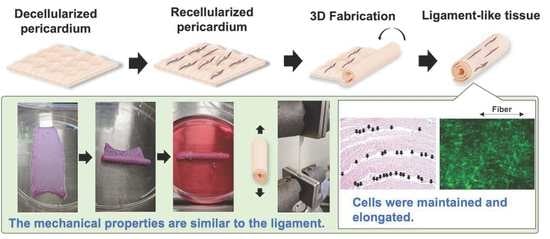
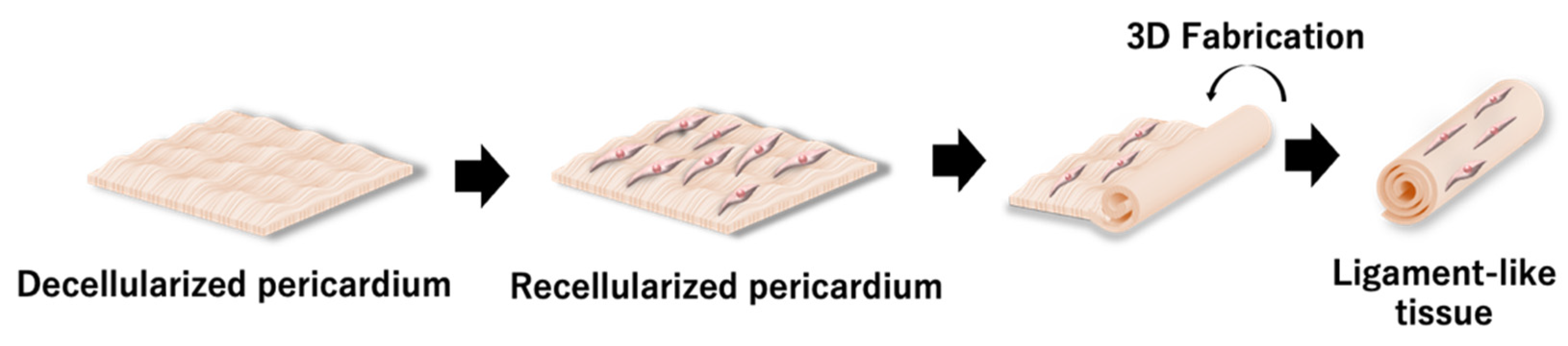
In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Decellularized Porcine Pericardium
2.3. Deoxyribonucleic Acid (DNA) Quantification
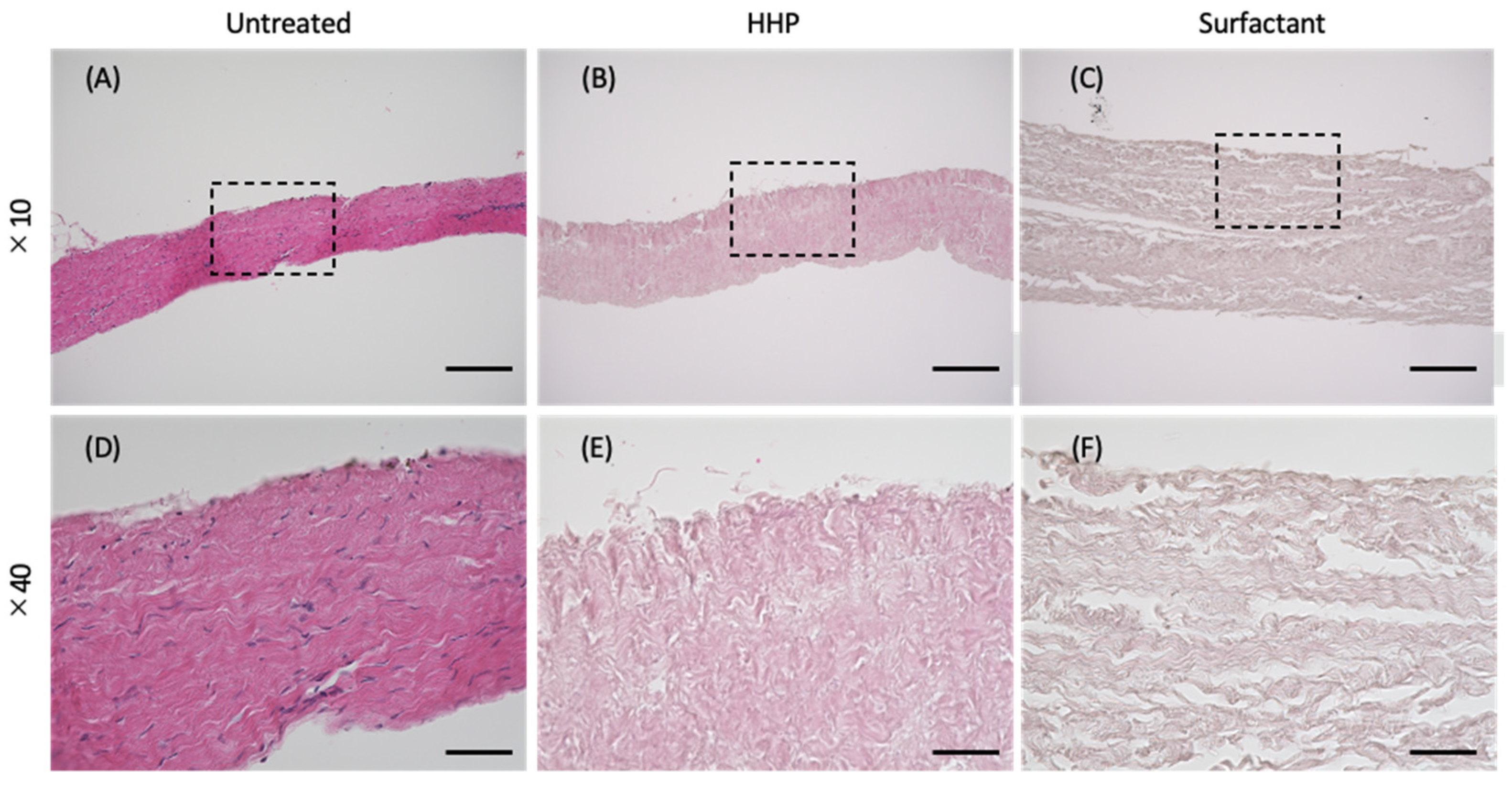
2.4. Hematoxylin–Eosin (H&E) Staining
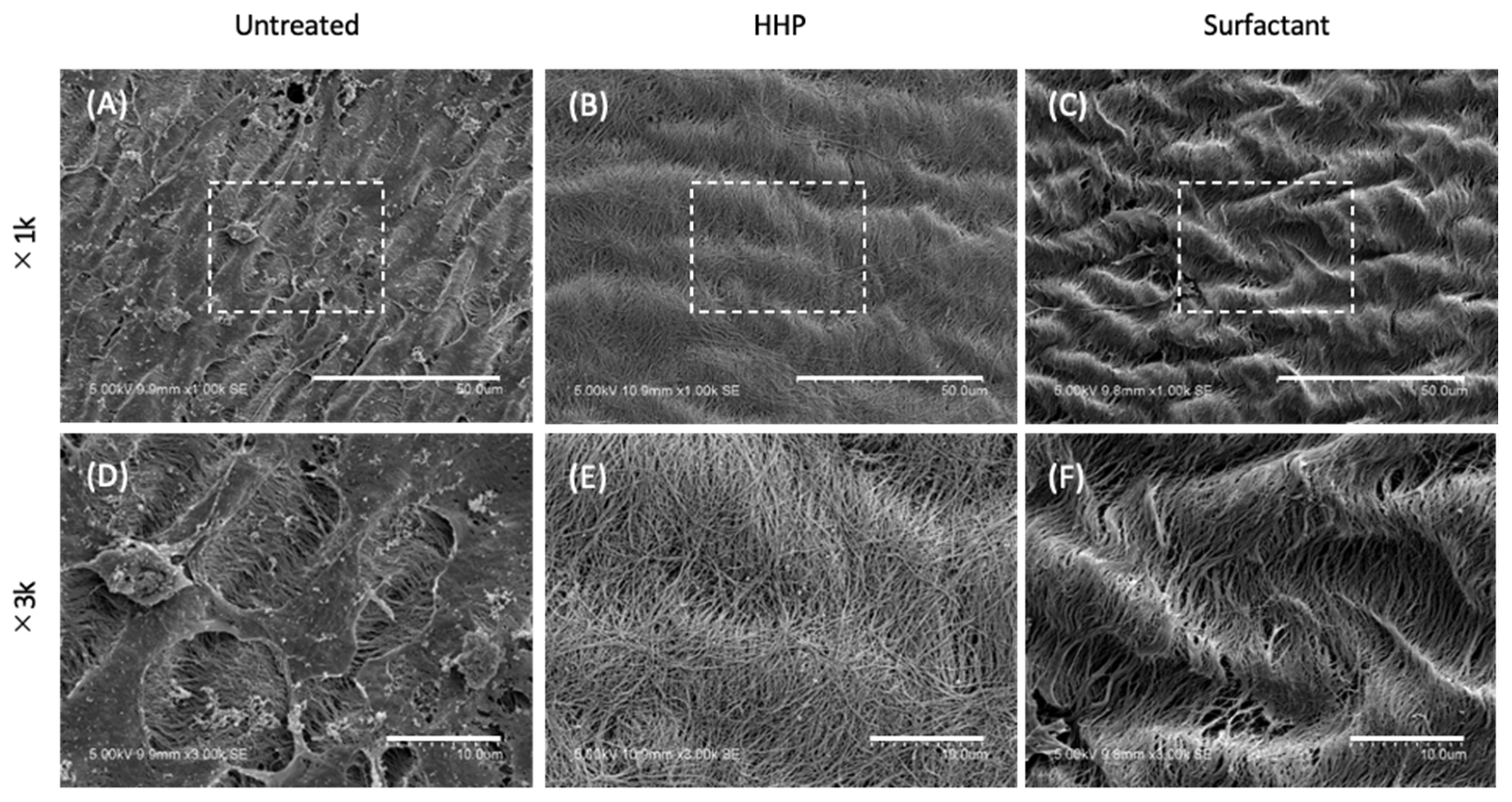
2.5. Scanning Electron Microscopy (SEM) Observation of the Pericardium
2.6. Mechanical Properties
2.7. Cell Culture
2.8. Cell Culture in the Cylindrically Formed Pericardium
2.9. Statistical Analysis
3. Results
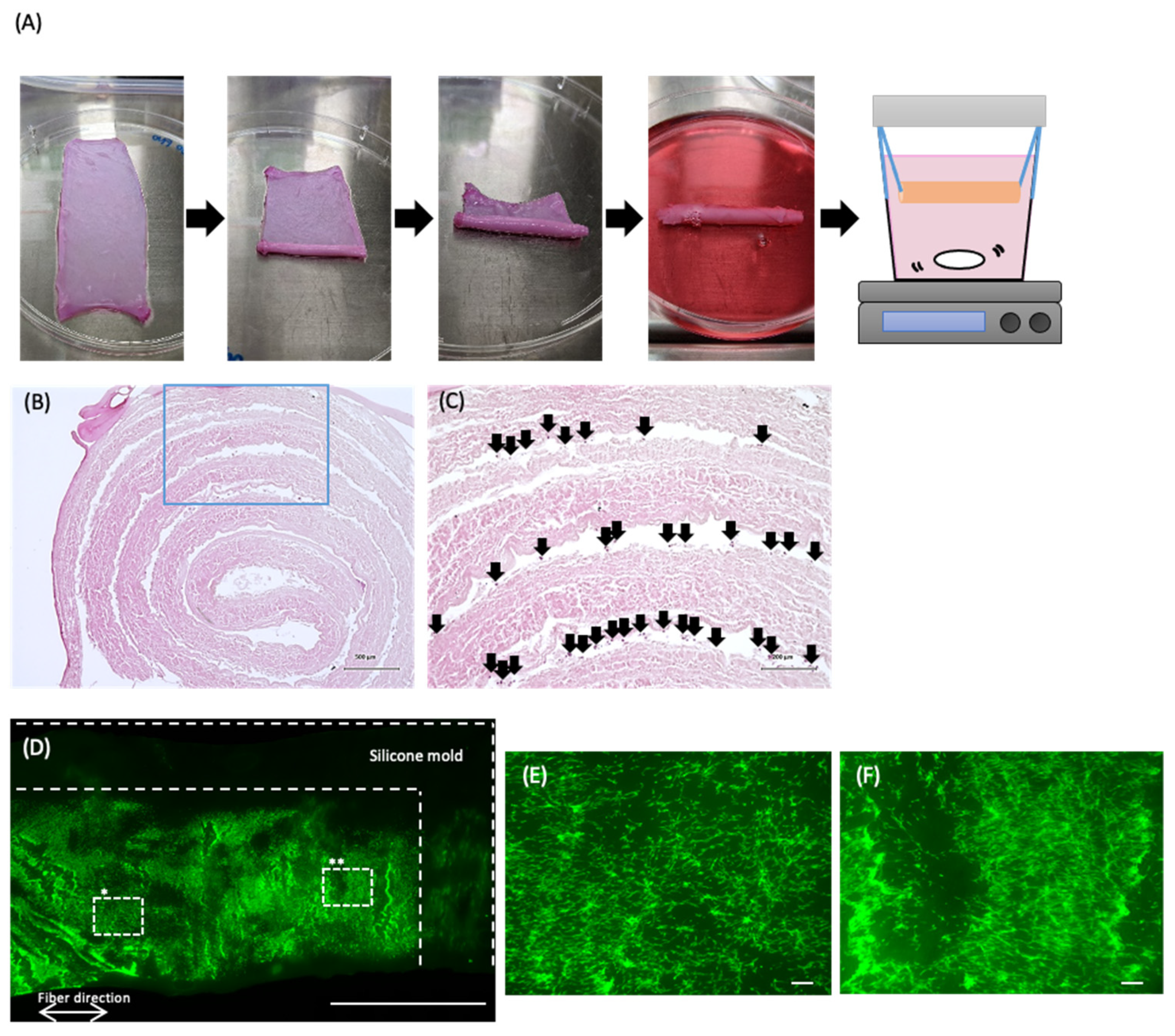
3.1. Preparation of the Decellularized Pericardium
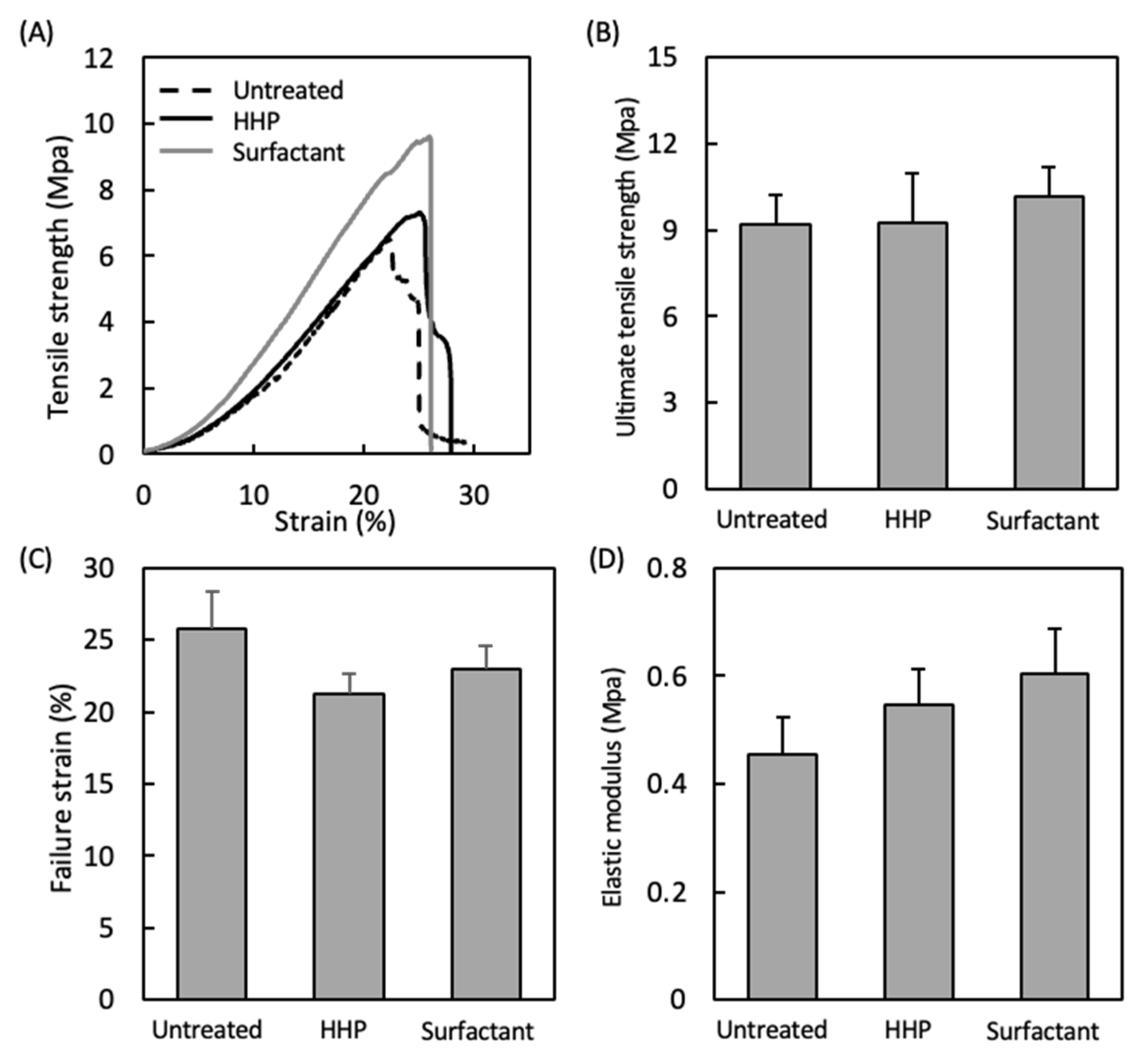
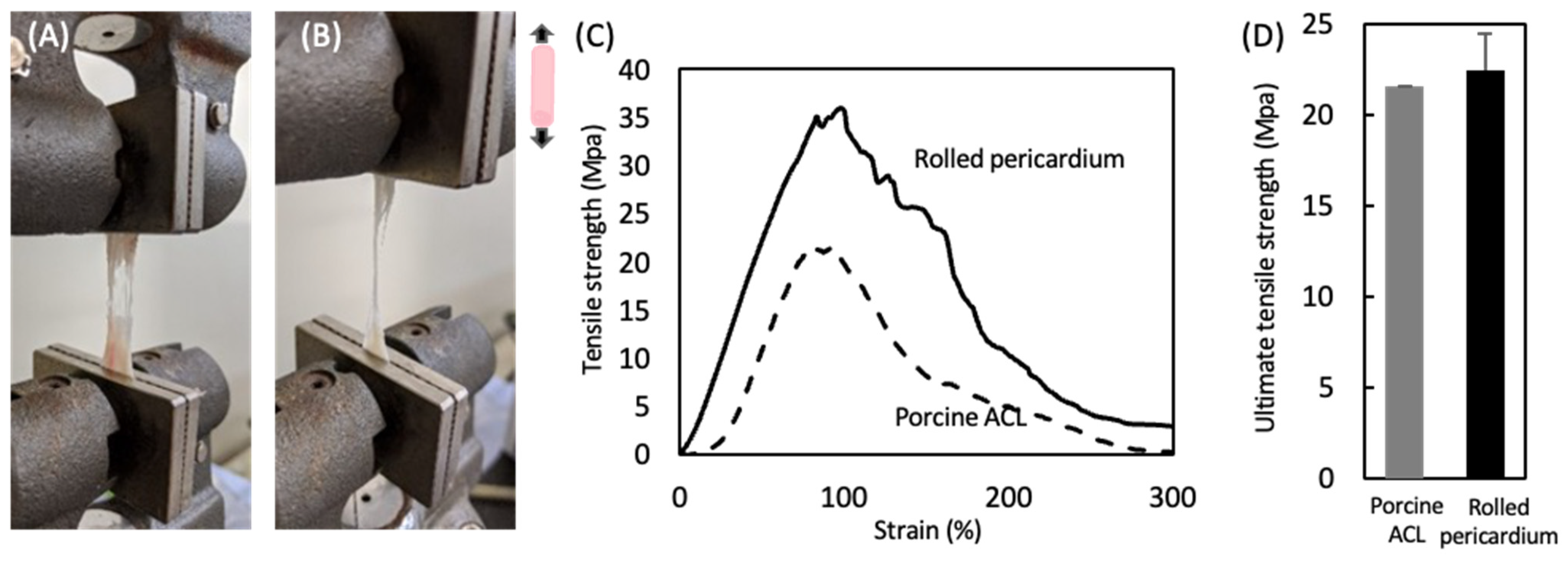
3.2. Mechanical Properties of the Decellularized Pericardium and 3D Reconstruction
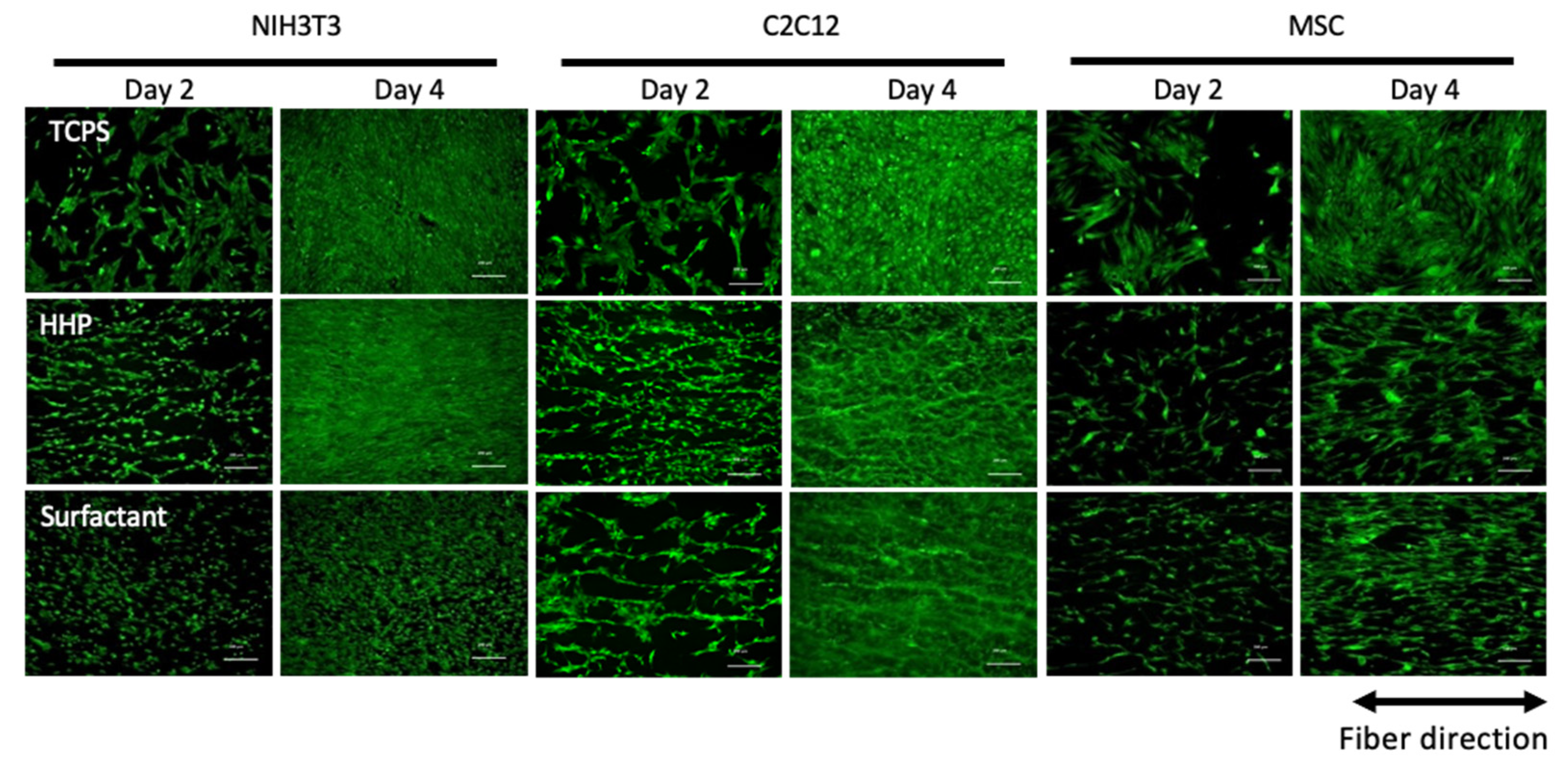
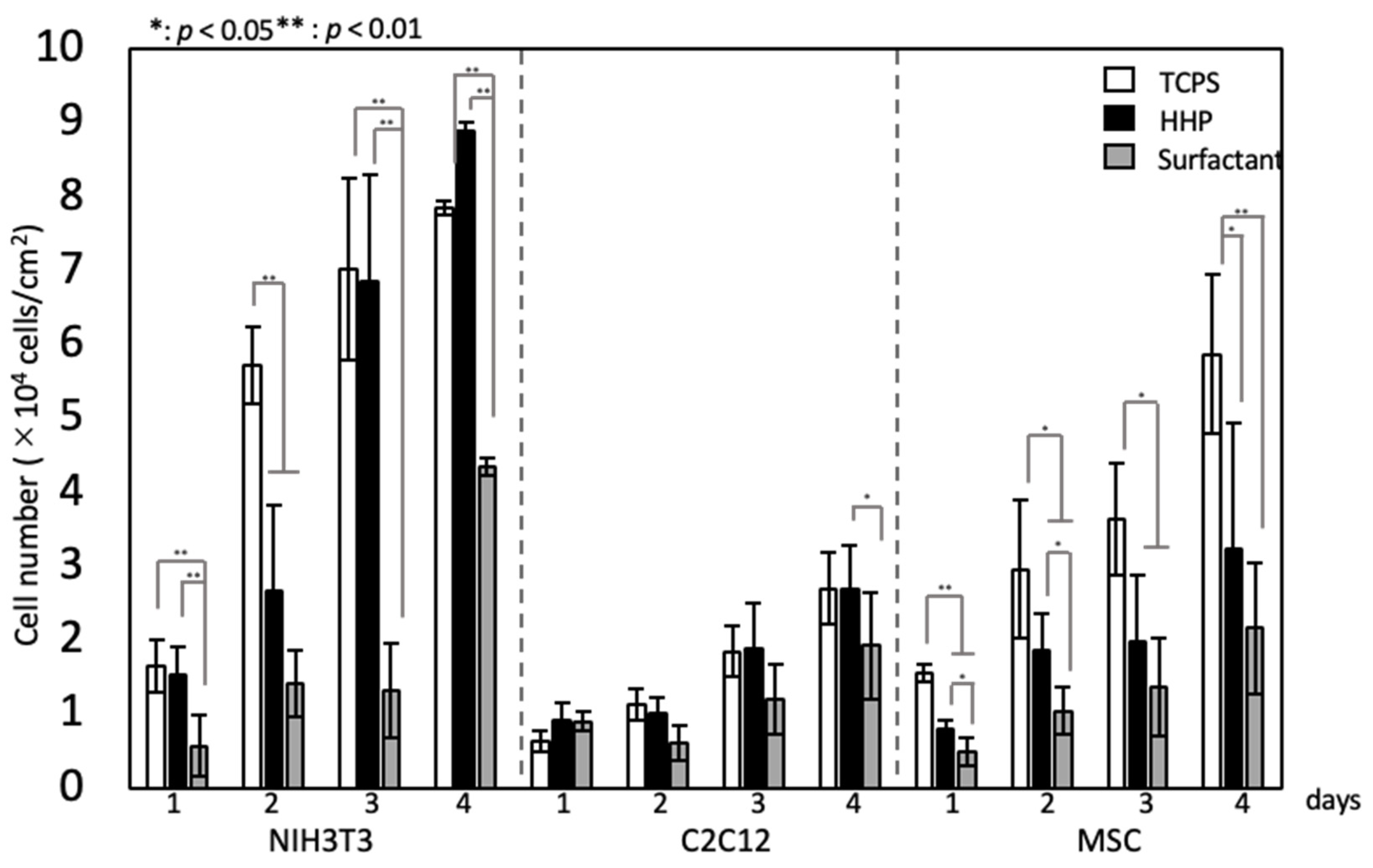
3.3. Recellularization of the Decellularized Pericardium and Hanging Circulation Culture of the 3D-Reconstructed Pericardium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HHP | High hydrostatic pressurization |
