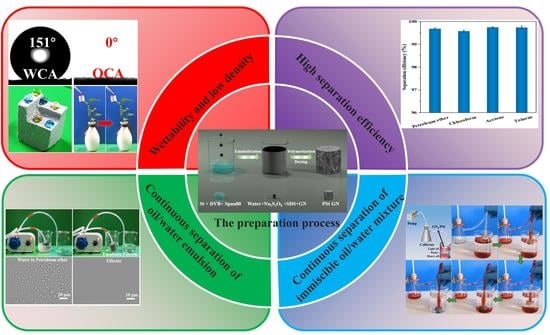
Facile Fabrication of Superhydrophobic Graphene/Polystyrene Foams for Efficient and Continuous Separation of Immiscible and Emulsified Oil/Water Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of GN/PSt Foams
2.3. Characterization
2.3.1. Oil Absorption Capacity
2.3.2. Oil/Water Mixture Separation Efficiency
2.3.3. Emulsified Oil/Water Mixture Separation Experiments
3. Results and Discussion
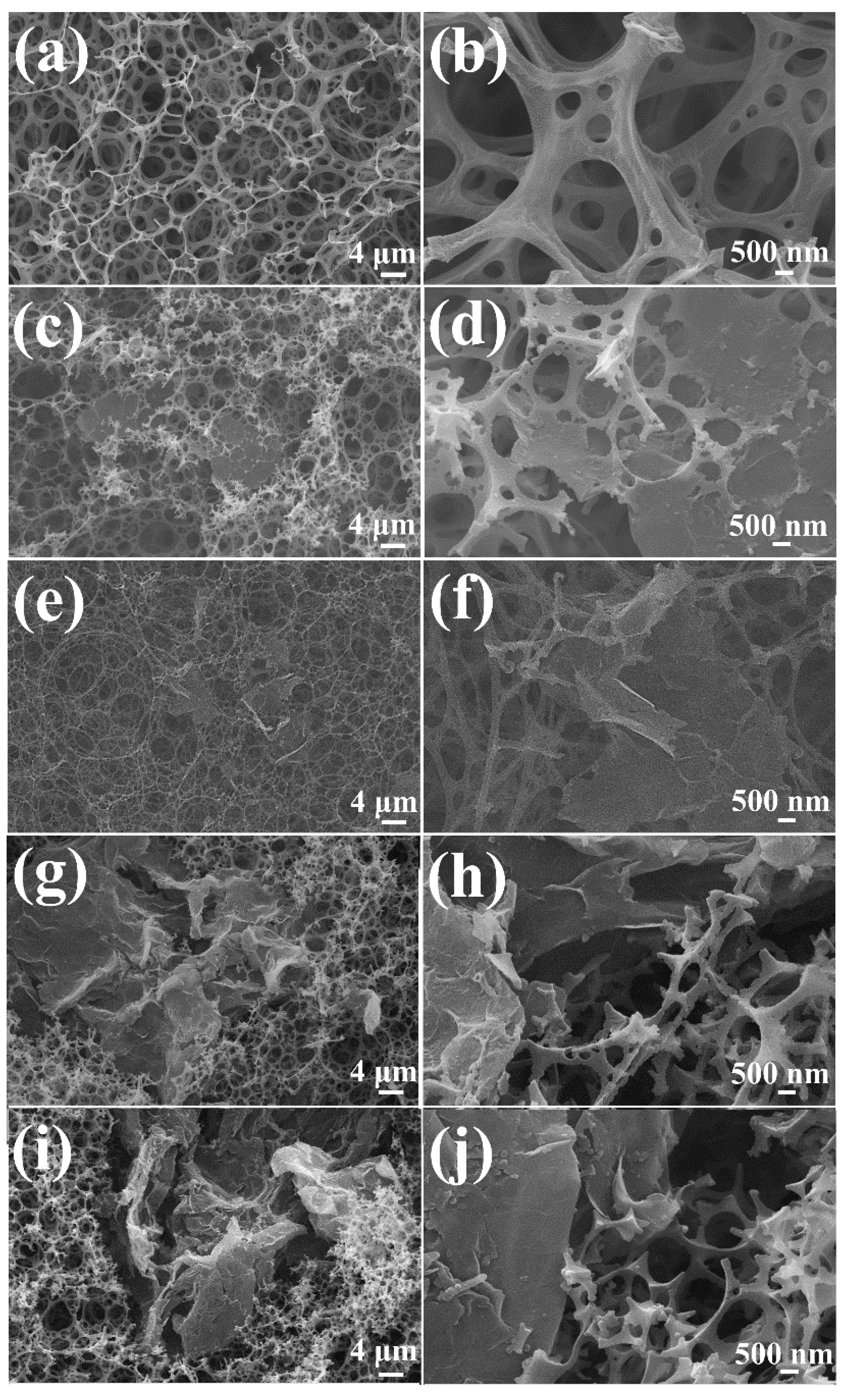
3.1. Characterizations of the GN/PSt Composites
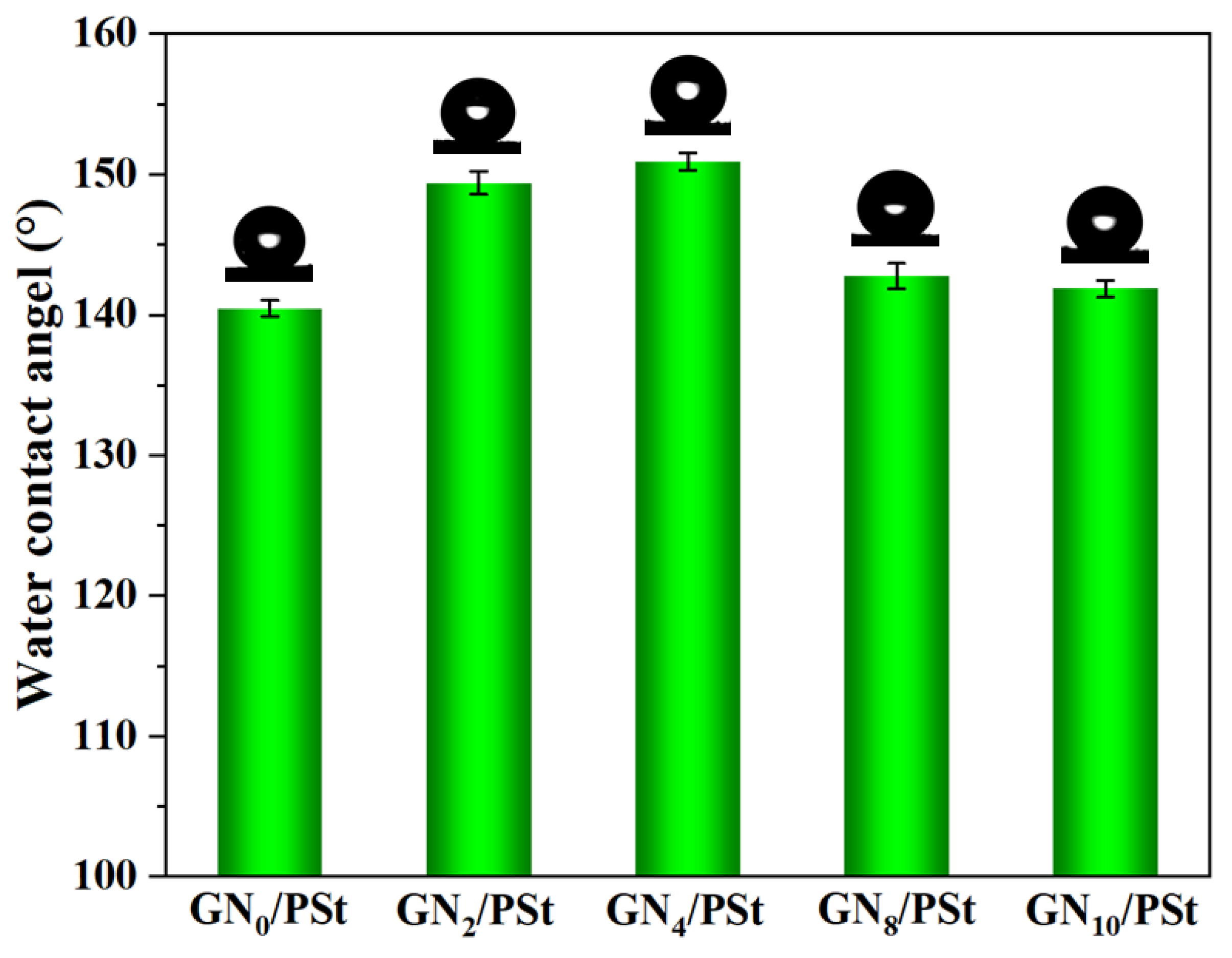
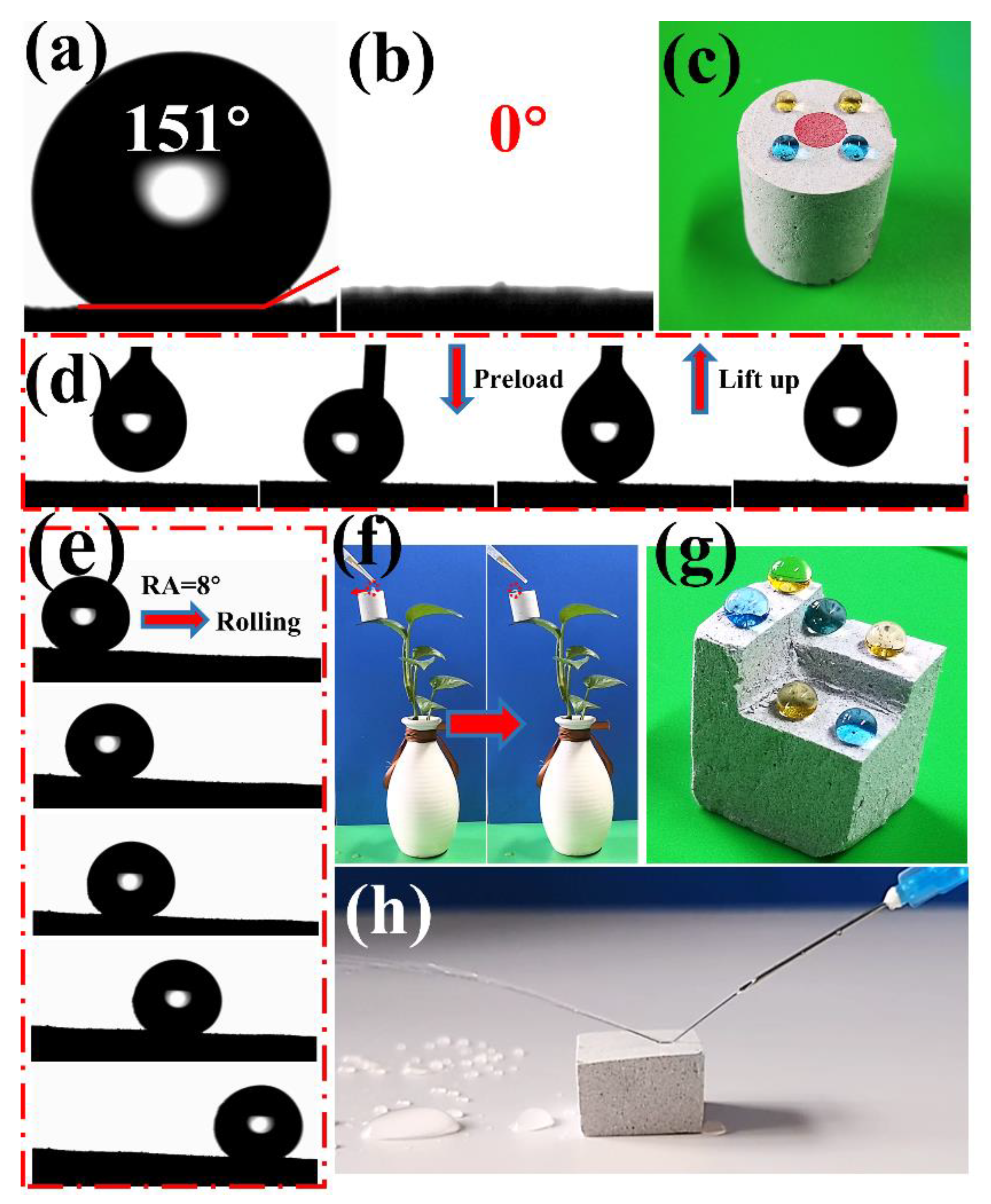
3.2. Hydrophobicity of the GN/PSt Composites
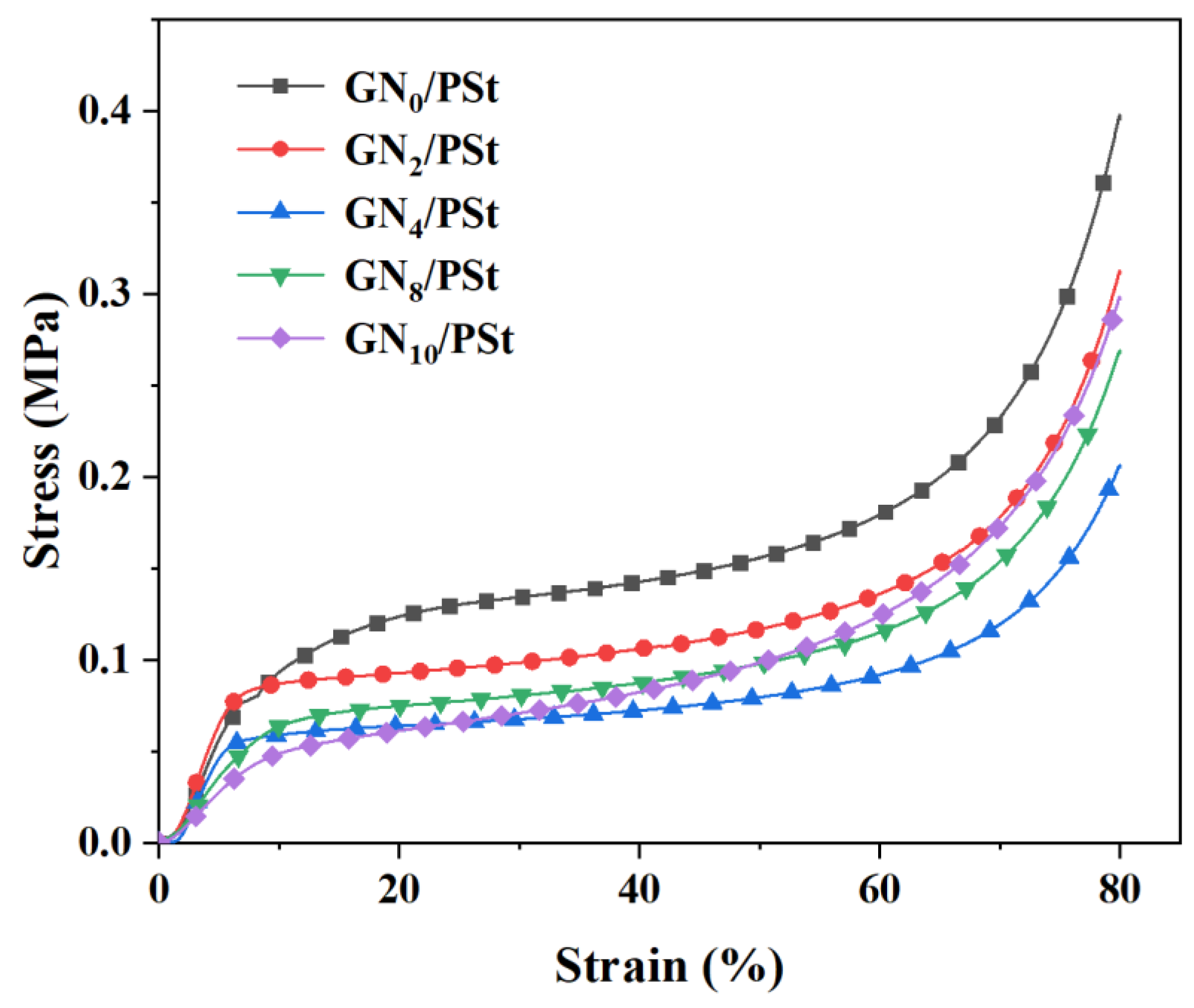
3.3. Mechanical Properties of GN/PSt Composites
3.4. Wettability of GN4/PSt Composites
3.5. Oil Absorption Capacity and Continuous Oil/Water Separation Using the GN4/PSt Composites
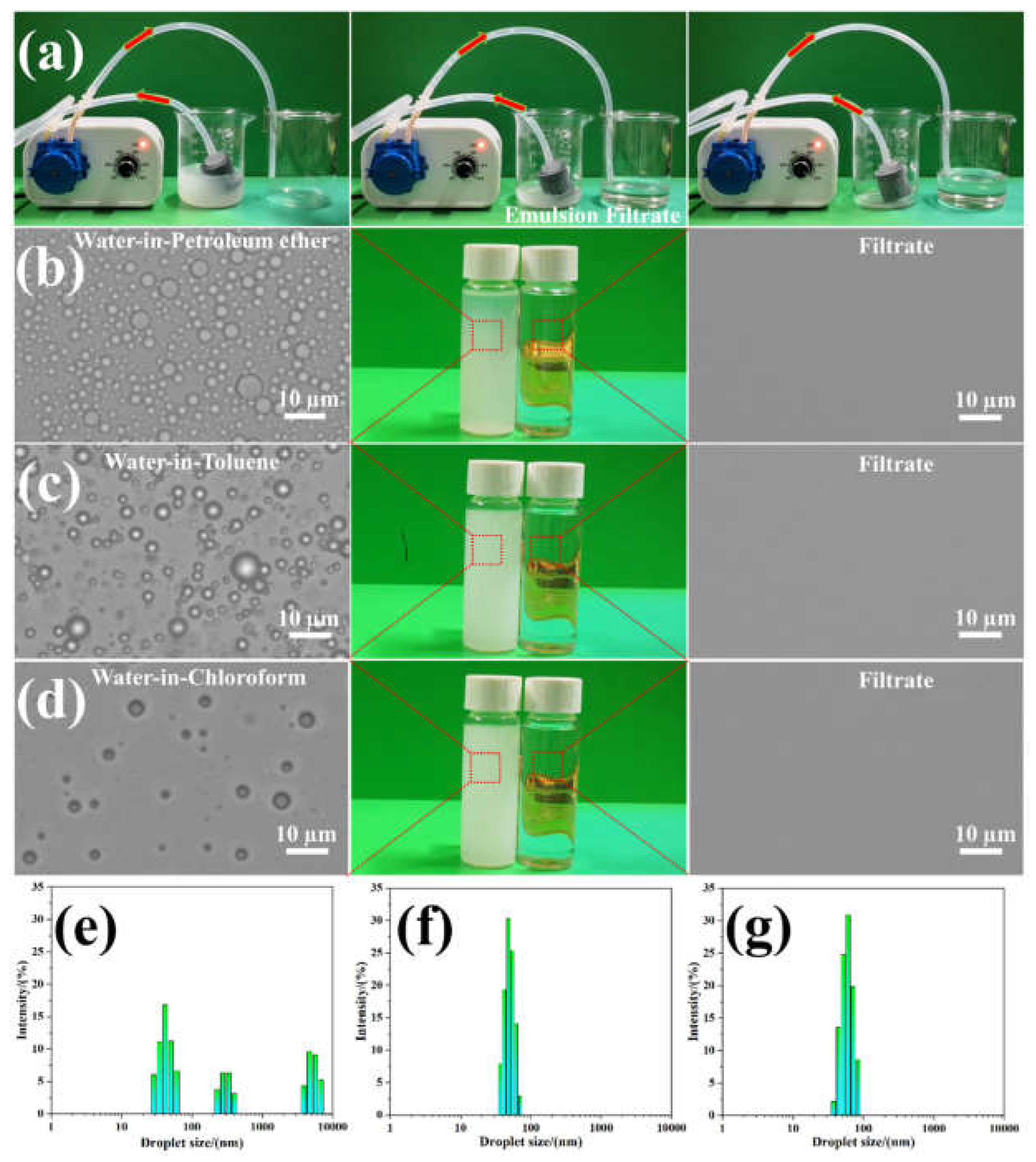
3.6. Continuous Separation of Surfactant-Stabilized Emulsions Using the GN4/PSt Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeda, R.; Wambaugh, Z.; Wang, Z.; Hyatt, C.; Liu, Z.; Buskey, E.J. Interactions between Zooplankton and Crude Oil: Toxic Effects and Bioaccumulation of Polycyclic Aromatic Hydrocarbons. PLoS ONE 2013, 8, e67212. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Esler, D.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B. Long-Term Ecosystem Response to the Exxon Valdez Oil Spill. Science 2003, 302, 2082–2086. [Google Scholar] [CrossRef] [Green Version]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent Improvements in Oily Wastewater Treatment: Progress, Challenges, and Future Opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef]
- Ezzat, A.O.; Atta, A.M.; Al-Lohedan, H.A. One-Step Synthesis of Amphiphilic Nonylphenol Polyethyleneimine for Demulsification of Water in Heavy Crude Oil Emulsions. ACS Omega 2020, 5, 9212–9223. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Jiang, X.; Mi, Y.; Kuang, J.; Huang, Z.; Yu, F.; Zhang, Z.; Yuan, H. Preparation of Oxidized Carbon Black Grafted with Nanoscale Silica and Its Demulsification Performance in Water-in-Oil Emulsion. