Study on the Synthesis and Properties of Waterborne Polyurea Modified by Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Agents
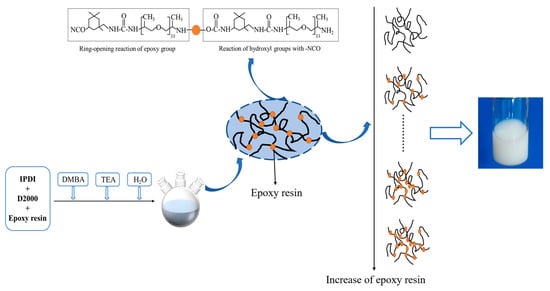
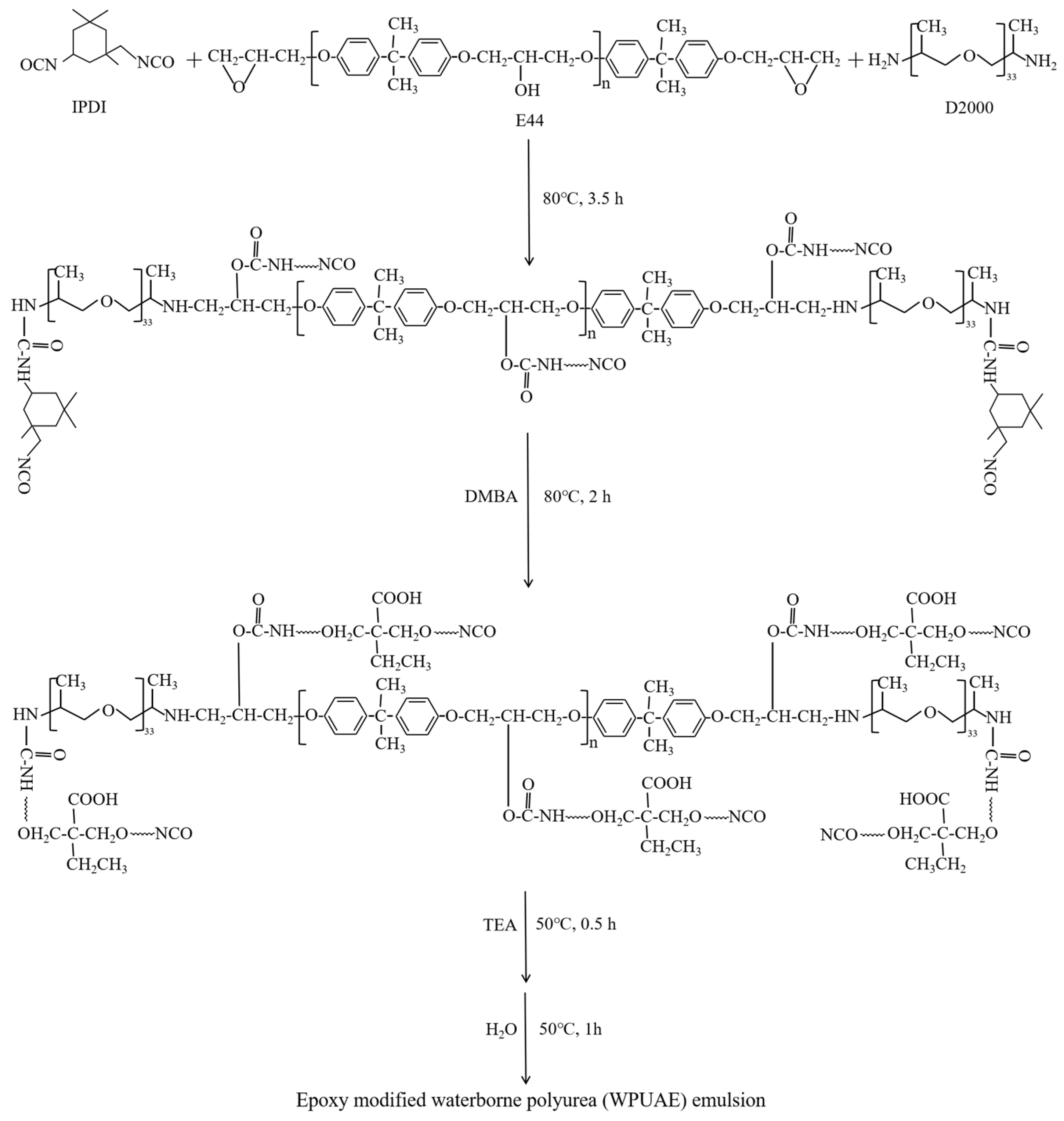
2.2. Preparation of Emulsions
2.3. Preparation of Films
2.4. Characterization
3. Results and Discussion
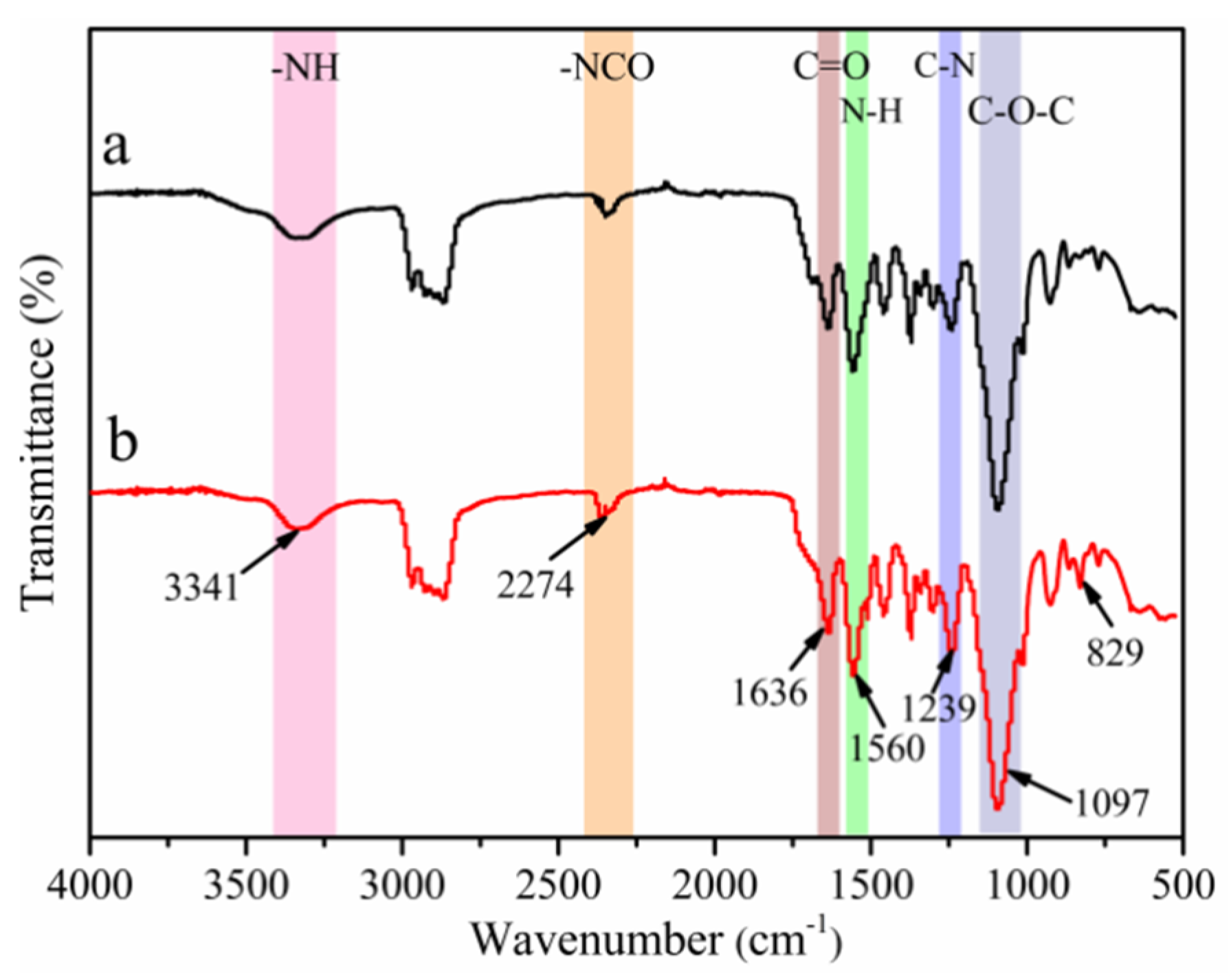
3.1. FT-IR Analysis
3.2. Basic Performances of WPUAE Emulsions
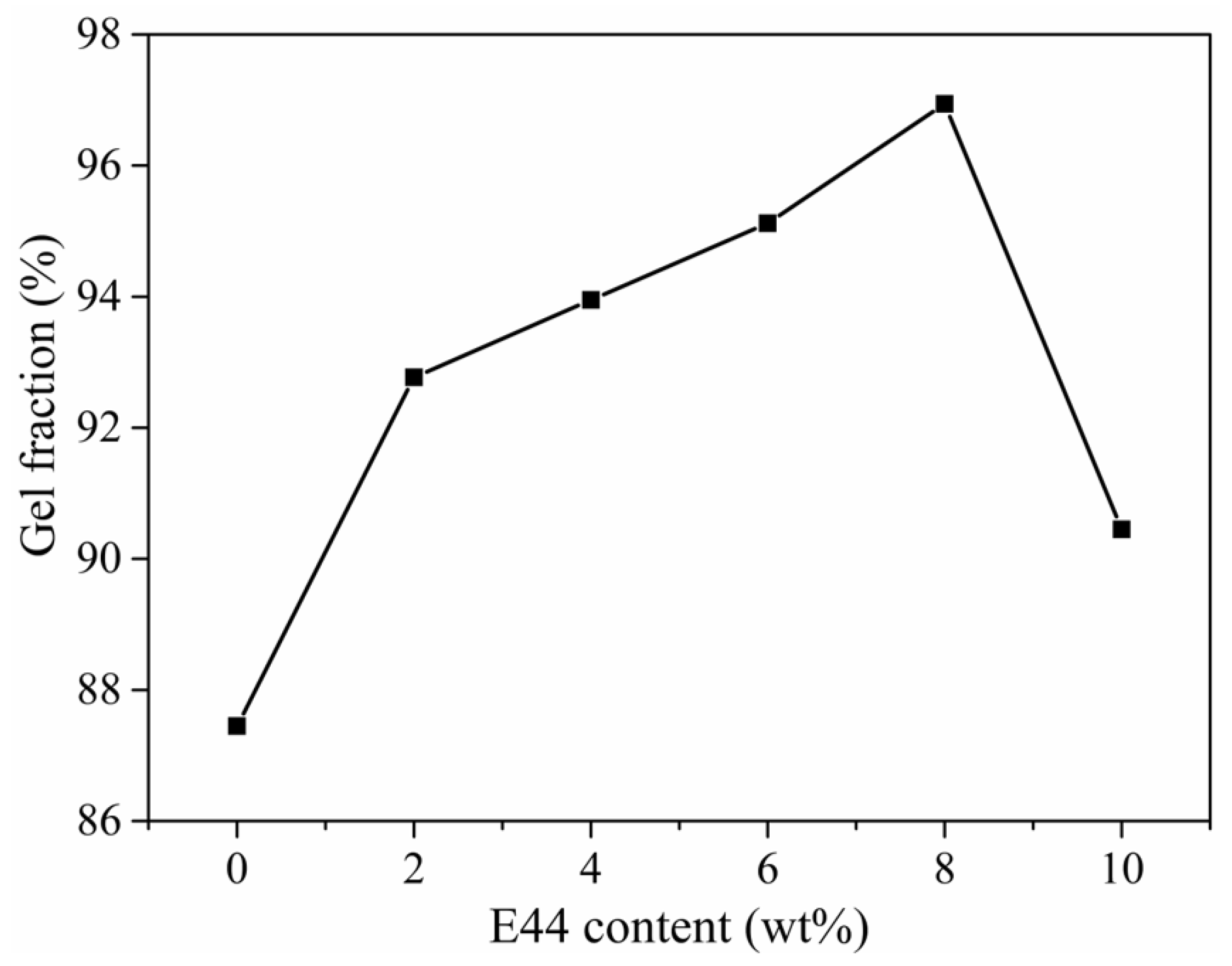
3.3. Gel Fraction Analysis
3.4. Adhesion Analysis
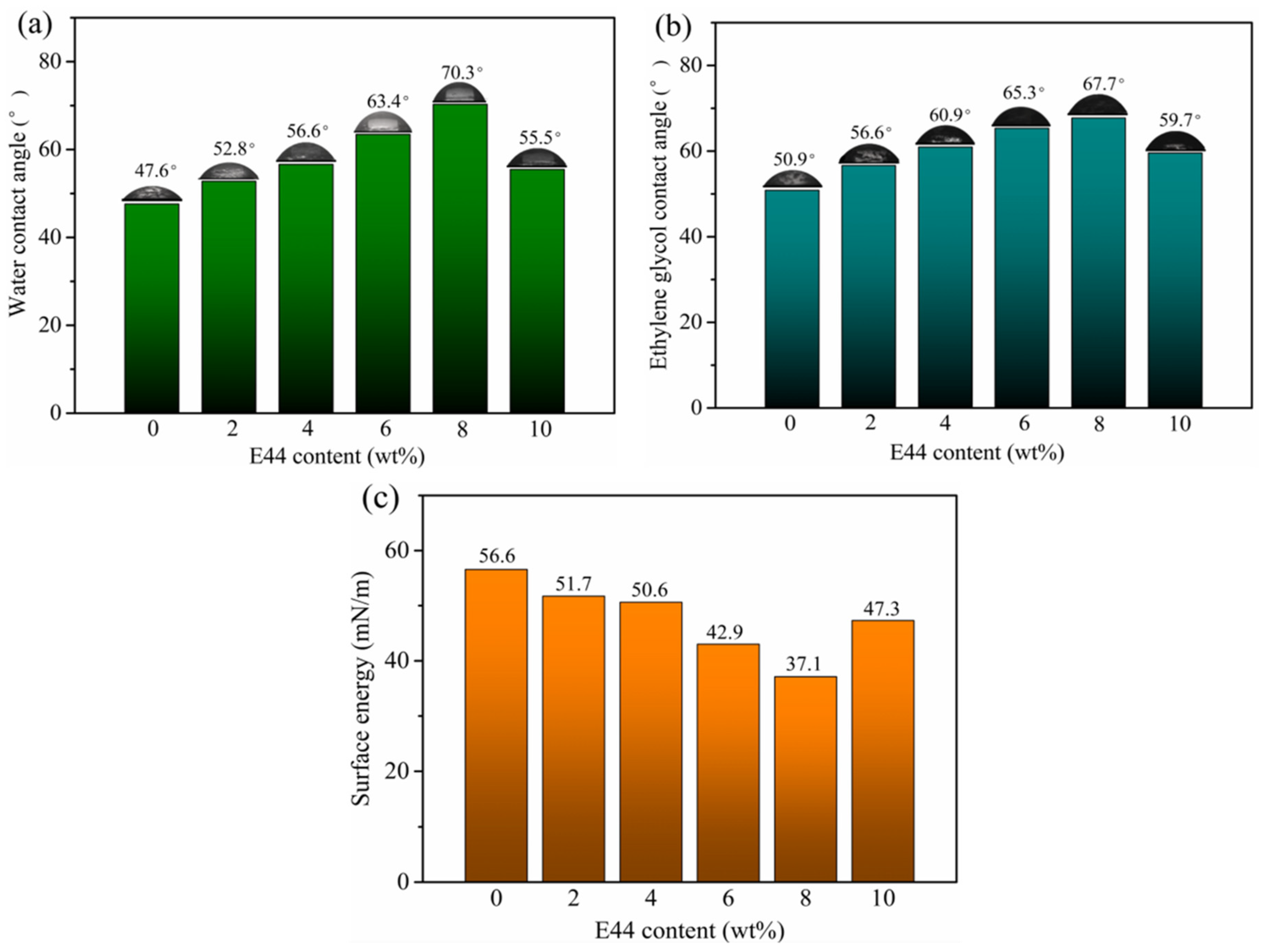
3.5. Contact Angle Analysis
3.6. Solvent Resistance Analysis
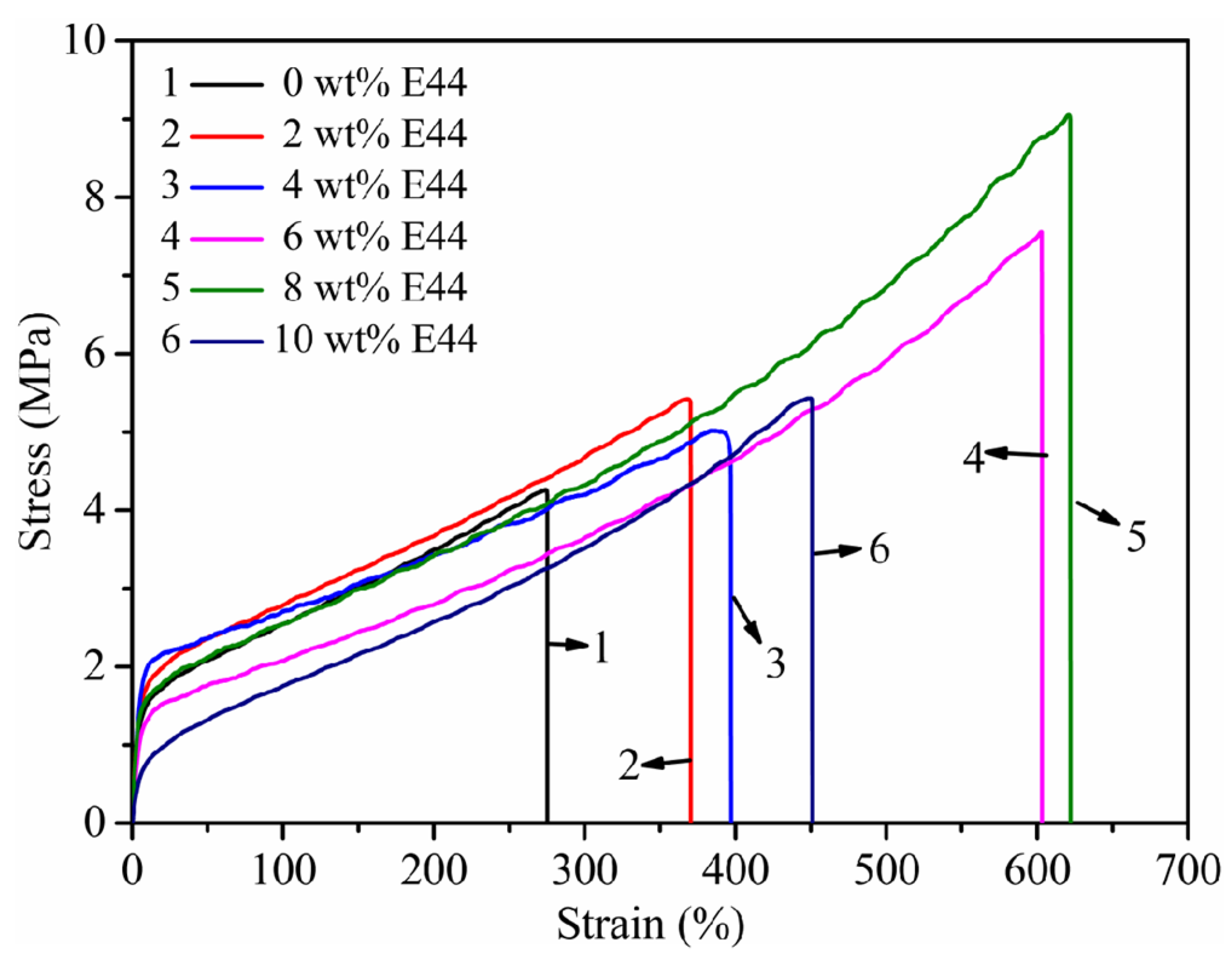
3.7. Mechanical Properties
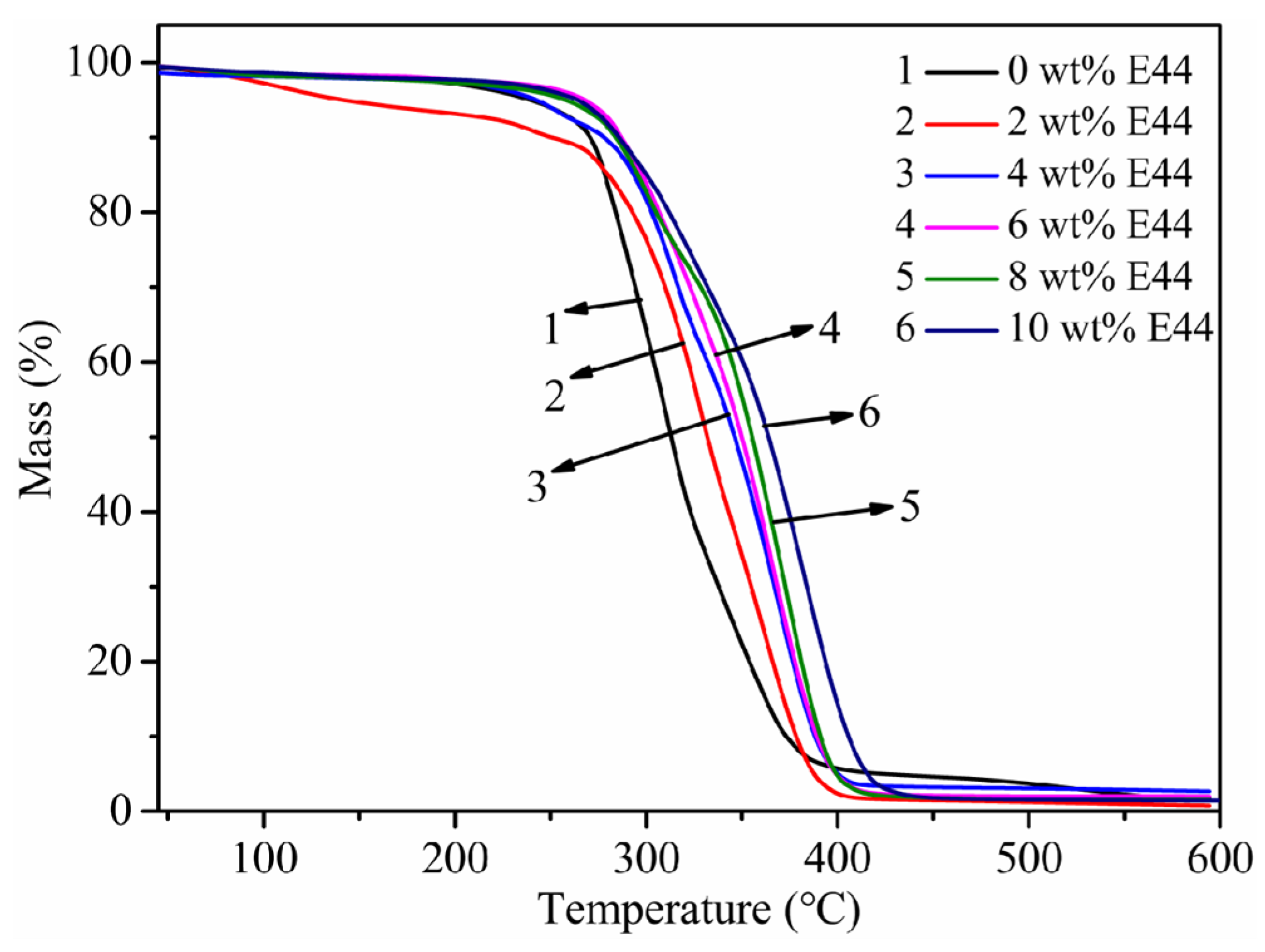
3.8. Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Im, D.; Jin, Y.; Jang, S.H.; Lim, K.T.; Lee, W.K. Synthesis and Properties of Eco-Friendly Coatings Using Waterborne Polyaspartic and Blocked Isocyanate. Mol. Cryst. Liq. Cryst. 2019, 688, 1–6. [Google Scholar] [CrossRef]
- Mishra, V.; Desai, J.; Patel, K.I. High-Performance Waterborne UV-Curable Polyurethane Dispersion Based on Thiol-Acrylate/Thiol-Epoxy Hybrid Networks. J. Coat. Technol. Res. 2017, 14, 1069–1081. [Google Scholar] [CrossRef]
- Jin, Z.M.; Guo, W.L.; Gao, C.H. Study on Synthesis and Properties of Waterborne Polyurethane Modified by Epoxy Resin. Appl. Mech. Mater. 2013, 395–396, 423–426. [Google Scholar] [CrossRef]
