Surface Modification of γ-Al2O3 Nanoparticles Using Conductive Polyaniline Doped by Dodecylbenzene Sulfonic Acid
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Synthesis of Pure PANDB
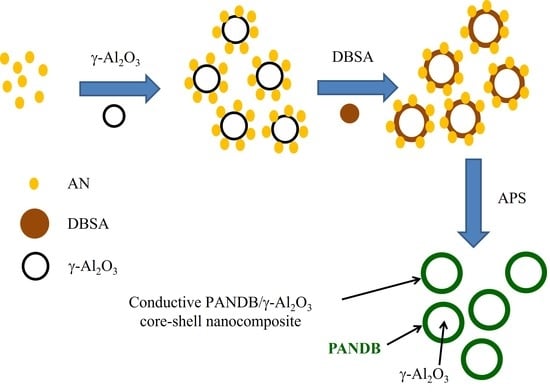
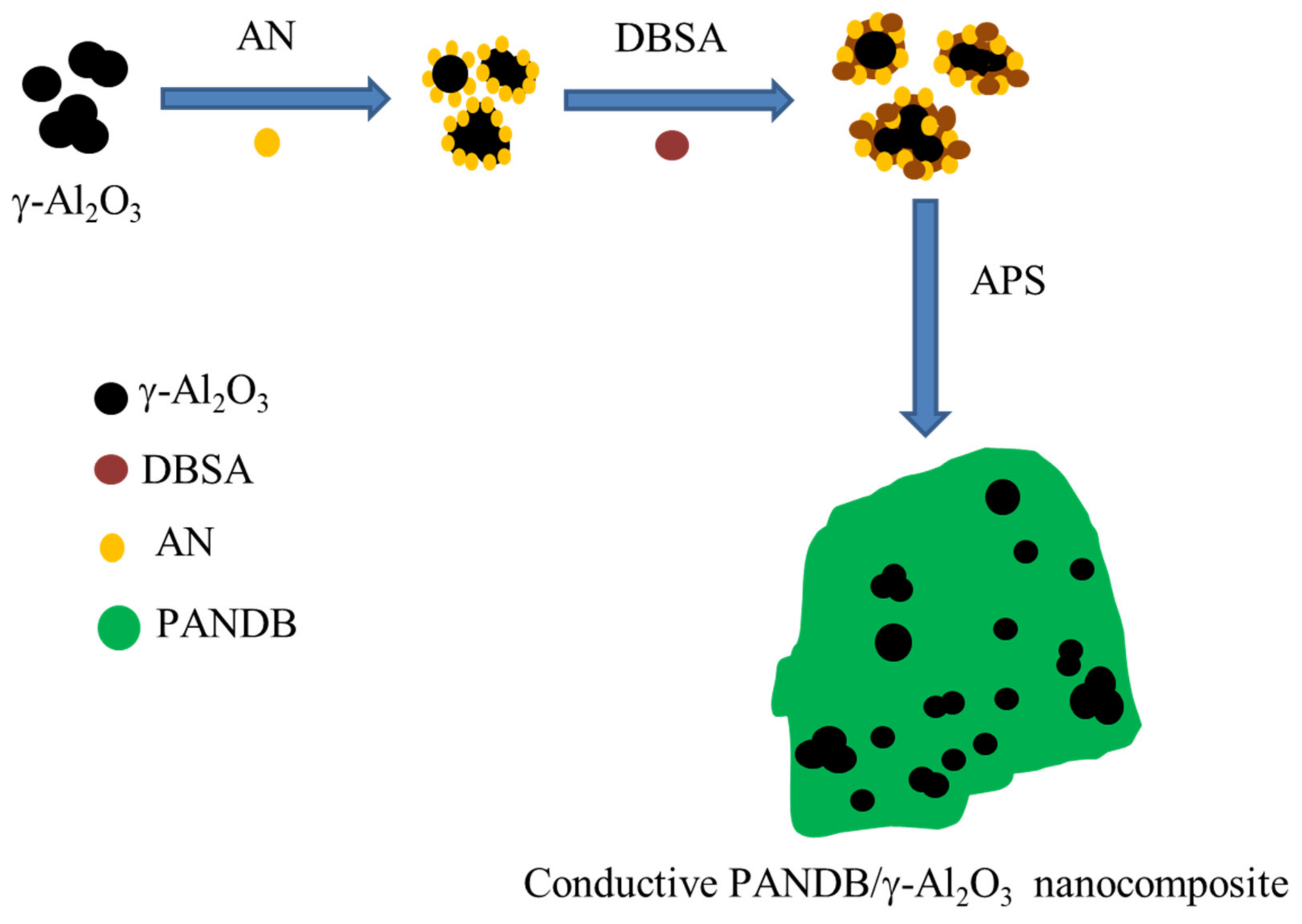
2.1.2. Synthesis of Conductive PANDB/γ-Al2O3 Core–Shell Nanocomposites
2.2. Characterization
2.2.1. Electrical Conductivity Analysis
2.2.2. FTIR Analysis
2.2.3. UV-Vis Analysis
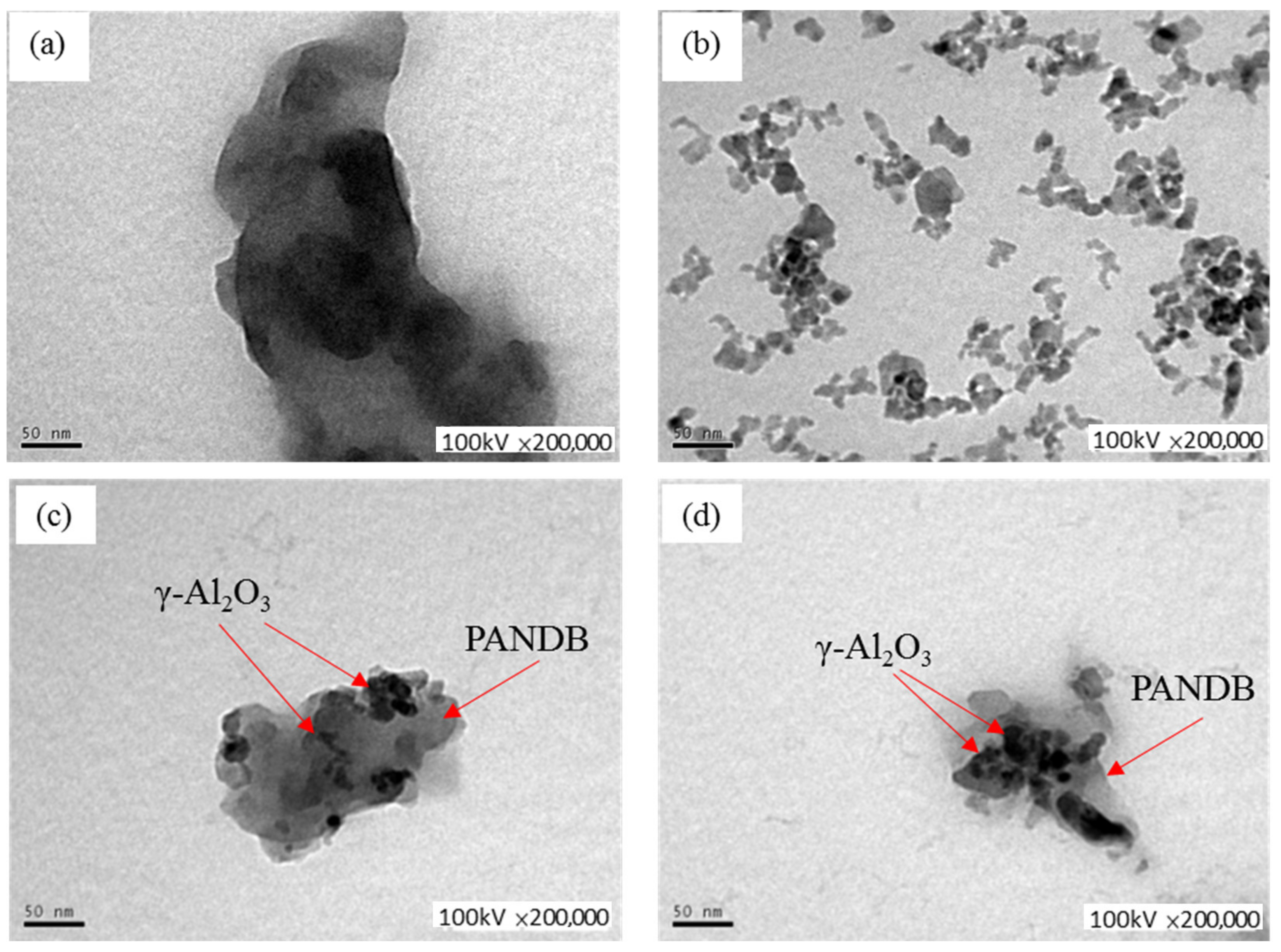
2.2.4. TEM Examination
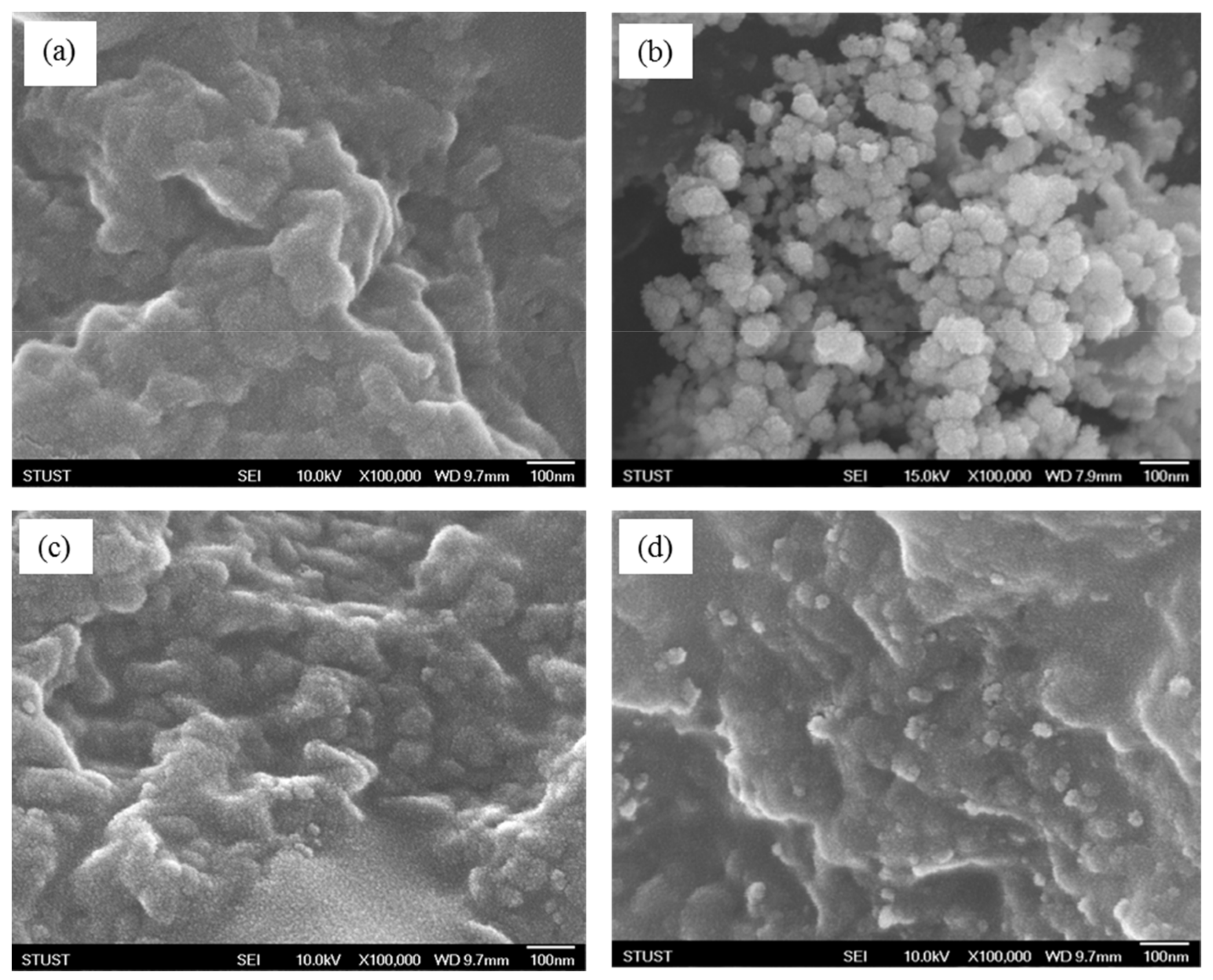
2.2.5. FE-SEM Examination
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.O.; Huang, X.W.; Nelson, W.; Kaner, R.B. Polyaniline nanofibers: Broadening applications for conducting polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Karthik, K.V.; Prasad, S.B.B.; Soni, S.K.; Jeong, H.M.; Raghu, A.V. Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Li, B.; Li, X.P.; Li, W.S.; Wang, Y.Q.; Uchaker, E.; Pei, Y.; Cao, X.; Li, S.; Huang, B.; Cao, G.Z. Mesoporous Tungsten Trioxide Polyaniline Nanocomposite as an Anode Material for High-Performance Lithium-Ion Batteries. Chem. Nano Mat. 2016, 2, 281–289. [Google Scholar] [CrossRef]
- Gao, F.J.; Mu, J.; Bi, Z.X.; Wang, S.; Li, Z.L. Recent advances of polyaniline composites in anticorrosive coatings: A review. Prog. Org. Coat. 2021, 151, 106071. [Google Scholar] [CrossRef]