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 582, 123878. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent Progress in Developing Advanced Membranes for Emulsified Oil/Water Separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Liu, N.; Zhang, W.; Yang, Y.; Cao, Y.; Lin, X.; Wei, Y.; Feng, L. Breathing Demulsification: A Three-Dimensional (3D) Free-Standing Superhydrophilic Sponge. ACS Appl. Mater. Interfaces 2015, 7, 22264–22271. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Thomas, A.G.; Yearsley, K.; Bolton, L.W.; Matthews, A.; Lewis, D.J. Renewable Adsorbent for the Separation of Surfactant-Stabilized Oil in Water Emulsions Based on Nanostructured Sawdust. ACS Sustain. Chem. Eng. 2019, 7, 18935–18942. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Wang, G.; He, Y.; Wang, H.; Zhang, L.; Yu, Q.; Peng, S.; Wu, X.; Ren, T.; Zeng, Z.; Xue, Q. A Cellulose Sponge with Robust Superhydrophilicity and Under-Water Superoleophobicity for Highly Effective Oil/Water Separation. Green Chem. 2015, 17, 3093–3099. [Google Scholar] [CrossRef]
- Li, X.; Cao, M.; Shan, H.; Tezel, F.H.; Li, B. Facile and Scalable Fabrication of Superhydrophobic and Superoleophilic PDMS-co-PMHS Coating on Porous Substrates for Highly Effective Oil/Water Separation. Chem. Eng. J. 2019, 358, 1101–1113. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Seeger, S. Tunable Bulk Material with Robust and Renewable Superhydrophobicity Designed via In-Situ Loading of Surface-Wrinkled Microparticles. Chem. Eng. J. 2020, 408, 127301. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhang, D.J.; Luo, X.; Lai, H.; Liu, Y.Y.; Jiang, L. Superwetting Shape Memory Microstructure: Smart Wetting Control and Practical Application. Adv. Mater. 2021, 33, 2001718–2001733. [Google Scholar] [CrossRef]
- Du, R.; Zhao, Q.; Zheng, Z.; Hu, W.; Zhang, J. 3D Self-Supporting Porous Magnetic Assemblies for Water Remediation and Beyond. Adv. Energy Mater. 2016, 6, 1600473. [Google Scholar] [CrossRef]
