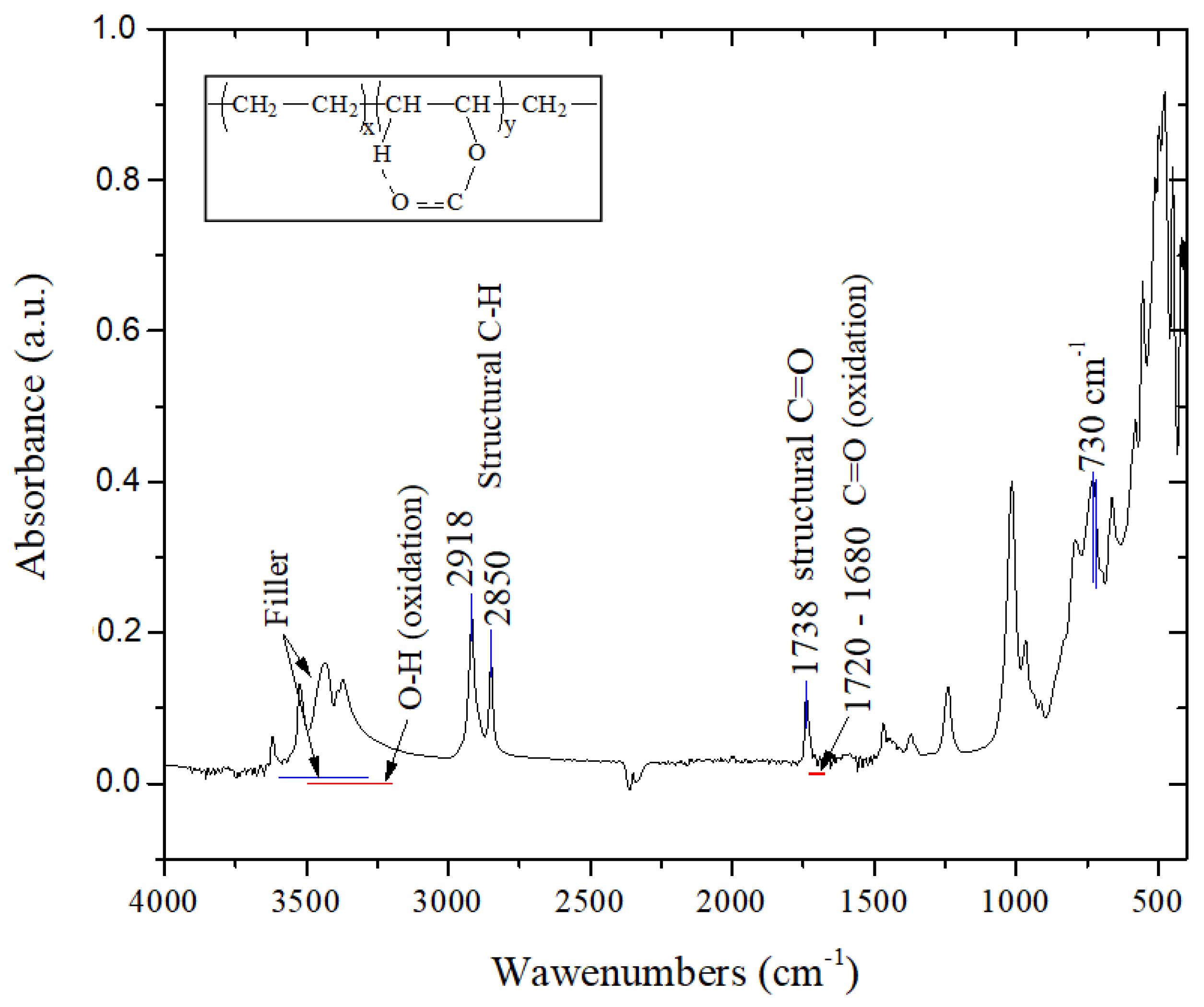
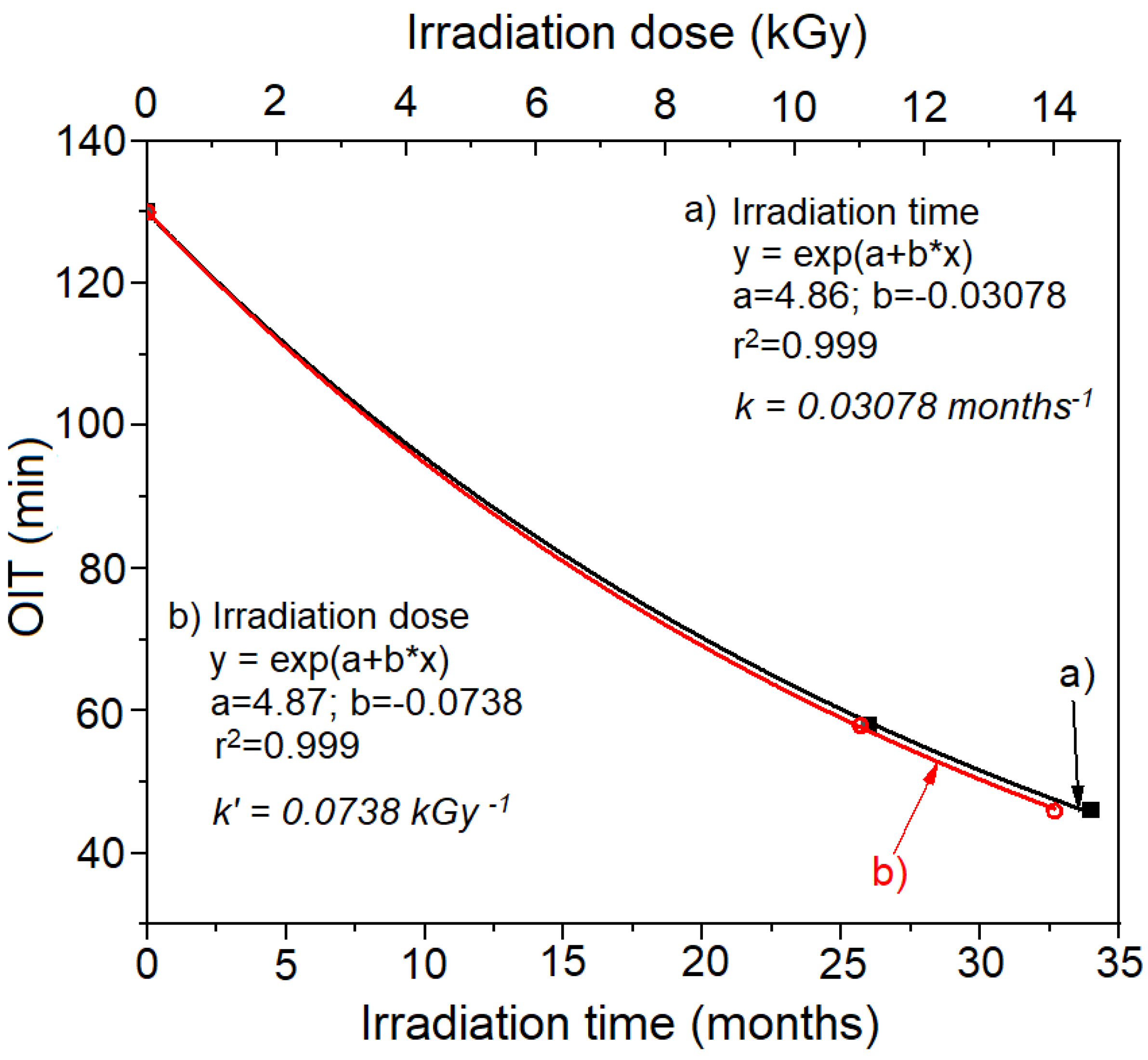

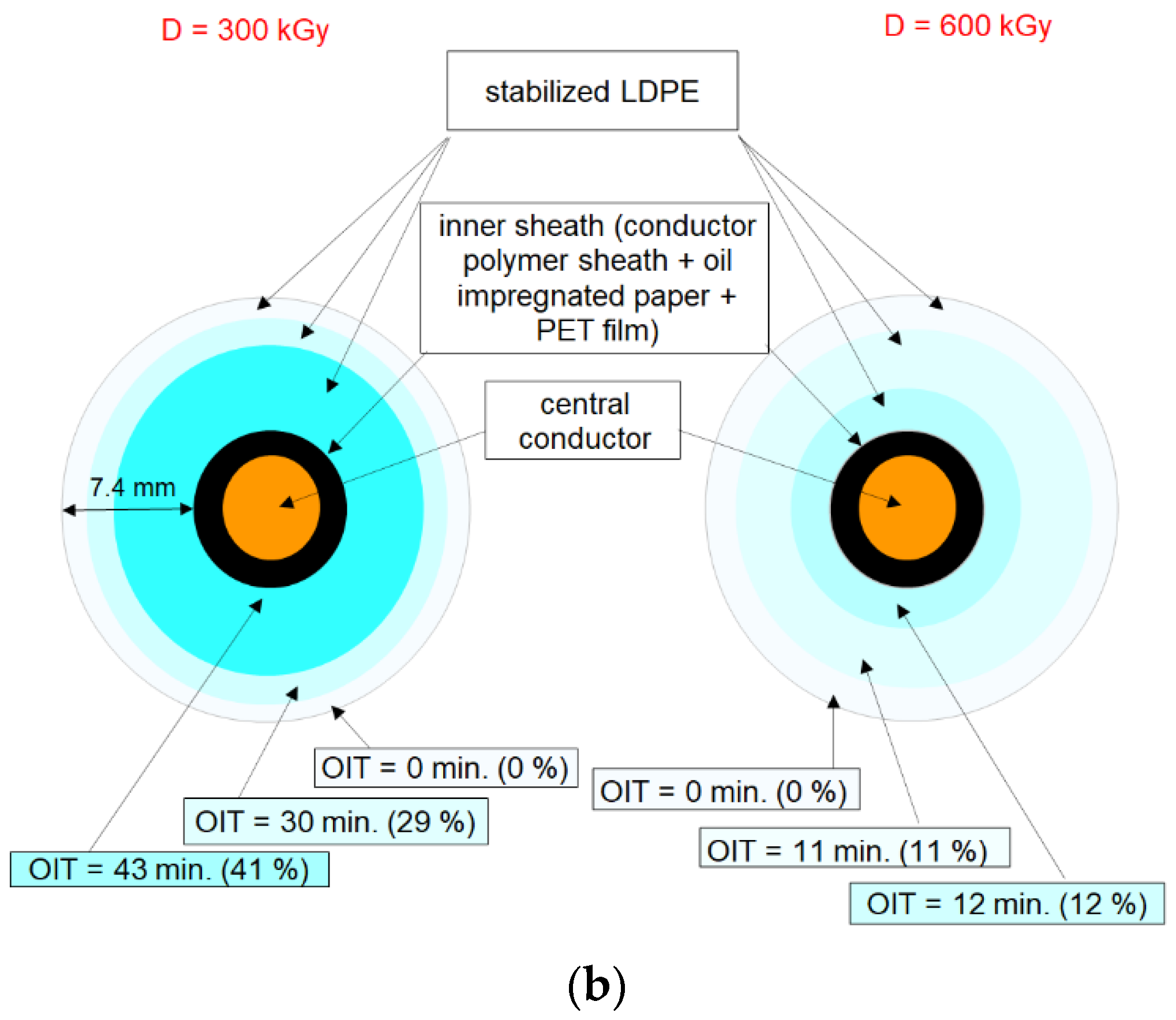
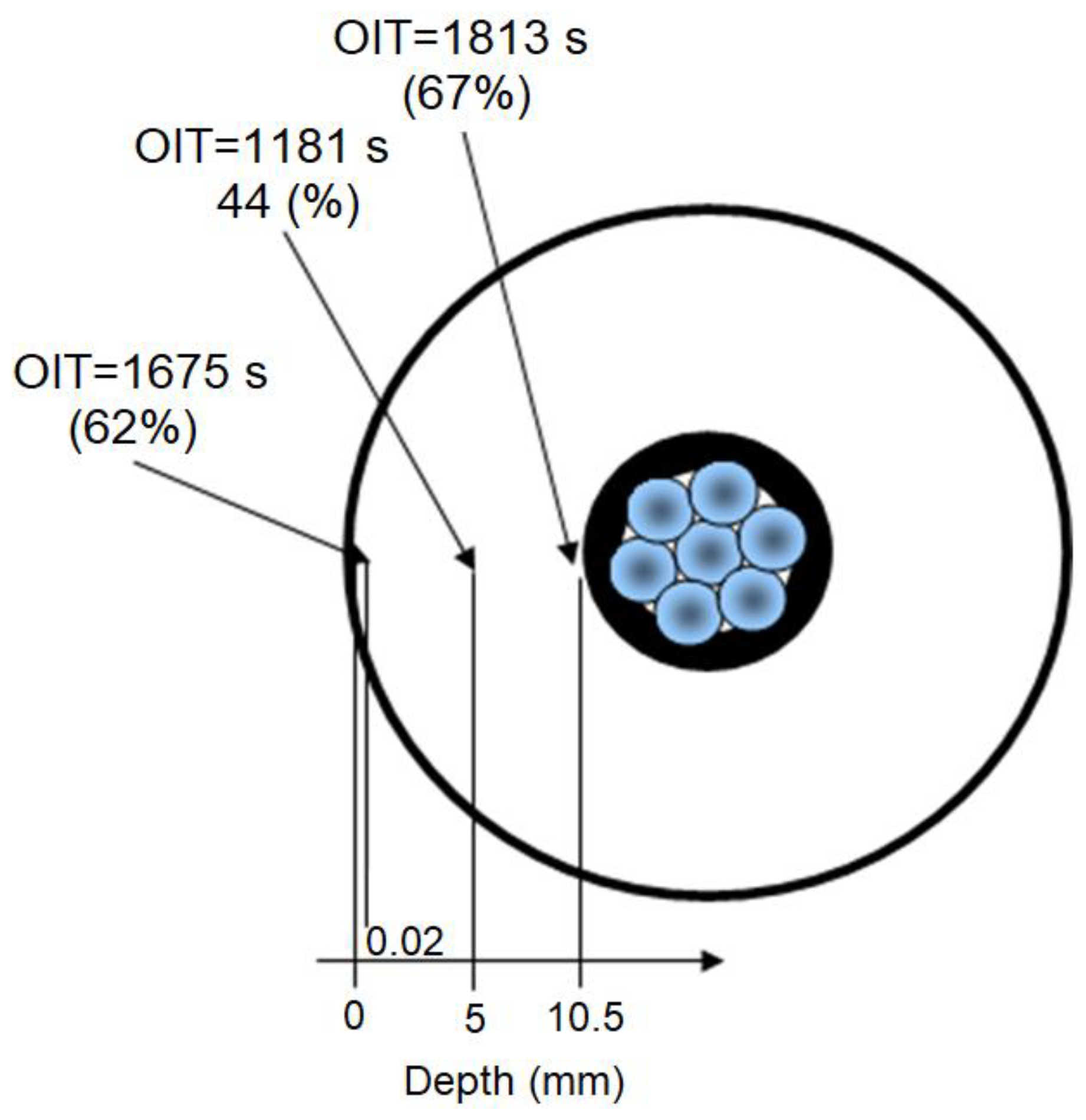

| Irradiation Conditions | Dose (kGy) | Dose Rate (kGy/h) | OIT (min) | k′ (kGy−1) | Dx (kGy) | tx (h) |
|---|---|---|---|---|---|---|
| Strap | 0 | 0.4 | 45 | 4.27 × 10−2 | 131 | 328 |
| 9.6 | 34 | |||||
| 28.8 | 8.7 | |||||
| 96 | 0.8 | |||||
| Bulk-free ends | 0 | 1.5 | 45 | 1.20 × 10−3 | 4629 | 3086 |
| 310 | 23.5 | |||||
| 630 | 25 | |||||
| Bulk-wax-encapsulated | 0 | 1.5 | 45 | 1.07 × 10−3 | 5196 | 3464 |
| 310 | 29 | |||||
| 630 | 26 |
| Irradiation Time (Months) | Dose * (kGy) | OIT (min) | tx (Months)/Dx (kGy) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| XLPE Color | |||||||||