- Joshi, M.; Jauhari, S. Glass Reinforcement of Various Epoxy Resins-Polyurea Systems. J. Materi. Eng. Perform. 2012, 21, 1346–1351. [Google Scholar] [CrossRef]
- Kausar, A. Shape Memory Interpenetrating Network Hybrids of Epoxy/Poly(Urea-Amide) and Organic Nanoparticle. J. Chin. Adv. Mater. Soc. 2017, 5, 158–173. [Google Scholar] [CrossRef]
- Wen, X.; Mi, R.; Huang, Y.; Cheng, J.; Pi, P.; Yang, Z. Crosslinked Polyurethane-Epoxy Hybrid Emulsion with Core-Shell Structure. J. Coat. Technol. Res. 2010, 7, 373–381. [Google Scholar] [CrossRef]
- Kalmagambetova, A.S.; Bogoyavlenskaya, T.A. Effect of the Modification of Polyurea by Glass-Microspheres on Its Performance. Glass Ceram. 2020, 77, 19–21. [Google Scholar] [CrossRef]
- Zhao, X.W.; Peng, J.; Gao, S.H.; Zhu, K.Y.; Zhao, Y.H.; Li, X.H.; Yuan, X.Y. Self-Healing Anti-Icing Coatings Prepared from PDMS Polyurea. Sci. China Technol. Sci. 2021, 64, 1535–1543. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wen, S.; Li, S.; Chen, Y.; Wang, J.; Wang, Y.; Wang, C.; Yu, X.; Mao, Y. Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance. Coatings 2021, 11, 251. [Google Scholar] [CrossRef]
- Arzhakov, M.S.; Yakovlev, P.P.; Yarysheva, A.Y.; Lopatkin, A.I.; Yaroslavov, A.A. Mechanical Properties of Insulation Coatings Based on Modified Polyurea. Dokl. Phys. Chem. 2021, 497, 25–27. [Google Scholar] [CrossRef]