- Li, L.X.; Zhang, J.P.; Wang, A.Q. Removal of Organic Pollutants from Water Using Superwetting Materials. Chem. Rec. 2018, 18, 118–136. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Zhang, N.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A Review on Oil/Water Mixture Separation Material. Ind. Eng. Chem. Res. 2020, 59, 14546–14568. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-Inspired Superwettability Systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Shen, L.; Pan, Y.; Fu, H. Fabrication of UV Curable Coating for Super Hydrophobic Cotton Fabrics. Polym. Eng. Sci. 2019, 59, E452–E459. [Google Scholar] [CrossRef]
- Shan, W.W.; Du, J.; Yang, K.; Ren, T.B.; Wan, D.C.; Pu, H.T. Superhydrophobic and Superoleophilic Polystyrene/Carbon Nanotubes Foam for Oil/Water Separation. J. Environ. Chem. Eng. 2021, 9, 106038–106046. [Google Scholar] [CrossRef]
- Li, Z.T.; Lin, B.; Jiang, L.W.; Lin, E.C.; Chen, J.; Zhang, S.J.; Tang, Y.W.; He, F.A.; Li, D.H. Effective Preparation of Magnetic Superhydrophobic Fe3O4/PU Sponge for Oil-Water Separation. Appl. Surf. Sci. 2018, 427, 56–64. [Google Scholar] [CrossRef]
- Kang, L.; Shi, L.; Zeng, Q.; Liao, B.; Wang, B.; Guo, X. Melamine Resin-Coated Lignocellulose Fibers with Robust Superhydrophobicity for Highly Effective Oil/Water Separation. Sep. Purif. Technol. 2021, 279, 119737. [Google Scholar] [CrossRef]
- Shang, Q.; Chen, J.; Hu, Y.; Yang, X.; Hu, L.; Liu, C.; Ren, X.; Zhou, Y. Facile Fabrication of Superhydrophobic Cross-Linked Nanocellulose Aerogels for Oil–Water Separation. Polymers 2021, 13, 625. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, J.; Lu, X.; Zhu, Y.; Jiang, L. In Situ Fastening Graphene Sheets into a Polyurethane Sponge for the Highly Efficient Continuous Cleanup of Oil Spills. Nano Res. 2017, 10, 1756–1766. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, N.; Chen, Z.; Jiang, B.; Sun, Y.; Yang, X.; Zhang, L. Fabrication of Meshes with Inverse Wettability Based on the TIO2 Nanowires for Continuous Oil/Water Separation. Chem. Eng. J. 2019, 380, 122524. [Google Scholar] [CrossRef]
