Characterization and Optimization of Real-Time Photoresponsive Gelatin for Direct Laser Writing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Mechanical Properties
2.4. Optical Properties
2.5. Physicochemical Properties
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, V.; Bangar, S.P.; Thakur, N.; Trif, M. Recent advancements in smart biogenic packaging: Reshaping the future of the food packaging industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzer, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Shamsuddin, I.M.; Jafar, J.A.; Shawai, A.S.A.; Yusuf, S.; Lateefah, M.; Aminu, I. Bioplastics as better alternative to petroplastics and their role in national sustainability: A review. Adv. Biosci. Bioeng. 2017, 5, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Le Guen, M.J.; Tucker, N.; Parker, K. Thermo-mechanical, morphological and water absorption properties of thermoplastic starch/cellulose composite foams reinforced with PLA. Cellulose 2019, 26, 4463–4478. [Google Scholar] [CrossRef]
- Souza, C.R.; Andrade, C.T. Processing and properties of thermoplastic starch and its blends with sodium alginate. J. Appl. Polym. Sci. 2001, 81, 412–420. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef]
- Rivadeneira-Velasco, K.E.; Utreras-Silva, C.A.; Díaz-Barrios, A.; Sommer-Márquez, A.E.; Tafur, J.P.; Michell, R.M. Green nanocomposites based on thermoplastic starch: A review. Polymers 2021, 13, 3227. [Google Scholar] [CrossRef]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current research and applications of starch based biodegradable films for food packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Estrada-Monje, A.; Alonso-Romero, S.; Zitzumbo-Guzmán, R.; Estrada-Moreno, I.A.; Zaragoza-Contreras, E.A. Thermoplastic starch-based blends with improved thermal and thermomechanical properties. Polymers 2021, 13, 4263. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Bansal, M.; Khan, G.; Yadav, S.K.; Singh, A.K.; Prakash, P.; Mishra, B. Development, optimization and evaluation of curcumin loaded biodegradable crosslinked gelatin film for the effective treatment of periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, X.; Kang, Q.; Zhu, L.; Qin, G.; Chen, Q. Freezing-tolerant and robust gelatin-based supramolecular conductive hydrogels with double-network structure for wearable sensors. Polym. Test. 2021, 93, 106879. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Hu, D.; Chao, X.; Zhou, Y.; Wang, J. An essential role of gelatin in the formation process of curling in long historical photos. Polymers 2021, 13, 3894. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of gelatin in food packaging: A review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Maiti, S.; Khillar, P.S.; Mishra, D.; Nambiraj, N.A.; Jaiswal, A.K. Physical and self–crosslinking mechanism and characterization of chitosan-gelatin-oxidized guar gum hydrogel. Polym. Test. 2021, 97, 107155. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Savić, S.; Pantelić, D.; Jakovijević, D. Real-time and postprocessing holographic effects in dichromated pullulan. Appl. Opt. 2002, 41, 4484–4488. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.A.C.M.; Ching. Y., C.; Abdullah, L.C.; Poh, S.C.; Chuah, C.H. Review of Bionanocomposite Coating Films andTheir Applications. Polymers 2016, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef] [PubMed]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Kafil, H.S.; Salehi, R. In situ synthesized chitosan−gelatin/ZnO nanocomposite scaffold with drug delivery properties: Higher antibacterial and lower cytotoxicity effects. J. Appl. Polym. Sci. 2019, 136, 47590. [Google Scholar] [CrossRef]
- Afnas, V.M.; Unnikrishnan, G.; Budhe, S.; Manaf, O.; Ameen, J. PVA/gelatin/chitin ternary blend as a humidity sensing material. J. Mater. Sci. Mater. Electron. 2022, 33, 2031–2043. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Scudder, L.L.; Halvorson, R.T.; Tresback, J.; Ferrier, J.P.; Sheehy, S.P.; Cho, A.; Kannan, S.; Sunyovszki, I.; Goss, J.A.; et al. Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications. Biofabrication 2018, 10, 25004. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current understanding of the Applications of photocrosslinked hydrogels in biomedical engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef]
- Pinto-Iguanero, B.; Olivares-Perez, A.; Mendez-Alvarado, A.W.; Fuentes-Tapia, I.; Trevino-Palacios, C.G. Non-hydroscopic vanilla doped dichromated gelatin holographic material. Opt. Mater. 2003, 22, 397–404. [Google Scholar] [CrossRef]
- Pantelić, D.; Murić, B. Improving the holographic sensitivity of dichromated gelatin in the blue–green part of the spectrum by sensitization with xanthene dyes. Appl. Opt. 2001, 40, 2871–2875. [Google Scholar] [CrossRef]
- Yao, J.; Cui, Z.; Gao, F.; Zhang, Y.; Guo, Y.; Du, C.; Zeng, H.; Qiu, C. Refractive micro lens array made of dichromate gelatin with gray-tone photolithography. Microelect. Eng. 2001, 57–58, 729–735. [Google Scholar] [CrossRef]