- Yu, P.; Li, G.; Zhang, L.; Zhao, F.; Chen, S.; Dmitriev, A.I.; Zhang, G. Regulating Microstructures of Interpenetrating Polyurethane-Epoxy Networks towards High-Performance Water-Lubricated Bearing Materials. Tribol. Int. 2019, 131, 454–464. [Google Scholar] [CrossRef]
- Zhan, B.; Li, Q.S.; Hong, W.; Xia, Y.Z.; Wang, G.W.; Xing, G.Z. Preparation and Properties of Epoxy Resin Modified Waterborne Polyurethane. Appl. Mech. Mater. 2013, 320, 607–610. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Li, N.; Huang, G.W.; Qu, C.B.; Xiao, H.M. Cryogenic Mechanical Properties of Epoxy Resin Toughened by Hydroxyl-Terminated Polyurethane. Polym. Test. 2019, 74, 45–56. [Google Scholar] [CrossRef]
- Patel, J.C.; Nayak, S.K.; Patel, H.S. Studies on Poly(Ester-Urea-Imide)s Containing Epoxy Segment. Int. J. Plast. Technol. 2017, 21, 227–238. [Google Scholar] [CrossRef]
- Li, R.; Leng, Z.; Partl, M.N.; Raab, C. Characterization and Modelling of Creep and Recovery Behaviour of Waterborne Epoxy Resin Modified Bitumen Emulsion. Mater. Struct. 2021, 54, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Kang, S.; Zhang, X. Synthesis and characterization of novel epoxy-modified waterborne polyurethanes and their use in carbon fiber sizing. J. Appl. Polym. Sci. 2012, 125, 3490–3499. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Jiang, W.; Li, S.; Sun, Q.; Zheng, J.; Qiu, J. Improvements of Heat Resistance and Adhesive Property of Condensed Poly-Nuclear Aromatic Resin via Epoxy Resin Modification. Pet. Sci. 2014, 11, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Li, C.; Wang, C.; Jiang, Y.; Liu, Y.; Xie, H. Natural and Acid-Treated Attapulgite Reinforced Soybean Oil-Based Polyurethane/Epoxy Resin Interpenetrating Polymer Networks. J. Therm. Anal. Calorim. 2019, 137, 1189–1198. [Google Scholar] [CrossRef]
- Ji, W.; Song, W.; Zheng, Y.; He, X. Improvement of Method for Determination of Isocyanate Group Content in Polyurethane Prepolymer. Appl. Mech. Mater. 2013, 303–306, 2533–2536. [Google Scholar] [CrossRef]
- Mao, L.; Shen, H.; Han, W.; Chen, L.; Li, J.; Tang, Y. Hybrid Polyurethane and Silane Sized Carbon Fibre/Epoxy Composites with Enhanced Impact Resistance. Compos. Part. A-Appl. S. 2019, 118, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Ma, J.; Huang, L.; Huang, L.; Chen, X.; Zeng, H.; Ma, S. Preparation and Properties of Corrosion-Resistant Coatings from Waterborne Polyurethane Modified Epoxy Emulsion. Front. Mater. 2019, 6, 185. [Google Scholar] [CrossRef] [Green Version]
- Sarafrazi, M.; Hamadanian, M.; Ghasemi, A.R. Optimize Epoxy Matrix with RSM/CCD Method and Influence of Multi-Wall Carbon Nanotube on Mechanical Properties of Epoxy/Polyurethane. Mech. Mater. 2019, 138, 103154. [Google Scholar] [CrossRef]
