Synthesis and Characterization of Porous Chitosan/Saccharomycetes Adsorption Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porous Chitosan/Saccharomycetes Microspheres
2.3. Characterization of Porous Chitosan/Saccharomycetes Microspheres
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Porosity
2.3.4. X-ray Diffraction (XRD)
2.3.5. Zeta Potential Analysis
2.3.6. Adsorption Experiment
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Formation of Porous Chitosan/Saccharomycetes Microspheres
3.2. FTIR Analysis
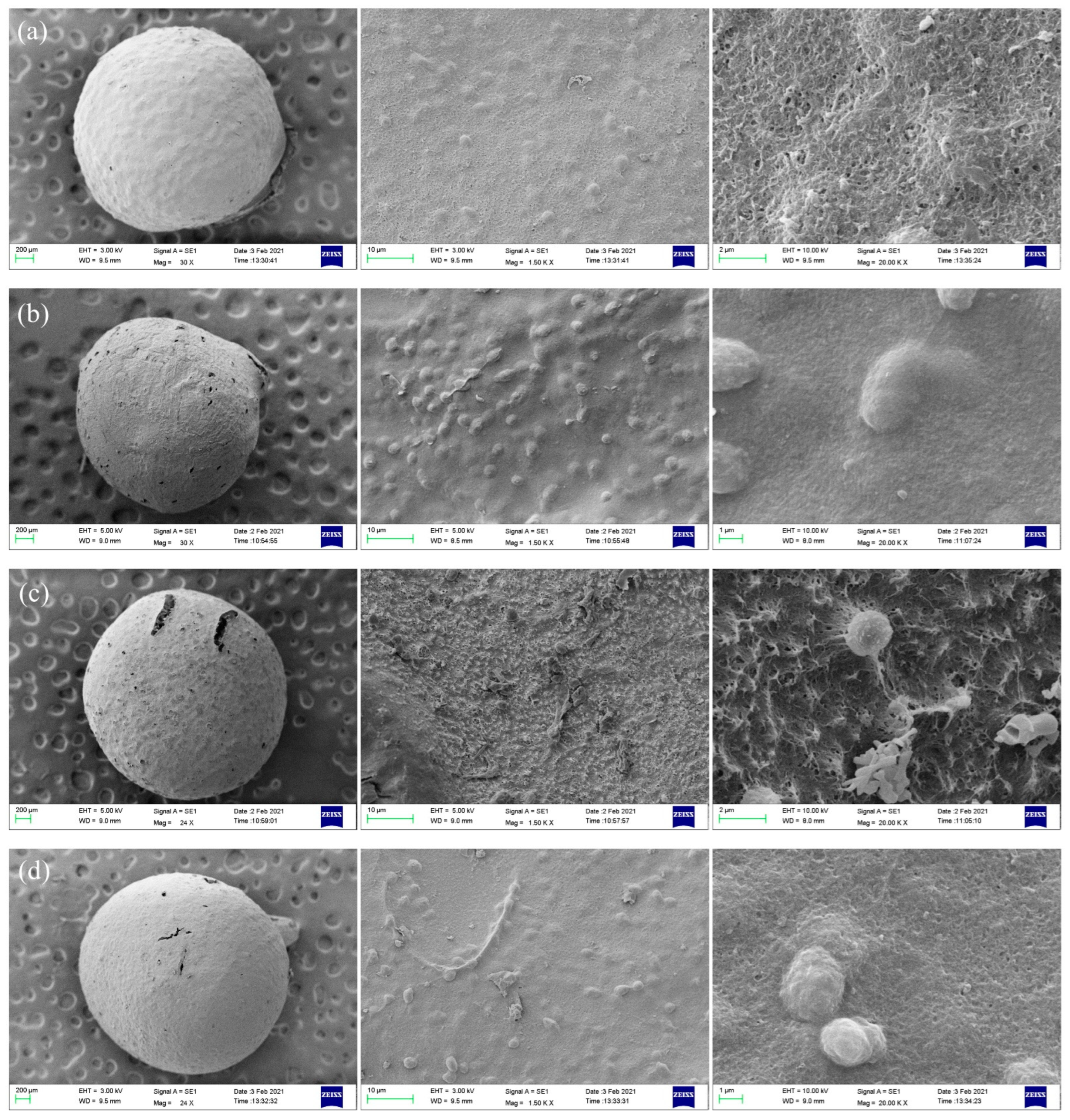
3.3. Morphology and Porosities of Porous Chitosan/Saccharomycetes Microspheres
3.4. XRD Analysis
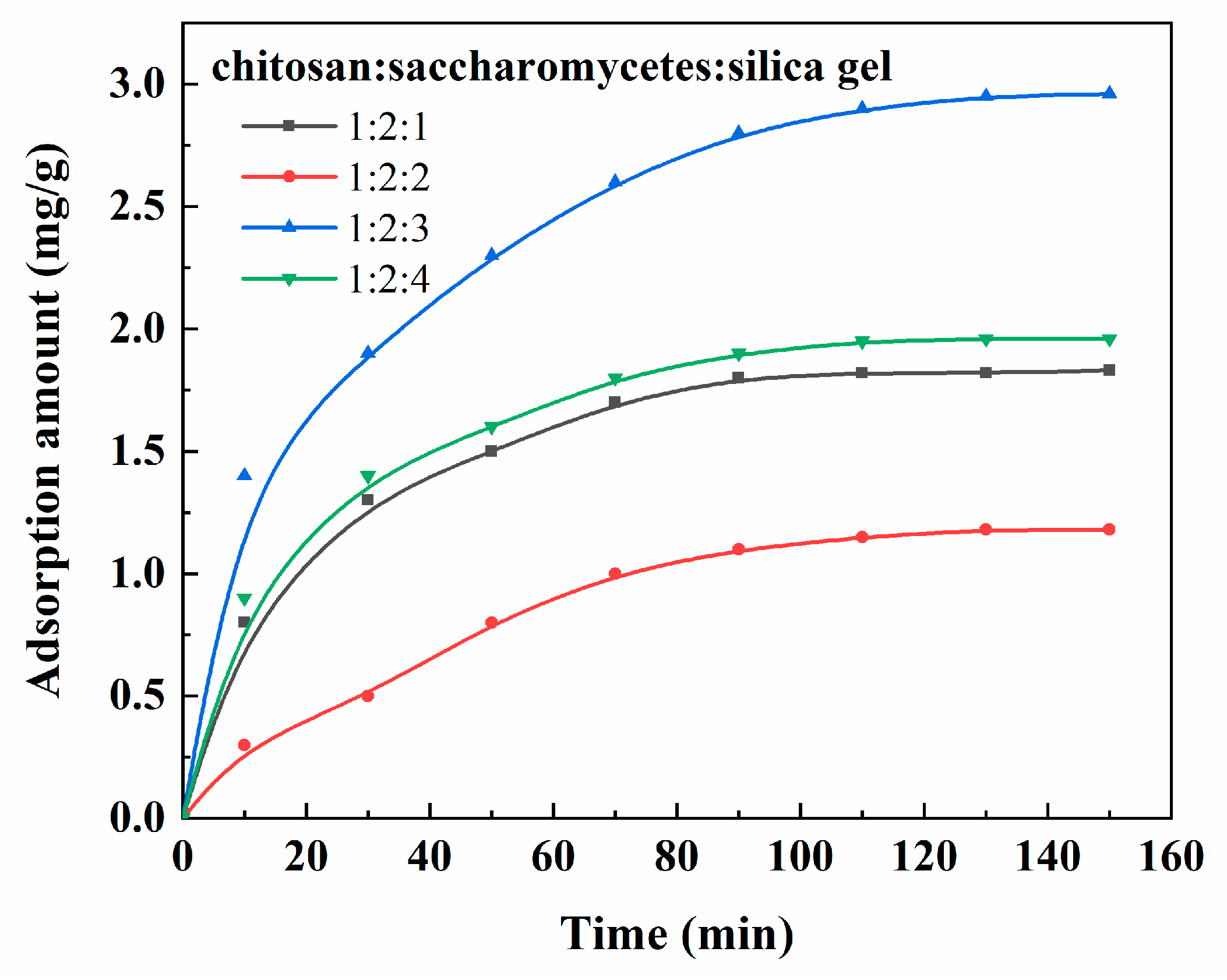
3.5. Application of Porous Chitosan/Saccharomycetes Microspheres
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Louvet, J.N.; Giammarino, C.; Potier, O.; Pons, M.N. Adverse effects of erythromycin on the structure and chemistry of activated sludge. Environ. Pollut. 2010, 158, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, J.; Nadelman, R.B.; Forseter, G.; McKenna, D.; Wormser, G.P. Doxycycline versus tetracycline therapy for Lyme disease associated with erythema migrans. J. Am. Acad. Dermatol. 1995, 32, 223–227. [Google Scholar] [CrossRef]
- Parikh, S.; Forest, W.; States, U.; Feldman, S.; Forest, W. Common use of prescription off-label therapy for acne in the pediatric population <12 years old. J. Am. Acad. Dermatol. 2014, 70, AB7. [Google Scholar] [CrossRef]
- Li, Y.; Lu, P.; Cheng, J.; Wang, Q.; He, C. Simultaneous Solid-Phase Extraction and Determination of Three Bisphenols in Water Samples and Orange Juice by a Porous β-Cyclodextrin Polymer. Food Anal. Methods 2018, 11, 1476–1484. [Google Scholar] [CrossRef]
- Qi, R.; Zhou, X.; Li, X.; Ma, J.; Lu, C.; Mu, J.; Zhang, X.; Jia, Q. Rapid identification of synthetic colorants in food samples by using indium oxide nanoparticle-functionalized porous polymer monolith coupled with HPLC-MS/MS. Analyst 2014, 139, 6168–6177. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Li, Y. Use of carbonaceous polysaccharide microspheres as templates for fabricating metal oxide hollow spheres. Chem. Eur. J. 2006, 12, 2039–2047. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Science, M.; Words, K. Control of Functions of Chitin and Chitosan by Chemical Modification. Trends Glycosci. Glycotechnol. 