- Yang, L.Y.; Xu, X.R.; Liu, M.D.; Chen, C.; Cui, J.; Chen, X.; Wu, K.; Sun, D.P. Wearable and flexible bacterial cellulose/polyaniline ammonia sensor based on a synergistic doping strategy. Sens. Actuators B Chem. 2021, 334, 129647. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Prabhakaran, P.V. Oligomeric phthalocyanine modified polyaniline—An electrode material for use in aqueous secondary batteries. Synth. Met. 1998, 97, 141–146. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Kang, S.G.; Lee, G.J.; Joo, J.S.; Chang, S.H. Electrochemical and physical characterization of lithium ionic salt doped polyaniline as a polymer electrode of lithium secondary battery. Synth. Met. 2000, 110, 213–217. [Google Scholar] [CrossRef]
- Parente, A.H.; Marques, E.T., Jr.; Azevedo, W.M.; Diniz, F.B.; Melo, E.H.; Filro, J.L.L. Glucose biosensor using glucose oxidase immobilized in polyaniline. Appl. Biochem. Biotechnol. 1992, 37, 267–273. [Google Scholar] [CrossRef]
- Leite, V.; Dasilva, V.L.; Azevedo, W.M.; Melo, E.H.; Filro, J.L.L. Increasing glucose determination range by flow injection analysis (FIA) using glucose oxidase immobilised on polyaniline. Biotechnol. Tech. 1994, 8, 133–136. [Google Scholar] [CrossRef]
- Li, P.; Tan, T.C.; Lee, J.Y. Corrosion protection of mild steel by electroactive polyaniline coatings. Synth. Met. 1997, 88, 237–242. [Google Scholar] [CrossRef]
- Pud, A.A.; Shapoval, G.S.; Kamarchik, P.; Ogurtsov, N.A.; Gromovaya, V.F.; Myronyuk, I.E.; Kontsur, Y.V. Electrochemical behavior of mild steel coated by polyaniline doped with organic sulfonic acids. Synth. Met. 1999, 107, 111–115. [Google Scholar] [CrossRef]