- Joshi, M.; Jauhari, S.; Desai, K.R. Studies on Heterocyclic Polyurea-Epoxy Resin Condensation Products. Res. Chem. Intermed. 2012, 38, 269–281. [Google Scholar] [CrossRef]
- Kausar, A. Interpenetrating Polymer Network and Nanocomposite IPN of Polyurethane/Epoxy: A Review on Fundamentals and Advancements. Polym. Plast. Technol. Mater. 2019, 58, 691–706. [Google Scholar] [CrossRef]
- He, L.; Attard, T.L.; Zhou, H.; Brooks, A. Integrating Energy Transferability into the Connection-Detail of Coastal Bridges Using Reinforced Interfacial Epoxy-Polyurea Reaction Matrix Composite. Compos. Struct. 2019, 216, 89–103. [Google Scholar] [CrossRef]
- Yang, G.; Wang, C.; Fu, H.; Yan, Z.; Yin, W. Waterborne Epoxy Resin-Polyurethane-Emulsified Asphalt: Preparation and Properties. J. Mater. Civ. Eng. 2019, 31, 04019265. [Google Scholar] [CrossRef]
- Bahramnia, H.; Mohammadian Semnani, H.; Habibolahzadeh, A.; Abdoos, H. Epoxy/Polyurethane Nanocomposite Coatings for Anti-Erosion/Wear Applications: A Review. J. Compos. Mater. 2020, 54, 3189–3203. [Google Scholar] [CrossRef]
- Lin, J.; Wu, X.; Zheng, C.; Zhang, P.; Li, Q.; Wang, W.; Yang, Z. A Novolac Epoxy Resin Modified Polyurethane Acylates Polymer Grafted Network with Enhanced Thermal and Mechanical Properties. J. Polym. Res. 2014, 21, 1–10. [Google Scholar] [CrossRef]