| Black | Brown | Blue | Green-Yellow | Black | Brown | Blue | Green-Yellow | ||
| 0 | 0 | 177 | 131 | 122 | 93 | − | − | − | − |
| 26 | 6 | 3.7 | 3.9 | 3.9 | 2.0 | 45/11 | 47/11 | 47/11 | 45/11 |
| 11 | 35 | 41 | 33 | 3.6 | 107/47 | 140/60 | 120/53 | 48/21 | |
| 22 | 24 | 47 | 68 | 7.5 | 86/75 | 160/140 | 250/220 | 62/55 | |
| 26 | 8.3 | 5.3 | 7.4 | 5.7 | 56/55 | 50/55 | 57/60 | 56/60 | |
| 105 | 0 | 0 | 0 | 0 | <26/<105 | ||||
| 34 | 11 | 3.5 | 3.7 | 2.9 | 2.2 | 53/15 | 59/17 | 57/16 | 55/16 |
| 14 | 29 | 29 | 30 | 11 | 126/50 | 150/62 | 145/59 | 105/41 | |
| 24 | 65 | 39 | 55 | 5.6 | 250/167 | 184/136 | 267/198 | 74/55 | |
| 31 | 4.7 | 4.0 | 3.8 | 3.5 | 65/59 | 62/57 | 64/59 | 64/59 | |