- Su, X.J.; Li, H.Q.; Lai, X.J.; Zhang, L.; Liao, X.F.; Wang, J.; Chen, Z.H.; He, J.; Zeng, X.R. Dual-Functional Superhydrophobic Textiles with Asymmetric Roll-Down/Pinned States for Water Droplet Transportation and Oil-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 4213–4221. [Google Scholar] [CrossRef]
- Li, Z.T.; He, F.A.; Lin, B. Preparation of Magnetic Superhydrophobic Melamine Sponge for Oil-Water Separation. Powder Technol. 2019, 345, 571–579. [Google Scholar] [CrossRef]
- Ge, J.; Ye, Y.D.; Yao, H.B.; Zhu, X.; Wang, X.; Wu, L.; Wang, J.L.; Ding, H.; Yong, N.; He, L.H.; et al. Pumping through Porous Hydrophobic/Oleophilic Materials: An Alternative Technology for Oil Spill Remediation. Angew. Chem. Int. Ed. Engl. 2014, 53, 3612–3616. [Google Scholar] [CrossRef]
- Dhar, M.; Das, A.; Shome, A.; Borbora, A.; Manna, U. Design of ‘Tolerant and Hard’ Superhydrophobic Coatings to Freeze Physical Deformation. Mater. Horiz. 2021, 10, 2717–2725. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.X.; Xu, J.C.; Zhu, Y.; Yang, D.Y.; Dai, Y.T. Design and Preparation of Efficient, Stable and Superhydrophobic Copper Foam Membrane for Selective Oil Absorption and Consecutive Oil–Water Separation. Mater. Des. 2018, 142, 83–92. [Google Scholar] [CrossRef]
- Liang, L.; Dong, Y.; Liu, Y.; Meng, X. Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation. Polymers 2019, 11, 2072. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Zhu, Y.; Li, H.; He, K.; Zeng, G.; Chen, A.; Huang, Z.; Huang, T.; Yuan, L.; Chen, G. Synthesis and Application of Modified Commercial Sponges for Oil-Water Separation. Chem. Eng. J. 2019, 373, 213–226. [Google Scholar] [CrossRef]
- Zeng, X.; Qian, L.; Yuan, X.; Zhou, C.; Li, Z.; Cheng, J.; Xu, S.; Wang, S.; Pi, P.; Wen, X. Inspired by Stenocara Beetles: From Water Collection to High-Efficiency Water-in-Oil Emulsion Separation. ACS Nano 2017, 11, 760–769. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Tao, Z.; Liu, X.; Wang, Z.; Yue, R.; Cui, Z. 3D Superhydrophobic Sponge with a Novel Compression Strategy for Effective Water-In-Oil Emulsion Separation and Its Separation Mechanism. Chem. Eng. J. 2019, 359, 149–158. [Google Scholar] [CrossRef]