2002, 14, 205–222. [Google Scholar]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of heavy metal ion adsorption on Chitosan/Sulfydryl-functionalized graphene oxide composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Wang, L.; Sun, Y.; Guan, Z.; Yang, S.; Zhou, K. Amphiphilic hollow carbonaceous microspheres with permeable shells. Angew. Chem. Int. Ed. 2010, 49, 4223–4227. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Liu, L.; He, L.; Yang, S. Amphiphilic hollow carbonaceous microspheres for the sorption of phenol from water. J. Hazard. Mater. 2011, 196, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Enhanced mechanical stability and sensitive swelling performance of chitosan/yeast hybrid hydrogel beads. New J. Chem. 2016, 40, 3350–3362. [Google Scholar] [CrossRef]
- Baldikova, E.; Prochazkova, J.; Stepanek, M.; Hajduova, J.; Pospiskova, K.; Safarikova, M.; Safarik, I. PMAA-stabilized ferrofluid/chitosan/yeast composite for bioapplications. J. Magn. Magn. Mater. 2017, 427, 29–33. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, D.; Yuan, C.; Yan, T.; Feng, S.; Nakanishi, H.; Gao, X.-D. Genetically engineered yeast spores as chitosan microspheres for intracellular delivery of immunostimulatory CpG oligodeoxynucleotides. J. Control. Release 2017, 259, e50. [Google Scholar] [CrossRef]
- Kim, B.J.; Choi, I.S.; Yang, S.H. Cytocompatible coating of yeast cells with antimicrobial chitosan through layer-by-layer assembly. Bull. Korean Chem. Soc. 2016, 37, 1850–1853. [Google Scholar] [CrossRef]
- Safarik, I.; Pospiskova, K.; Maderova, Z.; Baldikova, E.; Horska, K.; Safarikova, M. Microwave-synthesized magnetic chitosan microparticles for the immobilization of yeast cells. Yeast 2015, 32, 239–243. [Google Scholar] [CrossRef]
- Hadi, P.; To, M.H.; Hui, C.W.; Lin, C.S.K.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.; Oh, K.H.; Pramar, Y. Preparation and evaluation of drug-containing chitosan beads. Drug Dev. Ind. Pharm. 1989, 15, 1475–1494. [Google Scholar] [CrossRef]
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J. Control. Release 1996, 39, 17–25. [Google Scholar] [CrossRef]