- Reghunadhan, A.; Datta, J.; Kalarikkal, N.; Thomas, S. Development of Nanoscale Morphology and Role of Viscoelastic Phase Separation on the Properties of Epoxy/Recycled Polyurethane Blends. Polymer 2017, 117, 96–106. [Google Scholar] [CrossRef]
- Attard, T.L.; He, L.; Zhou, H. Improving Damping Property of Carbon-Fiber Reinforced Epoxy Composite through Novel Hybrid Epoxy-Polyurea Interfacial Reaction. Compos. Part B Eng. 2019, 164, 720–731. [Google Scholar] [CrossRef]
- Van den Brand, J.; Van Gils, S.; Beentjes, P.C.J.; Terryn, H.; Sivel, V.; de Wit, J.H.W. Improving the Adhesion between Epoxy Coatings and Aluminium Substrates. Prog. Org. Coat. 2004, 51, 339–350. [Google Scholar] [CrossRef]
- Kuan, H.C.; Dai, J.B.; Ma, J. A Reactive Polymer for Toughening Epoxy Resin. J. Appl. Polym. Sci. 2010, 115, 3265–3272. [Google Scholar] [CrossRef]
- Zhu, G.L.; Han, D.; Yuan, Y.; Chen, F.; Fu, Q. Improving Damping Properties and Thermal Stability of Epoxy/Polyurethane Grafted Copolymer by Adding Glycidyl POSS. Chin. J. Polym. Sci. 2018, 36, 1297–1302. [Google Scholar] [CrossRef]
- Sun, J.; Fang, H.; Wang, H.; Yang, S.; Xiao, S.; Ding, Y. Waterborne Epoxy-Modified Polyurethane-Acrylate Dispersions with Nano-Sized Core-Shell Structure Particles: Synthesis, Characterization, and Their Coating Film Properties. J. Polym. Eng. 2017, 37, 113–123. [Google Scholar] [CrossRef]