- Calixto, S.; Scholl, M.S. Relief optical microelements fabricated with dichromated gelatin. Appl. Opt. 1997, 36, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Gao, B.; Chen, T.; Si, J.; Li, C.; Chen, F.; Hou, X. Fabrication of concave spherical microlenses on silicon by femtosecond laser irradiation and mixed acid etching. Opt. Express 2014, 22, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Marcinkevičius, A.; Juodkazis, S.; Watanabe, M.; Miwa, M.; Matsuo, S.; Misawa, H.; Nishii, J. Femtosecond laser-assisted three-dimensional microfabrication in silica. Opt. Lett. 2001, 26, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a photosensitive material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jonesd, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Wickramaarachchi, K.; Minakshi Sundaram, M.; Henry, D.J.; Gao, X. Alginate Biopolymer Effect on the Electrodeposition of Manganese Dioxide on Electrodes for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Murić, B.D.; Pantelić, D.V.; Vasiljević, D.M.; Panić, B.M. Properties of microlenses produced on a layer of tot’hema and eosin sensitized gelatin. Appl. Opt. 2007, 46, 8527–8532. [Google Scholar] [CrossRef]
- Murić, B.; Pantelić, D.; Vasiljević, D.; Panić, B. Microlens fabrication on tot’hema sensitized gelatin. Opt. Mater. 2008, 30, 1217–1220. [Google Scholar] [CrossRef]
- Murić, B.D.; Pantelić, D.V.; Vasiljević, D.M.; Savić-Šević, S.N.; Jelenković, B.M. Application of tot’hema eosin sensitized gelatin as a potential eye protection filter against direct laser radiation. Curr. Appl. Phys. 2016, 16, 57–62. [Google Scholar] [CrossRef]
- Murić, B.; Pantelić, D.; Vasiljević, D.; Zarkov, B.; Jelenković, B.; Pantović, S.; Rosić, M. Sensitized gelatin as a versatile biomaterial with tunable mechanical and optical properties. Phys. Scr. 2013, T157, 14018. [Google Scholar] [CrossRef]
- Krmpot, A.J.; Tserevelakis, G.J.; Murić, B.D.; Filippidis, G.; Pantelić, D.V. 3D imaging and characterization of microlenses and microlens arrays using nonlinear microscopy. J. Phys. D Appl. Phys. 2013, 46, 195101. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Qi, Y.-N.; Yin, X.-J.; Yang, X.; Chen, C.-M.; Yu, J.-Y.; Yu, J.-C.; Lin, Y.-M.; Hui, F.; Liu, P.-L.; et al. Polymer-Based Device Fabrication and Applications Using Direct Laser Writing Technology. Polymers 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: www.vidal.fr/medicaments/tot-hema-sol-buv-en-ampoule-16626.html (accessed on 10 February 2022).
- Keinonen, T.; Riihola, P.; Huttu, K.; Parkkonen, S. Dye films for optical demonstrations in the undergraduate laboratory. Opt. Mater. 1998, 11, 79–86. [Google Scholar] [CrossRef]
- ISO 527–3:2018; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/70307.html (accessed on 12 March 2022).
- Radmilović, M.D.; Murić, B.D.; Grujić, D.; Zarkov, B.; Nenadić, M.Z.; Pantelić, D.V. Rapid direct laser writing of microoptical components on a meltable biocompatible gel. Opt. Quantum Electron. 2022, 54, 361. [Google Scholar] [CrossRef]
- Tabatabaei, S.D.; Ghiasi, F.; Gahruie, H.H.; Hosseini, S.M.H. Effect of emulsified oil droplets and glycerol content on the physicochemical properties of Persian gum-based edible films. Polym. Test. 2022, 106, 107427. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Meeren, P.V.; Osseini, S.M.H. Study on hydrophobic modification of basil seed gum-based (BSG) films by octenyl succinate anhydride. Carbohydr.Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; García, M.A.; Pinotti, A. Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Fernández-Martín, F.; Montero, M.P. Effect of different protein extracts from Dosidicus gigas muscle co-products on edible films development. Food Hydrocoll. 2013, 33, 118–131. [Google Scholar] [CrossRef]
- Siepmann, J.; Streubel, A.; Peppas, N.A. Understanding and predicting drug delivery from hydrophilic matrix tablets using the “Sequential layer” model. Pharm. Res. 2002, 19, 306–314. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Rachel, K.; Melo, T.; Sabry, D.A.; Sassaki, G.L.; Alexandre, H.; Rocha, O. Does the use of chitosan contribute to oxalate kidney stone formation. Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Farahnaky, A.; Dadfar, S.M.M.; Shahbazi, M. Physical and mechanical properties of gelatin–clay nanocomposite. J. Food Eng. 2014, 122, 78–83. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, A practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–23. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, S.-L. Advances in simultaneous DSC–FTIR microspectroscopy for rapid solid-state chemicalstability studies: Some dipeptide drugs as examples. Adv. Drug Deliv. Rev. 2012, 64, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Zhong, F. Tailoring physical properties of transglutaminase-modified gelatin films by varying drying temperature. Food Hydrocoll. 2016, 58, 20–28. [Google Scholar] [CrossRef]
- Dai, C.A.; Chen, Y.-F.; Liu, M.-W. Thermal properties measurements of renaturated gelatin using conventional and temperature modulated differential scanning calorimetry. Appl. Polym. Sci. 2006, 99, 1795–1801. [Google Scholar] [CrossRef]
- Mendieta-Taboada, O.; Sobral, P.J.A.; Carvalho, R.A.; Habitante, A.M.B.Q. Thermomechanical properties of biodegradable films based on blends of gelatin and poly(vinyl alcohol). Food Hydrocoll. 2008, 22, 1485–1492. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Murić, B.; Pantelić, D.; Vasiljević, D.; Panić, B.; Jelenković, B. Thermal analysis of microlens formation on a sensitized gelatin layer. Appl. Opt. 2009, 48, 3854–3859. [Google Scholar] [CrossRef]
- Hong, C.-C.; Choi, J.-W.; Ahn, C.H. A novel in-plane microfluidic mixer with modified Tesla structures. Lab Chip 2004, 4, 109–113. [Google Scholar] [CrossRef]
- Tesla, N. Valvular Conduit. U.S. Patent 1329559, 3 February 1920. Available online: https://www.freepatentsonline.com/1329559.html (accessed on 12 March 2022).