- Subramaniam, C.K.; Kaiser, A.B.; Gilberd, P.W.; Wessling, B. Electronic transport properties of polyaniline/PVC blends. J. Polym. Sci. Pol. Phys. 1993, 31, 1425–1430. [Google Scholar] [CrossRef]
- Gustafsson, G.; Cao, Y.; Treacy, G.M.; Klavetter, F.; Colaneri, N.; Heeger, A.J. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992, 357, 477–479. [Google Scholar] [CrossRef]
- Yang, Z.; Qiu, M.N.; Yu, Y.; Wen, B.Y.; Cheng, L.L. A Novel Polyaniline-Coated Bagasse Fiber Composite with Core–Shell Heterostructure Provides Effective Electromagnetic Shielding Performance. ACS Appl. Mater. Interf. 2017, 9, 809–818. [Google Scholar]
- Kowsari, E.; Faraghi, G. Ultrasound and ionic-liquid-assisted synthesis and characterization of polyaniline/Y2O3 nanocomposite with controlled conductivity. Ultrason. Sonochem. 2010, 17, 718–725. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Li, H.J.; Zhang, X.M.; Liu, N.K.; Tan, W.L.; Zhang, X.; Zhang, L.L. Facile synthesis of NiCo2O4@Polyaniline core–shell nanocomposite for sensitive determination of glucose. Biosens. Bioelectron. 2016, 75, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Z.; Lu, W.; Huang, T.; Hu, S. Preparation of core-shell structure T-ZnOw/polyaniline composites via graft polymerization. Mater. Chem. Phys. 2009, 115, 258–262. [Google Scholar] [CrossRef]
- Gai, L.; Du, G.; Zuo, Z.; Wang, Y.; Liu, D.; Liu, H. Controlled synthesis of hydrogen Titanate-Polyaniline composite nanowires and their resistance-temperature characteristics. J. Phys. Chem. C 2009, 113, 7610–7615. [Google Scholar] [CrossRef]