- Bodmeier, R.; Paeratakul, O. Spherical agglomerates of water-insoluble drugs. J. Pharm. Sci. 1989, 78, 964–967. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A. Nifedipine loaded chitosan microspheres prepared by emulsification phase-separation. Biotech. Histochem. 2003, 78, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Thanoo, B.C.; Sunny, M.C.; Jayakrishnan, A. Cross-linked Chitosan Microspheres: Preparation and Evaluation as a Matrix for the Controlled Release of Pharmaceuticals. J. Pharm. Pharmacol. 1992, 44, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Aiedeh, K.; Gianasi, E.; Bertasi, V.; Zecchi, V. Indomethacin loaded chitosan microspheres. Correlation between the erosion process and release kinetics. J. Microencapsul. 1996, 13, 463–472. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Shahi, V.K. An improved process for separation of proteins using modified chitosan-silica cross-linked charged ultrafilter membranes under coupled driving forces: Isoelectric separation of proteins. J. Colloid Interface Sci. 2008, 319, 252–262. [Google Scholar] [CrossRef]
- Santos, D.E.S.; Neto, C.G.T.; Fonseca, J.L.C.; Pereira, M.R. Chitosan macroporous asymmetric membranes-Preparation, characterization and transport of drugs. J. Memb. Sci. 2008, 325, 362–370. [Google Scholar] [CrossRef]
- Li, S.; Chen, Q.; Liu, M. Kinetics and equilibrium studies on adsorption of dimethylformamide by macroporous chitosan membranes. Adv. Mater. Res. 2011, 233–235, 1141–1145. [Google Scholar]
- Yu, X.; Zhang, C.; Ni, Y.; Zheng, S. Crosslinked epoxy microspheres: Preparation, surface-initiated polymerization, and use as macroporous silica porogen. J. Appl. Polym. Sci. 2013, 128, 2829–2839. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Cui, J.; He, W.; Liu, H.; Liao, S.; Yue, Y. Ordered hierarchical mesoporous anatase TiO2 from yeast biotemplates. Colloids Surf. B Biointerfaces 2009, 74, 274–278. [Google Scholar] [CrossRef]
- Xu, J.; Song, W.; Wu, N.; Tong, J.; Ren, L. Preparation and characterization of chitosan/polyvinyl porous alcohol aerogel microspheres with stable physicochemical properties. Int. J. Biol. Macromol. 2021, 187, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhu, T.; He, F.; Ou, Z.; Xu, J.; Ren, L. Preparation and characterization of functional films based on chitosan and corn starch incorporated tea polyphenols. Coatings 2021, 11, 817. [Google Scholar] [CrossRef]
- Song, W.; Xu, j.; Gao, L.; Zhang, Q.; Tong, J.; Ren, L. Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Appl. Sci. 2021, 11, 4217. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Meenakshi, S. Preparation and characterization of silica gel/chitosan composite for the removal of Cu(II) and Pb(II). Int. J. Biol. Macromol. 2012, 50, 650–657. [Google Scholar] [CrossRef]