- Murić, B.D.; Panić, B.M. Microlenses with focal length controlled by chemical processes. Phys. Scr. 2012, T 149, 14071. [Google Scholar] [CrossRef]
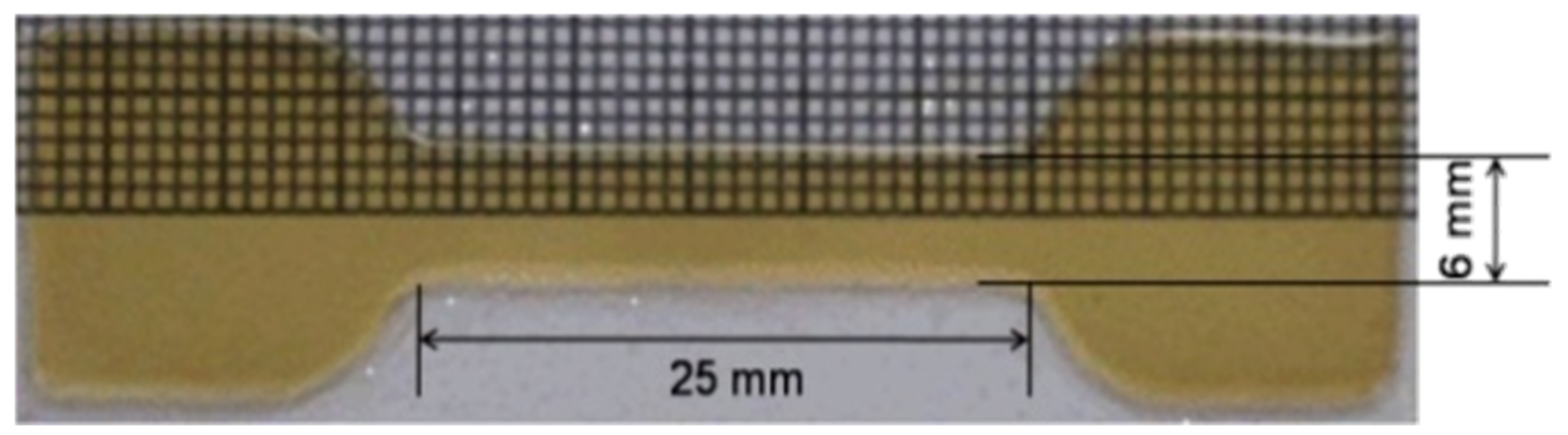
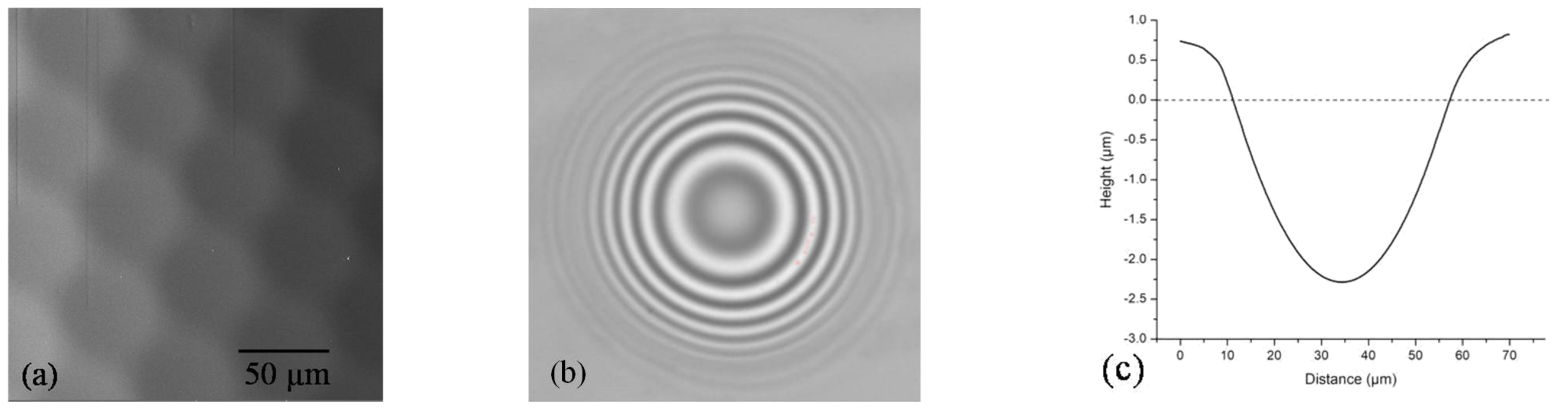

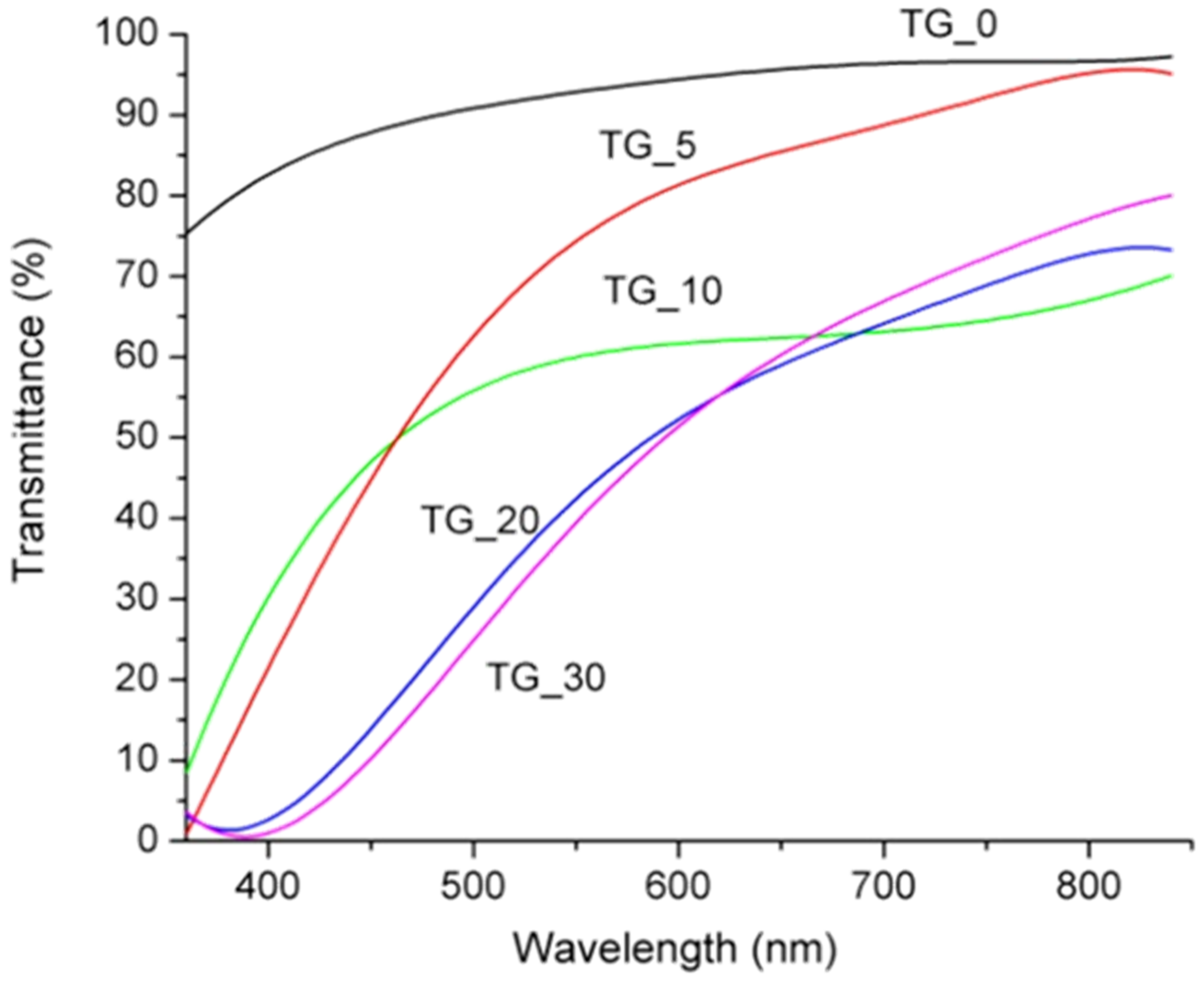
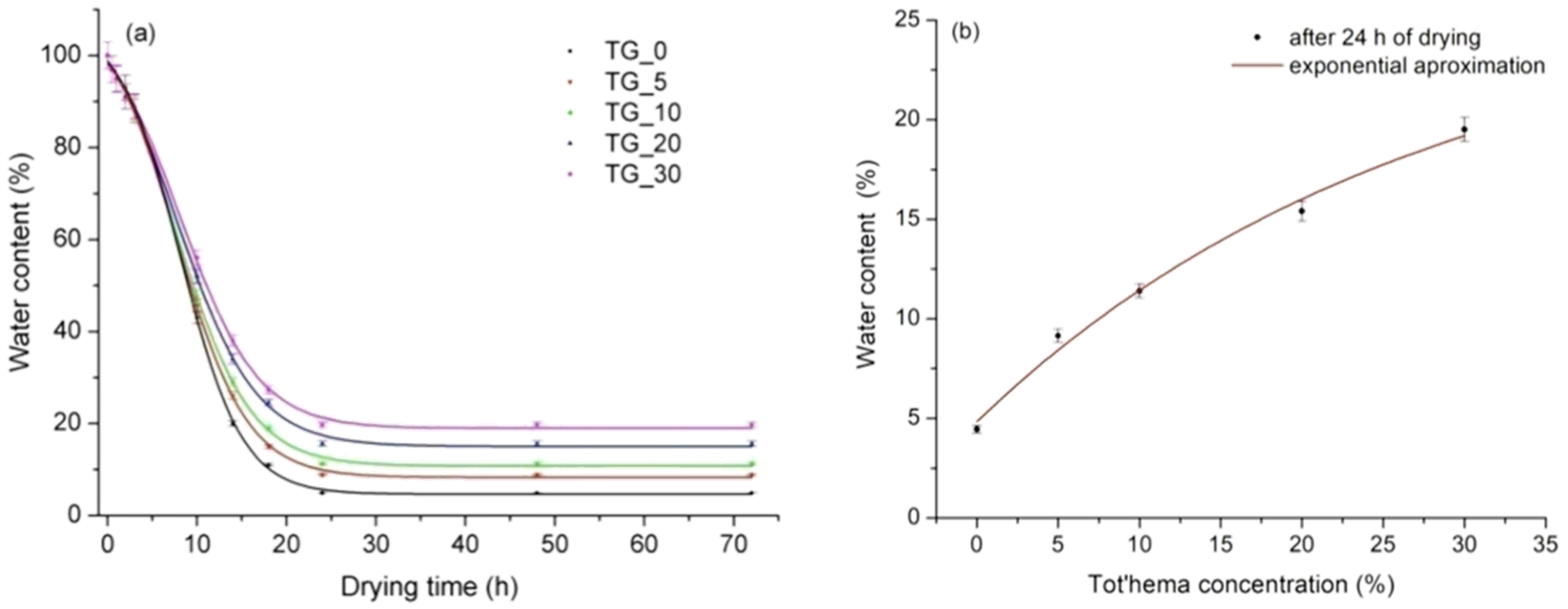