- Jia, L.; Qi, P.; Shi, K.; Liu, X.; Ma, W.; Lin, S.; Zhang, F.; Jia, X.; Cai, Q.; Yang, X. High Performance Epoxy-Based Composites for Cryogenic Use: An Approach Based on Synergetic Strengthening Effects of Epoxy Grafted Polyurethane and MWCNTs-NH2. Compos. Sci. Technol. 2019, 184, 107865. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.; Zhu, Z. Modification of Aqueous Acrylic-Polyurethane via Epoxy Resin Postcrosslinking. J. Appl. Polym. Sci. 2003, 88, 470–475. [Google Scholar] [CrossRef]
- Manjula Dhevi, D.; Jaisankar, S.N.; Pathak, M. Effect of New Hyperbranched Polyester of Varying Generations on Toughening of Epoxy Resin through Interpenetrating Polymer Networks Using Urethane Linkages. Eur. Polym. J. 2013, 49, 3561–3572. [Google Scholar] [CrossRef]


| E44 Content (wt%) | Raw Material (g) | ||||
|---|---|---|---|---|---|
| IPDI | D2000 | E44 | DMBA | TEA | |
| 0 | 7.00 | 12.50 | 0.00 | 0.78 | 0.60 |
| 2 | 7.00 | 12.50 | 0.41 | 0.78 | 0.60 |
| 4 | 7.00 | 12.50 | 0.82 | 0.78 | 0.60 |
| 6 | 7.00 | 12.50 | 1.25 | 0.78 | 0.60 |
| 8 | 7.00 | 12.50 | 1.70 | 0.78 | 0.60 |
| 10 | 7.00 | 12.50 | 2.18 | 0.78 | 0.60 |
| E44 Content (wt%) | Average Particle Size (nm) | Viscosity (KU) | Solid Content (%) | Storage Stability |
|---|---|---|---|---|
| 0 | 204 | 47.1 | 51 | no precipitation |