| H&E | Hematoxylin–eosin |
| SDS | Sodium dodecyl sulfate |
| SDC | Sodium deoxycholate |
| FBS | Fatal bovine serum |
| ACL | Anterior cruciate ligament |
| TCPS | Tissue culture polystylene |
References
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Hideyoshi, S.; Jiang, H.B.; Matsumura, Y.; Dziki, J.L.; LoPresti, S.T.; Huleihel, L.; Faria, G.N.F.; Fuhrman, L.C.; Lodono, R.; et al. Injectable, Porous, Biohybrid Hydrogels Incorporating Decellularized Tissue Components for Soft Tissue Applications. Acta Biomater. 2018, 73, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Andrée, B.; Bär, A.; Haverich, A.; Hilfiker, A. Small Intestinal Submucosa Segments as Matrix for Tissue Engineering: [Review]. Tissue Eng. Part B Rev. 2013, 19, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Quinta, C.; Kondob, Y.; Mansonc, R.L.; Lawsonc, J.H.; Dardikb, A.; Niklason, L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 2011, 108, 9214–9219. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef] [Green Version]
- Woods, T.; Gratzer, P.F. Effectiveness of Three Extraction Techniques in the Development of a Decellularized Bone-Anterior Cruciate Ligament-Bone Graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef]
- Jones, G.; Herbert, A.; Berry, H.; Edwards, J.H.; Fisher, J.; Ingham, E. Decellularization and Characterization of Porcine Superflexor Tendon: A Potential Anterior Cruciate Ligament Replacement. Tissue Eng. Part A 2017, 23, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Schulze-Tanzil, G.; Al-Sadi, O.; Ertel, W.; Lohan, A. Decellularized Tendon Extracellular Matrix-A Valuable Approach for Tendon Reconstruction? Cells. 2012, 1, 1010–1028. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, T.; Kishida, A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater. Sci. Eng. 2017, 3, 1236–1244. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and Characterization of Decellularized Cornea Using High-Hydrostatic Pressurization for Corneal Tissue Engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef]