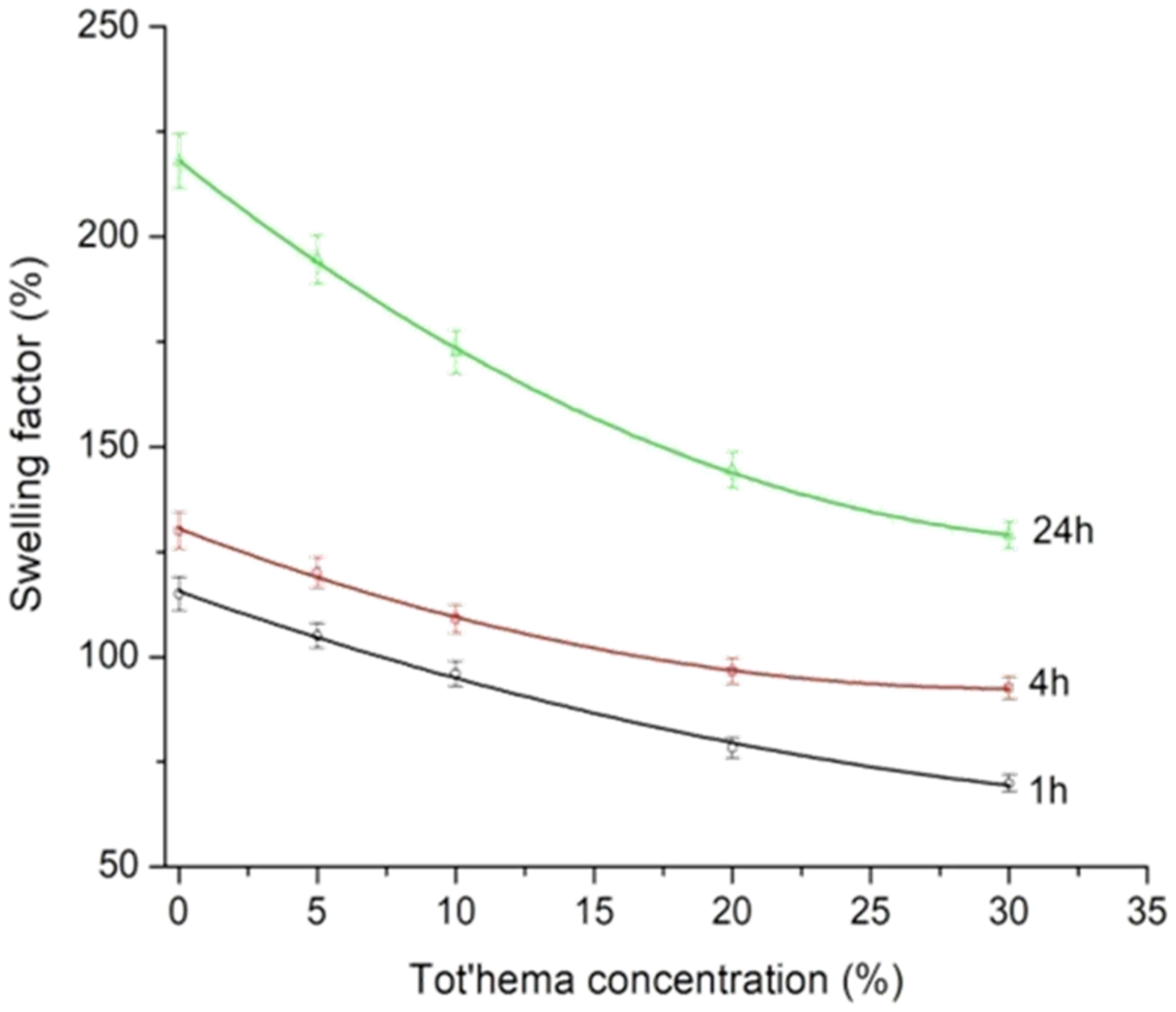
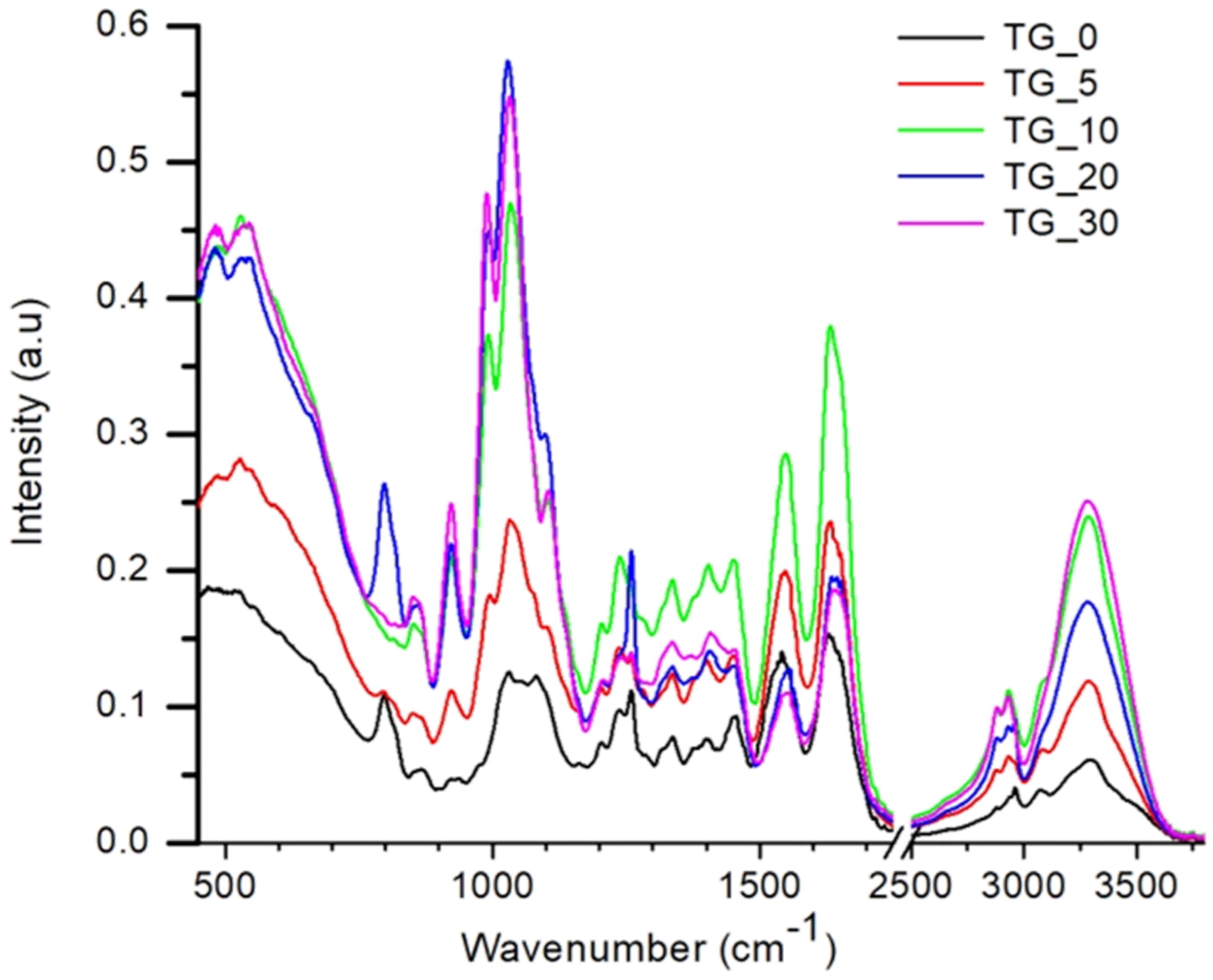
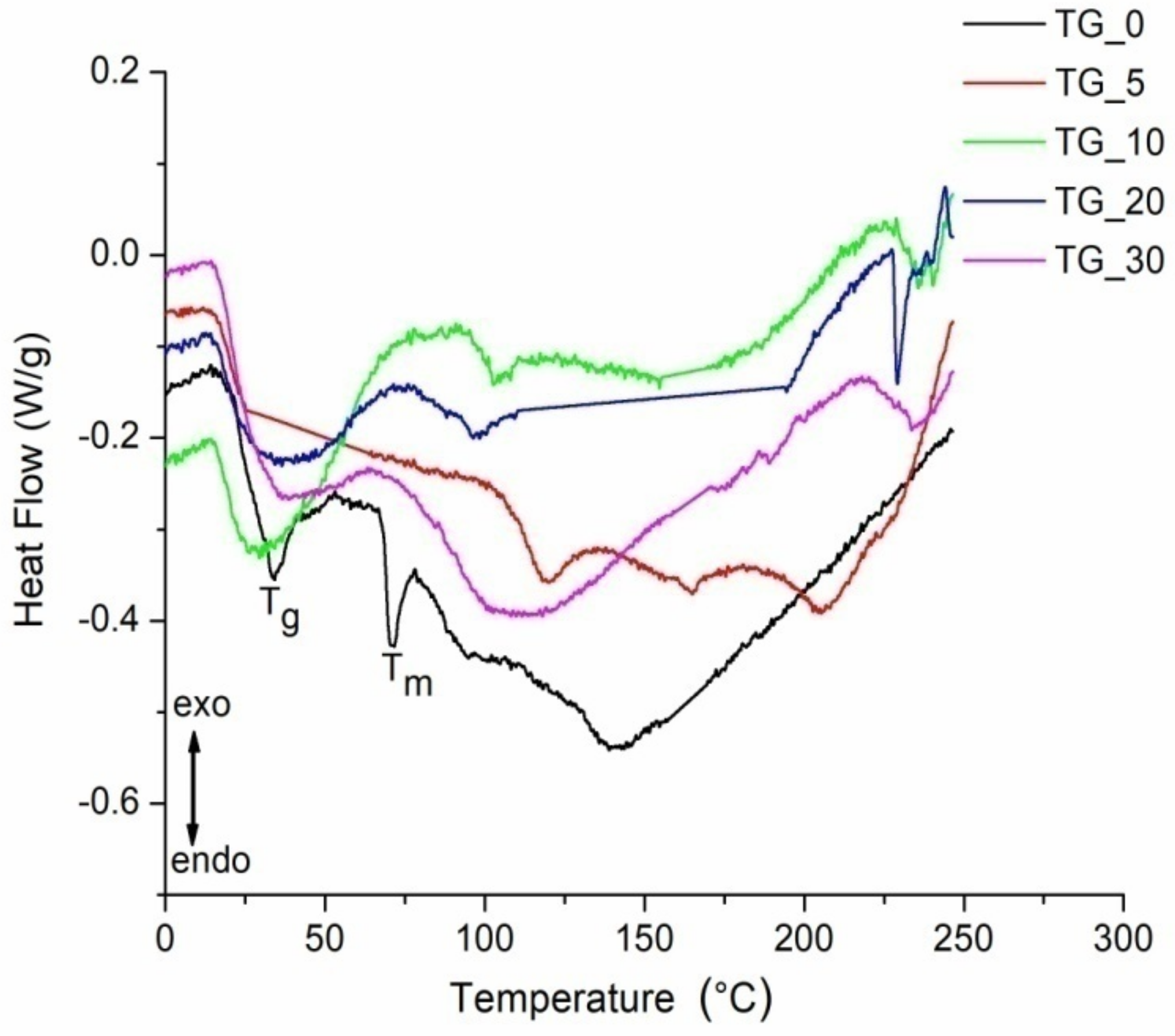
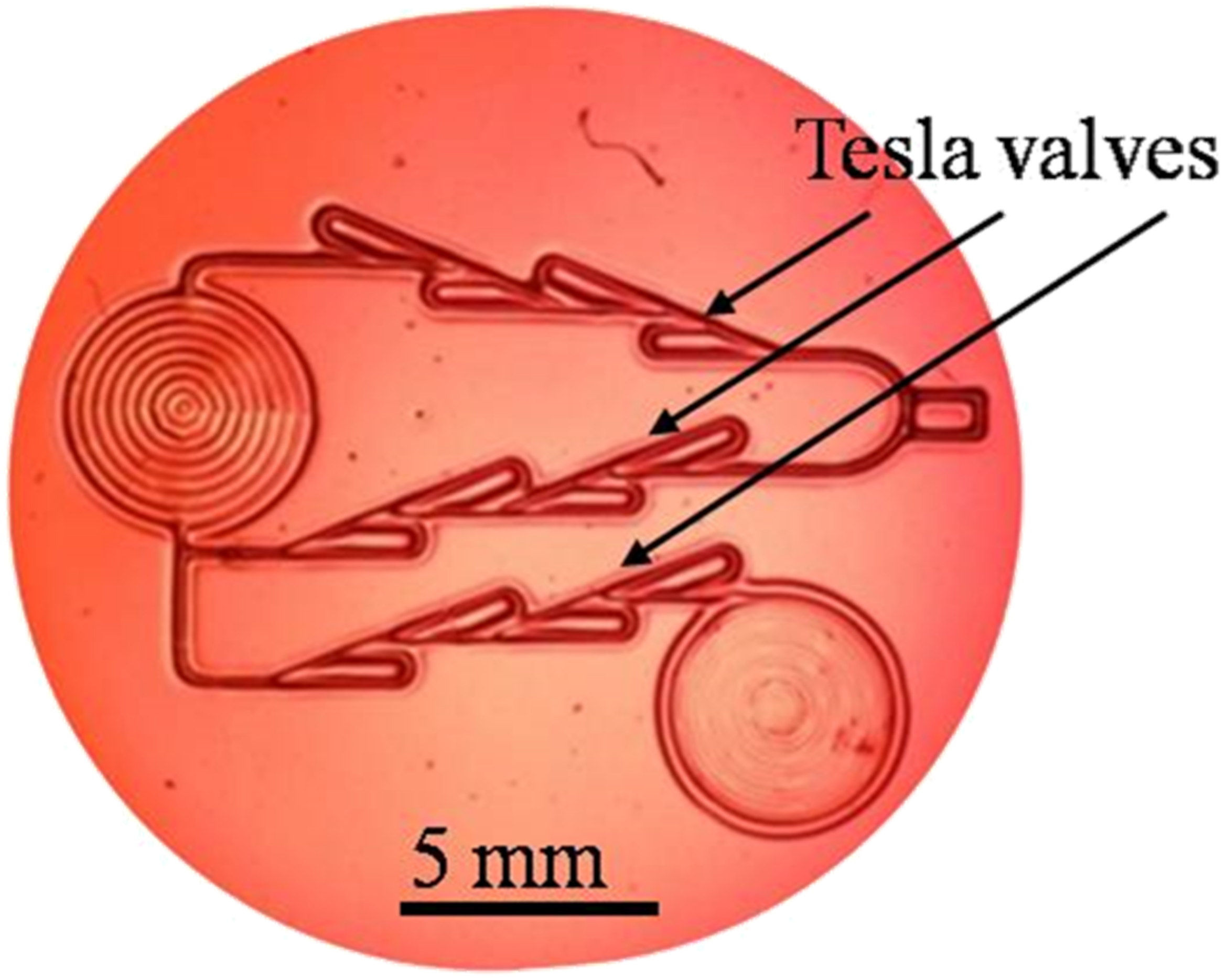
| Film | Tot’hema (%) | Thickness (µm) | Stress at Break (MPa) | Strain at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| TG_0 | 0 | 25 ± 10 | 58 ± 1 | 2 ± 0.1 | 1933 ± 40 |