- Viswanathan, P.; Johnson, D.W.; Hurley, C.; Cameron, N.R.; Battaglia, G. 3D Surface Functionalization of Emulsion-Templated Polymeric Foams. Macromolecules 2014, 47, 7091–7098. [Google Scholar] [CrossRef]
- Moon, S.; Kim, J.Q.; Kim, B.Q.; Chae, J.; Choi, S.Q. Processable Composites with Extreme Material Capacities: Toward Designer High Internal Phase Emulsions and Foams. Chem. Mater. 2020, 32, 4838–4854. [Google Scholar] [CrossRef]
- Li, D.; Bu, X.; Xu, Z.; Luo, Y.; Bai, H. Bioinspired Multifunctional Cellular Plastics with a Negative Poisson’s Ratio for High Energy Dissipation. Adv. Mater. 2020, 32, e2001222. [Google Scholar] [CrossRef]
- Gui, H.; Zhang, T.; Guo, Q. Nanofibrous, Emulsion-Templated Syndiotactic Polystyrenes with Superhydrophobicity for Oil Spill Cleanup. ACS Appl. Mater. Interfaces 2019, 11, 36063–36072. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, H.; Ngai, T. Pickering High Internal Phase Emulsions Templated Super-Hydrophobic–Oleophilic Elastic Foams for Highly Efficient Oil/Water Separation. ACS Appl. Polym. Mater. 2020, 2, 5664–5673. [Google Scholar] [CrossRef]
- Dong, B.; Wang, F.; Abadikhah, H.; Hao, L.Y.; Xu, X.; Khan, S.A.; Wang, G.; Agathopoulos, S. Simple Fabrication of Concrete with Remarkable Self-Cleaning Ability, Robust Superhydrophobicity, Tailored Porosity, and Highly Thermal and Sound Insulation. ACS Appl. Mater. Interfaces 2019, 11, 42801–42807. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, X.; Zhao, C.; Zhu, L.; Li, Y.; Wu, Y.; Li, Z. Superhydrophobic and Magnetic PS/Fe3O4 Sponge for Remote Oil Removal under Magnetic Driven, Continuous Oil Collection, and Oil/Water Emulsion Separation. J. Mater. Sci. 2022, 57, 336–350. [Google Scholar] [CrossRef]
- Azman, M.; Chungprempree, J.; Preechawong, J.; Sapsrithong, P.; Nithitanakul, M. Layer-by-Layer (LbL) Surface Augmented Modification of Poly(Styrene/Divinylbenzene)High Internal Phase Emulsion for Carbon Dioxide Capture. Polymers 2021, 13, 2247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, Y.; Zhang, Y.; Jiang, W.; Wang, T.; Fu, J. Dual-Templating Synthesis of Compressible and Superhydrophobic Spongy Polystyrene for Oil Capture. Chem. Eng. J. 2018, 354, 245–253. [Google Scholar] [CrossRef]