- Pan, L.; Pu, L.; Shi, Y.; Song, S.; Xu, Z.; Zhang, R.; Zheng, Y. Synthesis of polyaniline nanotubes with a reactive template of manganese oxide. Adv. Mater. 2007, 19, 461–464. [Google Scholar] [CrossRef]
- Zhang, D.H. Preparation of Core–Shell Structured Alumina–Polyaniline Particles and Their Application for Corrosion Protection. J. Appl. Polym. Sci. 2006, 101, 4372–4377. [Google Scholar] [CrossRef]
- Resan, S.A.; Essa, A.F. Preparation and study of the optical properties for polyaniline-Al2O3 nanocomposite. Mater. Today Proc. 2021, 45, 5819–5822. [Google Scholar] [CrossRef]
- Bekhti, M.A.; Belardja, M.S.E.; Lafiah, M.; Chouli, F.; Benyoucef, A. Enhanced Tailored of Thermal Stability, Optical and Electrochemical Properties of PANI Matrix Containing Al2O3 Hybrid Materials Synthesized through In Situ Polymerization. Polym. Compos. 2021, 42, 6–14. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, Y.C.; Yen, F.S. Synthesis and Characterization of Conducting PANDB/χ-Al2O3 Core-Shell Nanocomposites by In Situ Polymerization. Polymers 2021, 13, 2787. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Ohashi, F.; Kameyama, T. Characterization and thermal degradation studies on polyaniline-intercalated montmorillonite nanocomposites prepared by a solvent-free mechanochemical route. J. Polym. Sci. Polym. Phys. 2005, 43, 2705–2714. [Google Scholar] [CrossRef]