- Iablonskii, P.; Cebotari, S.; Tudorache, I.; Granados, M.; Morticelli, L.; Goecke, T.; Klein, N.; Korossis, S.; Hilfiker, A.; Haverich, A. Tissue-Engineered Mitral Valve: Morphology and Biomechanics. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 712–719; discussion 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.C.; Zhang, T.; Amadio, P.C.; An, K.N.; Moran, S.L.; Gingery, A.; Zhao, C. Lateral Slit Delivery of Bone Marrow Stromal Cells Enhances Regeneration in the Decellularized Allograft Flexor Tendon. J. Orthop. Transl. 2019, 19, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Tozer, S.; Duprez, D. Tendon and Ligament: Development, Repair and Disease. Birth Defects Res. C Embryo Today 2005, 75, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; Altamimi, A.; García Sánchez, M.G.; Tamburrinia, R.; Asthana, A.; Gazia, C.; Orlando, G. Utility of Extracellular Matrix Powders in Tissue Engineering. Organogenesis 2018, 14, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.J.; Jiang, Y.L.; Zhang, C.H.; Zhang, Y.; Yang, J.L.; Cui, J.; Zhang, Y.J.; Yao, X.; Luo, J.C.; Qin, T.W. Fabrication and Characterization of a Decellularized Bovine Tendon Sheet for Tendon Reconstruction. J. Biomed. Mater. Res. A 2017, 105, 2299–2311. [Google Scholar] [CrossRef]