| 2 | 259 | 61.8 | 54 | no precipitation |
| 4 | 328 | 68.7 | 55 | no precipitation |
| 6 | 416 | 87.7 | 57 | no precipitation |
| 8 | 417 | 90.9 | 56 | no precipitation |
| 10 | 669 | 102.6 | 51 | precipitation |
| E44/wt% | 0 | 2 | 4 | 6 | 8 | 10 |
|---|---|---|---|---|---|---|
| T10%/°C | 251 | 271 | 278 | 288 | 284 | 291 |
| T50%/°C | 312 | 332 | 334 | 350 | 355 | 365 |
| T95%/°C | 313 | 361 | 362 | 365 | 365 | 373 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, J.; Wang, S.; Wen, S.; Chen, K.; Xie, C.; Yuan, C. Study on the Synthesis and Properties of Waterborne Polyurea Modified by Epoxy Resin. Polymers 2022, 14, 2283. https://doi.org/10.3390/polym14112283
Wang J, Wang J, Wang S, Wen S, Chen K, Xie C, Yuan C. Study on the Synthesis and Properties of Waterborne Polyurea Modified by Epoxy Resin. Polymers. 2022; 14(11):2283. https://doi.org/10.3390/polym14112283
Chicago/Turabian StyleWang, Jing, Jihu Wang, Song Wang, Shaoguo Wen, Kaimin Chen, Chen Xie, and Chunping Yuan. 2022. "Study on the Synthesis and Properties of Waterborne Polyurea Modified by Epoxy Resin" Polymers 14, no. 11: 2283. https://doi.org/10.3390/polym14112283
APA StyleWang, J., Wang, J., Wang, S., Wen, S., Chen, K., Xie, C., & Yuan, C. (2022). Study on the Synthesis and Properties of Waterborne Polyurea Modified by Epoxy Resin. Polymers, 14(11), 2283. https://doi.org/10.3390/polym14112283