- Benaddi, A.O.; Cohen, O.; Matyjaszewski, K.; Silverstein, M.S. RAFT Polymerization within High Internal Phase Emulsions: Porous Structures, Mechanical Behaviors, and Uptakes. Polymer 2021, 213, 123327. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, C.; Zhu, L.; Huang, H.; Li, Y.; Xiang, D.; Li, H.; Wu, Y.; Huang, S. Thermally Stable and Superhydrophobic Foam Prepared by High-Internal Phase Emulsion for Oil/Water Separation with Good Recycling Capacity. J. Appl. Polym. Sci. 2021, 139, 51521. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Gao, A.; Cui, J.; Zhao, S.; Yan, Y. Robust Graphene/Poly(vinyl alcohol) Janus Aerogels with a Hierarchical Architecture for Highly Efficient Switchable Separation of Oil/Water Emulsions. ACS Appl. Mater. Interfaces 2019, 11, 36638–36648. [Google Scholar] [CrossRef]
- Zhang, N.; Zhong, S.; Zhou, X.; Jiang, W.; Wang, T.; Fu, J. Superhydrophobic P (St-DVB) Foam Prepared by the High Internal Phase Emulsion Technique for Oil Spill Recovery. Chem. Eng. J. 2016, 298, 117–124. [Google Scholar] [CrossRef]
- Li, C.; Jin, M.; Wan, D. Evolution of a Radical-Triggered Polymerizing High Internal Phase Emulsion into an Open-Cellular Monolith. Macromol. Chem. Phys. 2019, 220, 1900216. [Google Scholar] [CrossRef]
- Wu, H.; Shi, J.; Ning, X.; Long, Y.Z.; Zheng, J. The High Flux of Superhydrophilic-Superhydrophobic Janus Membrane of cPVA-PVDF/PMMA/GO by Layer-by-Layer Electrospinning for High Efficiency Oil-Water Separation. Polymers 2022, 14, 621. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, X.; Wang, H.; Du, Q. Macroporous Graphene Oxide–Polymer Composite Prepared through Pickering High Internal Phase Emulsions. ACS Appl. Mater. Interfaces 2013, 5, 7974–7982. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Q.; Zhao, Y.; Wang, H.; Heng, Y. A Convenient and Versatile Strategy for the Functionalization of Silica Foams Using High Internal Phase Emulsion Templates as Microreactors. ACS Appl. Mater. Interfaces 2020, 12, 14607–14619. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, B.; Pu, Z.; Liu, T.; Feng, Y.; Han, W.; Liu, C.; Shen, C. Flexible and Robust Porous Thermoplastic Polyurethane/Reduced Graphene Oxide Monolith with Special Wettability for Continuous Oil/Water Separation in Harsh Environment. Sep. Purif. Technol. 2021, 266, 118553. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, J.E.; Liu, Y.Y.; Liu, K.; Situ, Z.Q.; Tian, L.F.; Luo, W.J.; Hong, P.Z.; Li, Y. Facile Fabrication of Compressible, Magnetic and Superhydrophobic Poly(DVB-MMA) Sponge for High-Efficiency Oil–Water Separation. J. Mater. Sci. 2020, 56, 3111–3126. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, B.; Ye, S.; Zhang, Y.; Wang, B.; Feng, Y.; Han, W.; Liu, C.; Shen, C. Magnetic, Superelastic and Superhydrophobic Porous Thermoplastic Polyurethane Monolith with Nano-Fe3O4 Coating for Highly Selective and Easy-Recycling Oil/Water Separation. Appl. Surf. Sci. 2021, 535, 147690. [Google Scholar] [CrossRef]
- Wang, J.; He, B.; Ding, Y.; Li, T.; Zhang, W.; Zhang, Y.; Liu, F.; Tang, C.Y. Beyond Superwetting Surfaces: Dual-Scale Hyperporous Membrane with Rational Wettability for “Nonfouling” Emulsion Separation via Coalescence Demulsification. ACS Appl. Mater. Interfaces 2021, 13, 4731–4739. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Hou, Y.; Zhang, L.; Li, M.; Wang, D.; Fu, S. Microstructure-Controllable Nanocomplexes Bulk Possessed with Durable Superhydrophobicity. Chem. Eng. J. 2020, 389, 124420. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, M.; Du, C.; Guo, H.; Bai, H.; Li, L. Poly(dimethylsiloxane) Oil Absorbent with a Three-Dimensionally Interconnected Porous Structure and Swellable Skeleton. ACS Appl. Mater. Interfaces 2013, 5, 10201–10206. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Wang, Y.; Zhao, X.; Yang, M.; Men, X. Durable Superwetting Materials through Layer-By-Layer Assembly: Multiple Separations Towards Water/Oil Mixtures, Water-In-Oil and Oil-In-Water Emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 571, 142–150. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, Y.; Wang, J.; He, B.; Yang, S.; Pan, K.; Liu, F. Carbonaceous Microsphere/Nanofiber Composite Superhydrophilic Membrane with Enhanced Anti-adhesion Property Towards Oil and Anionic Surfactant: Membrane Fabrication and Applications. Sep. Purif. Technol. 2020, 235, 116189. [Google Scholar] [CrossRef]
- Lv, W.Y.; Mei, Q.; Xiao, J.; Du, M.; Zheng, Q. 3D Multiscale Superhydrophilic Sponges with Delicately Designed Pore Size for Ultrafast Oil/Water Separation. Adv. Funct. Mater. 2017, 27, 1704293. [Google Scholar] [CrossRef]
- Wang, F.; Pi, J.; Li, J.Y.; Song, F.; Feng, R.; Wang, X.L.; Wang, Y.Z. Highly-Efficient Separation of Oil and Water Enabled by a Silica Nanoparticle Coating with pH-Triggered Tunable Surface Wettability. J. Colloid Interface Sci. 2019, 557, 65–75. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Huang, H.; Li, J.; Li, Y.; Xiang, D.; Wu, Y.; Wang, G.; Qin, M. Facile Fabrication of Superhydrophobic Graphene/Polystyrene Foams for Efficient and Continuous Separation of Immiscible and Emulsified Oil/Water Mixtures. Polymers 2022, 14, 2289. https://doi.org/10.3390/polym14112289
Zhao C, Huang H, Li J, Li Y, Xiang D, Wu Y, Wang G, Qin M. Facile Fabrication of Superhydrophobic Graphene/Polystyrene Foams for Efficient and Continuous Separation of Immiscible and Emulsified Oil/Water Mixtures. Polymers. 2022; 14(11):2289. https://doi.org/10.3390/polym14112289
Chicago/Turabian StyleZhao, Chunxia, Haoran Huang, Jiaxin Li, Yuntao Li, Dong Xiang, Yuanpeng Wu, Ge Wang, and Mingwang Qin. 2022. "Facile Fabrication of Superhydrophobic Graphene/Polystyrene Foams for Efficient and Continuous Separation of Immiscible and Emulsified Oil/Water Mixtures" Polymers 14, no. 11: 2289. https://doi.org/10.3390/polym14112289
APA StyleZhao, C., Huang, H., Li, J., Li, Y., Xiang, D., Wu, Y., Wang, G., & Qin, M. (2022). Facile Fabrication of Superhydrophobic Graphene/Polystyrene Foams for Efficient and Continuous Separation of Immiscible and Emulsified Oil/Water Mixtures. Polymers, 14(11), 2289. https://doi.org/10.3390/polym14112289