- Spang, M.T.; Christman, K.L. Extracellular Matrix Hydrogel Therapies: In Vivo Applications and Development. Acta Biomater. 2018, 68, 1–14. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular Matrix as a Biological Scaffold Material: Structure and Function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Negishi, J.; Hashimoto, Y.; Yamashita, A.; Zhang, Y.; Kimura, T.; Kishida, A.; Funamoto, S. Evaluation of Small-Diameter Vascular Grafts Reconstructed from Decellularized Aorta Sheets. J. Biomed. Mater. Res. A 2017, 105, 1293–1298. [Google Scholar] [CrossRef]
- Wu, P.; Nakamura, N.; Morita, H.; Nam, K.; Fujisato, T.; Kimura, T.; Kishida, A. A Hybrid Small-Diameter Tube Fabricated from Decellularized Aortic Intima-Media and Electrospun Fiber for Artificial Small-Diameter Blood Vessel. J. Biomed. Mater. Res. A 2019, 107, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, A.; Tudorache, I.; Cebotari, S.; Ringes-Lichtenberg, S.; Sturz, G.; Hoeffler, K.; Hurscheler, C.; Brandes, G.; Hilfiker, A.; Haverich, A. In Vitro Re-Endothelialization of Detergent Decellularized Heart Valves under Simulated Physiological Dynamic Conditions. Biomaterials 2006, 27, 4221–4229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized Matrices in Regenerative Medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nakamura, N.; Kimura, T.; Nam, K.; Fujisato, T.; Funamoto, S.; Higami, T.; Kishida, A. Decellularized Porcine Aortic Intima-Media as a Potential Cardiovascular Biomaterial. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Laker, L.; Dohmen, P.M.; Smit, F.E. Synergy in a Detergent Combination Results in Superior Decellularized Bovine Pericardial Extracellular Matrix Scaffolds. J. Biomed. Mater. Res. A 2020, 108, 2571–2578. [Google Scholar] [CrossRef]
- Butler, D.L.; Guan, Y.; Kay, M.D.; Cummings, J.F.; Feder, S.M.; Levy, M.S. Location-Dependent Variations in the Material Properties of the Anterior Cruciate Ligament. J. Biomech. 