| 144 | 0 | 0 | 0 | 0 | <26/<105 | ||||
| Irradiation Conditions | Dose (kGy) | Dose Rate (kGy/h) | OIT (min) | k′ (kGy−1) | Dx (kGy) | tx |
|---|---|---|---|---|---|---|
| Bulk-free ends | 0 | 0.4 | 372 | 1.36 × 10−2 | 567 | 1418 h |
| 9.6 | 308 | |||||
| 28.8 | 258 | |||||
| 96 | 101 | |||||
| Bulk-free ends | 0 | 1.5 | 342 | 2.36 × 10−3 | 3266 | 8165 h |
| 310 | 143 | |||||
| 630 | 94 | |||||
| Bulk-wax-encapsulated | 0 | 1.5 | 342 | 02.19 × 10−3 | 3520 | 8800 h |
| 310 | 238 | |||||
| 630 | 90 | |||||
| One year in SPS (bulk-free ends) | 0 | N/A | 246 | − | − | ~22 years |
| N/A | 177 |
| Irradiation Time (Months) | Dose * (kGy) | OIT (min) | tx (Months)/Dx (kGy) |
|---|---|---|---|
| 0 | 0 | 130 | − |
| 26 | 6 | 8 | 61/14 |
| 11 | 58 | 206/90 | |
| 22 | 11 | 75/58 | |
| 26 | 25 | 100/100 | |
| 105 | 33 | 121/246 | |
| 34 | 11 | 7 | 69/21 |
| 14 | 46 | 214/90 | |
| 24 | 8 | 76/57 | |
| 31 | 19 | 113/106 | |
| 144 | 1 | 58/246 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungulescu, E.-M.; Setnescu, R.; Ilie, S.; Taborelli, M. On the Use of Oxidation Induction Time as a Kinetic Parameter for Condition Monitoring and Lifetime Evaluation under Ionizing Radiation Environments. Polymers 2022, 14, 2357. https://doi.org/10.3390/polym14122357
Lungulescu E-M, Setnescu R, Ilie S, Taborelli M. On the Use of Oxidation Induction Time as a Kinetic Parameter for Condition Monitoring and Lifetime Evaluation under Ionizing Radiation Environments. Polymers. 2022; 14(12):2357. https://doi.org/10.3390/polym14122357
Chicago/Turabian StyleLungulescu, Eduard-Marius, Radu Setnescu, Sorin Ilie, and Mauro Taborelli. 2022. "On the Use of Oxidation Induction Time as a Kinetic Parameter for Condition Monitoring and Lifetime Evaluation under Ionizing Radiation Environments" Polymers 14, no. 12: 2357. https://doi.org/10.3390/polym14122357
APA StyleLungulescu, E.-M., Setnescu, R., Ilie, S., & Taborelli, M. (2022). On the Use of Oxidation Induction Time as a Kinetic Parameter for Condition Monitoring and Lifetime Evaluation under Ionizing Radiation Environments. Polymers, 14(12), 2357. https://doi.org/10.3390/polym14122357