- De León-Almazan, C.M.; Estrada-Moreno, I.A.; Páramo-García, U.; Rivera-Armenta, J.L. Polyaniline/clay nanocomposites. A comparative approach on the doping acid and the clay spacing technique. Synth. Met. 2018, 236, 61–67. [Google Scholar] [CrossRef]
- Yin, W.; Ruckenstein, E. Soluble polyaniline co-doped with dodecyl benzene sulfonic acid and hydrochloric acid. Synth. Met. 2000, 108, 39–46. [Google Scholar] [CrossRef]
- Qi, L.; Xie, A.; Chen, T.; Han, J.; Yu, L.; Zhang, M. Direct access to xylene solution of polyanilines via emulsion polymerization-extraction method facilitating the preparation of conductive film materials. Mater. Lett. 2019, 254, 361–363. [Google Scholar] [CrossRef]
- Cao, Y.; Andreatta, A.; Heeger, A.J.; Smith, P. Influence of chemical polymerization conditions on the properties of polyaniline. Polymers 1989, 30, 2305–2311. [Google Scholar] [CrossRef]
- Chen, C.H. Thermal studies of polyaniline doped with dodecyl benzene sulfonic acid directly prepared via aqueous dispersions. J. Polym. Res. 2002, 9, 195–200. [Google Scholar] [CrossRef]
- Haba, Y.; Segal, E.; Narkis, M.; Titelman, G.I.; Siegmann, A. Polyaniline–DBSA/polymer blends prepared via aqueous dispersions. Synth. Met. 2000, 110, 189–193. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, M. Composite films of nanostructured polyaniline with poly (vinyl alcohol). Synth. Met. 2002, 128, 83–89. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, T.; Kan, J.Q. Effect of methanol on morphology of polyaniline. Eur. Polym. J. 2007, 43, 395–402. [Google Scholar] [CrossRef]