1992, 25, 511–518. [Google Scholar] [CrossRef]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Directed Endothelial Cell Morphogenesis in Micropatterned Gelatin Methacrylate Hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of Aligned and Random Fibers with Different Diameter on Cell Behaviors. Colloids Surf. B Biointerfaces 2018, 171, 461–467. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Shi, J.; Wei, J.; Wang, L.; Tu, X.; Tang, Y.; Chen, Y. Fabrication of elastomer pillar arrays with height gradient for cell culture studies. Microelectron. Eng. 2017, 175, 50–55. [Google Scholar] [CrossRef]
- Kimura, T.; Kondo, M.; Hashimoto, Y.; Fujisato, T.; Nakamura, N.; Kishida, A. Surface Topography of PDMS Replica Transferred from Various Decellularized Aortic Lumens Affects Cellular Orientation. ACS Biomater. Sci. Eng. 2019, 5, 5721–5726. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of Tissues and Organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bao, M.; Bruekers, S.M.C.; Huck, W.T.S. Collagen Gels with Different Fibrillar Microarchitectures Elicit Different Cellular Responses. ACS Appl. Mater. Interfaces 2017, 9, 19630–19637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Kimura, T.; Yoshida, Y.; Kobayashi, M.; Hashimoto, Y.; Takahashi, H.; Shimizu, T.; Anzai, S.; Nakamura, N.; Kishida, A. In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration. Polymers 2022, 14, 2351. https://doi.org/10.3390/polym14122351
Suzuki M, Kimura T, Yoshida Y, Kobayashi M, Hashimoto Y, Takahashi H, Shimizu T, Anzai S, Nakamura N, Kishida A. In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration. Polymers. 2022; 14(12):2351. https://doi.org/10.3390/polym14122351
Chicago/Turabian StyleSuzuki, Mika, Tsuyoshi Kimura, Yukina Yoshida, Mako Kobayashi, Yoshihide Hashimoto, Hironobu Takahashi, Tatsuya Shimizu, Shota Anzai, Naoko Nakamura, and Akio Kishida. 2022. "In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration" Polymers 14, no. 12: 2351. https://doi.org/10.3390/polym14122351
APA StyleSuzuki, M., Kimura, T., Yoshida, Y., Kobayashi, M., Hashimoto, Y., Takahashi, H., Shimizu, T., Anzai, S., Nakamura, N., & Kishida, A. (2022). In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration. Polymers, 14(12), 2351. https://doi.org/10.3390/polym14122351