| TG_5 | 5 | 35 ± 10 | 37.92 ± 0.76 | 4 ± 0.2 | 948 ± 10 |
| TG_10 | 10 | 50 ± 10 | 4.89 ± 0.10 | 40 ± 2 | 12.23 ± 0.06 |
| TG_20 | 20 | 100 ± 10 | 1.71 ± 0.03 | 140 ± 6 | 1.22 ± 0.02 |
| TG_30 | 30 | 200 ± 10 | 0.72 ± 0.01 | 224 ± 10 | 0.32 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murić, B.D.; Pantelić, D.V.; Radmilović, M.D.; Savić-Šević, S.N.; Vasović, V.O. Characterization and Optimization of Real-Time Photoresponsive Gelatin for Direct Laser Writing. Polymers 2022, 14, 2350. https://doi.org/10.3390/polym14122350
Murić BD, Pantelić DV, Radmilović MD, Savić-Šević SN, Vasović VO. Characterization and Optimization of Real-Time Photoresponsive Gelatin for Direct Laser Writing. Polymers. 2022; 14(12):2350. https://doi.org/10.3390/polym14122350
Chicago/Turabian StyleMurić, Branka D., Dejan V. Pantelić, Mihajlo D. Radmilović, Svetlana N. Savić-Šević, and Vesna O. Vasović. 2022. "Characterization and Optimization of Real-Time Photoresponsive Gelatin for Direct Laser Writing" Polymers 14, no. 12: 2350. https://doi.org/10.3390/polym14122350
APA StyleMurić, B. D., Pantelić, D. V., Radmilović, M. D., Savić-Šević, S. N., & Vasović, V. O. (2022). Characterization and Optimization of Real-Time Photoresponsive Gelatin for Direct Laser Writing. Polymers, 14(12), 2350. https://doi.org/10.3390/polym14122350