- Abdiryim, T.; Zhang, X.G.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Gao, Y.; Shan, D.; Cao, F.; Gong, J.; Li, X.; Ma, H.Y.; Su, Z.M.; Qu, L.Y. Silver/polyaniline composite nanotubes: One-step synthesis and electrocatalytic activity for neurotransmitter dopamine. J. Phys. Chem. C 2009, 113, 15175–15181. [Google Scholar] [CrossRef]




| Weight Ratio of AN/γ-Al2O3 | Conductivity of PANDB and PANDB/γ-Al2O3 Nanocomposites (S/cm) |
|---|---|
| Pure γ-Al2O3 | - |
| Pure PANDB | 0.82 |
| 3/1 | 0.72 |
| 3/2 | 0.58 |
| 3/3 | 0.57 |
| 3/4 | 0.55 |
| 3/5 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lin, Y.-C.; Lin, H.-M. Surface Modification of γ-Al2O3 Nanoparticles Using Conductive Polyaniline Doped by Dodecylbenzene Sulfonic Acid. Polymers 2022, 14, 2232. https://doi.org/10.3390/polym14112232
Chen C-H, Lin Y-C, Lin H-M. Surface Modification of γ-Al2O3 Nanoparticles Using Conductive Polyaniline Doped by Dodecylbenzene Sulfonic Acid. Polymers. 2022; 14(11):2232. https://doi.org/10.3390/polym14112232
Chicago/Turabian StyleChen, Cheng-Ho, Ying-Chen Lin, and Hung-Mao Lin. 2022. "Surface Modification of γ-Al2O3 Nanoparticles Using Conductive Polyaniline Doped by Dodecylbenzene Sulfonic Acid" Polymers 14, no. 11: 2232. https://doi.org/10.3390/polym14112232
APA StyleChen, C.-H., Lin, Y.-C., & Lin, H.-M. (2022). Surface Modification of γ-Al2O3 Nanoparticles Using Conductive Polyaniline Doped by Dodecylbenzene Sulfonic Acid. Polymers, 14(11), 2232. https://doi.org/10.3390/polym14112232