- Hasmath Farzana, M.; Meenakshi, S. Photocatalytic aptitude of titanium dioxide impregnated chitosan beads for the reduction of Cr(VI). Int. J. Biol. Macromol. 2015, 72, 1265–1271. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, D.; Wang, A. Chitosan-g-poly(acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal. Chim. Acta 2011, 687, 193–200. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Gümüşoĝlu, T.; Ari, G.A.; Deligöz, H. Investigation of salt addition and acid treatment effects on the transport properties of ionically cross-linked polyelectrolyte complex membranes based on chitosan and polyacrylic acid. J. Memb. Sci. 2011, 376, 25–34. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Guo, J.; Shi, K.; Shang, L.; Xiao, J.; Zhao, Y. Pollens derived magnetic porous particles for adsorption of low-density lipoprotein from plasma. Bioact. Mater. 2021, 6, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ren, Y.; Cui, M.; Lee, Y.; Kim, J.; Kwon, O.; Ji, W.; Khim, J. Arsenic adsorption on two types of powdered and beaded coal mine drainage sludge adsorbent. Chemosphere 2021, 272, 129560. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, Y.; Zhao, Y. Physicochemical, Microstructural, and Antibacterial Properties of β-Chitosan and Kudzu Starch Composite Films. J. Food Sci. 2012, 77, E280–E286. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch-chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Zhang, Q.; Guan, Y.; Li, W.; Xie, S.; Tong, J.; Li, M.; Ren, L. Synthesis and Characterization of Porous Chitosan/Saccharomycetes Adsorption Microspheres. Polymers 2022, 14, 2292. https://doi.org/10.3390/polym14112292
Song W, Zhang Q, Guan Y, Li W, Xie S, Tong J, Li M, Ren L. Synthesis and Characterization of Porous Chitosan/Saccharomycetes Adsorption Microspheres. Polymers. 2022; 14(11):2292. https://doi.org/10.3390/polym14112292
Chicago/Turabian StyleSong, Wei, Qingzhu Zhang, Yuxin Guan, Wanyan Li, Siyu Xie, Jin Tong, Mo Li, and Lili Ren. 2022. "Synthesis and Characterization of Porous Chitosan/Saccharomycetes Adsorption Microspheres" Polymers 14, no. 11: 2292. https://doi.org/10.3390/polym14112292
APA StyleSong, W., Zhang, Q., Guan, Y., Li, W., Xie, S., Tong, J., Li, M., & Ren, L. (2022). Synthesis and Characterization of Porous Chitosan/Saccharomycetes Adsorption Microspheres. Polymers, 14(11), 2292. https://doi.org/10.3390/polym14112292






