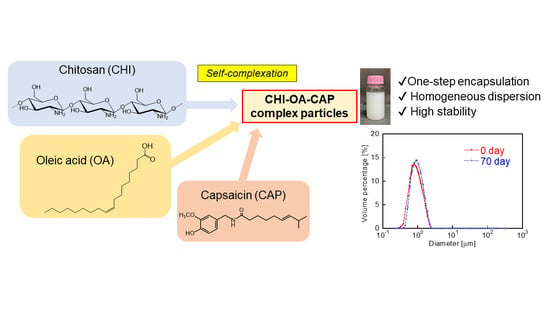
One-Step Encapsulation of Capsaicin into Chitosan–Oleic Acid Complex Particles: Evaluation of Encapsulation Ability and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
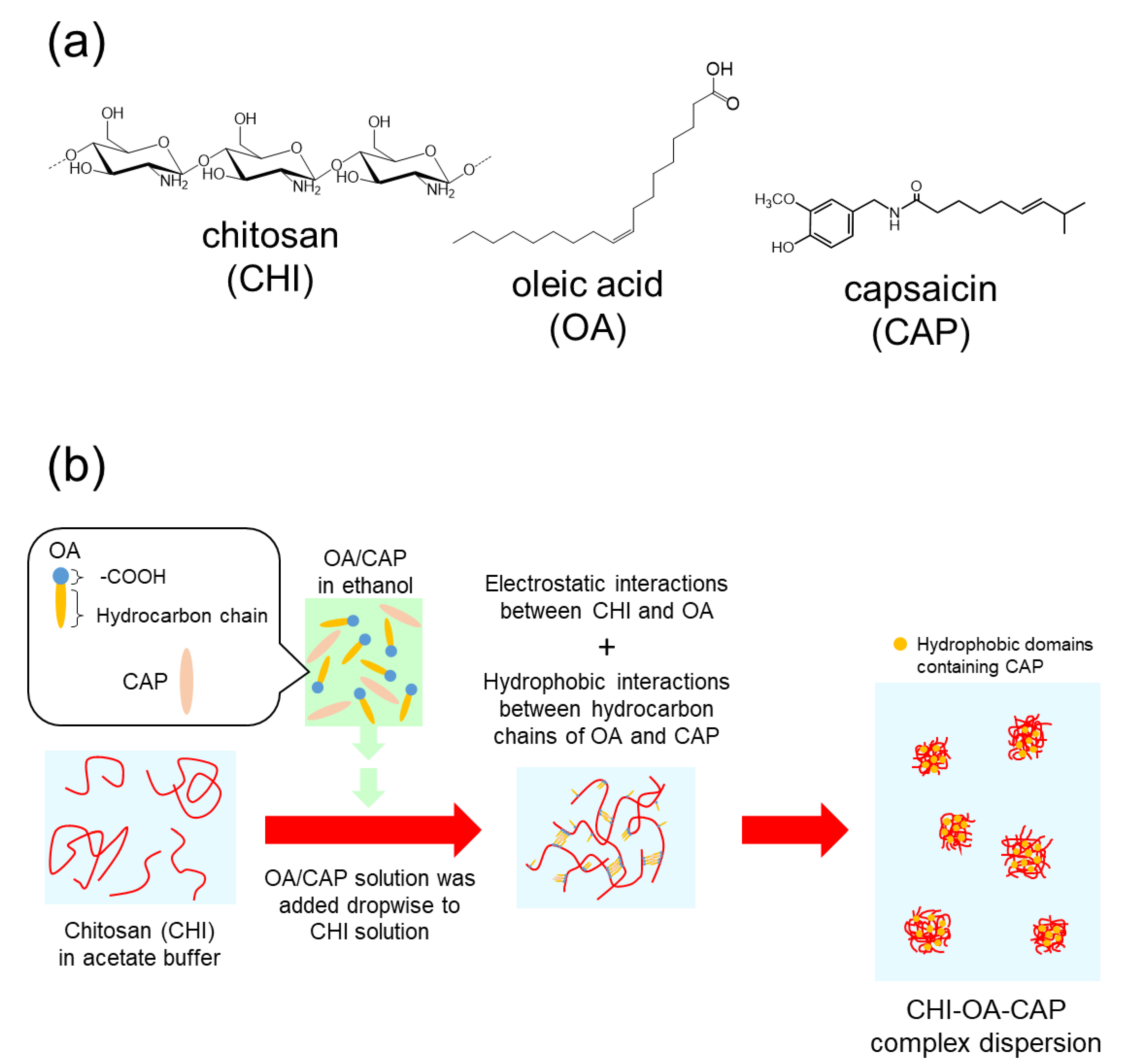
2.2. Encapsulation of CAP into CHI–OA Complex Particles
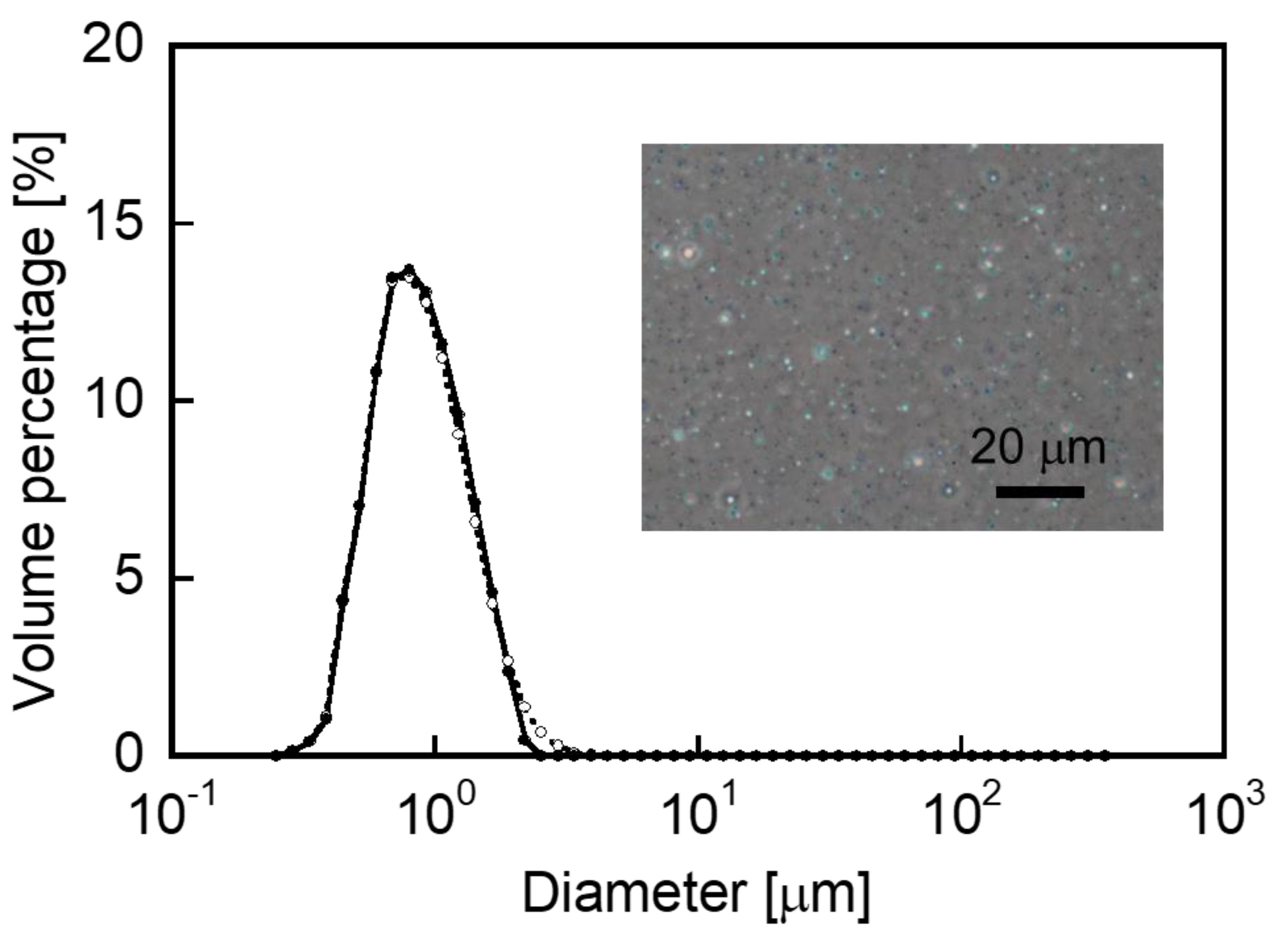
2.3. Measurement of Particle Diameter
2.4. Determination of the Amount of CAP Encapsulated
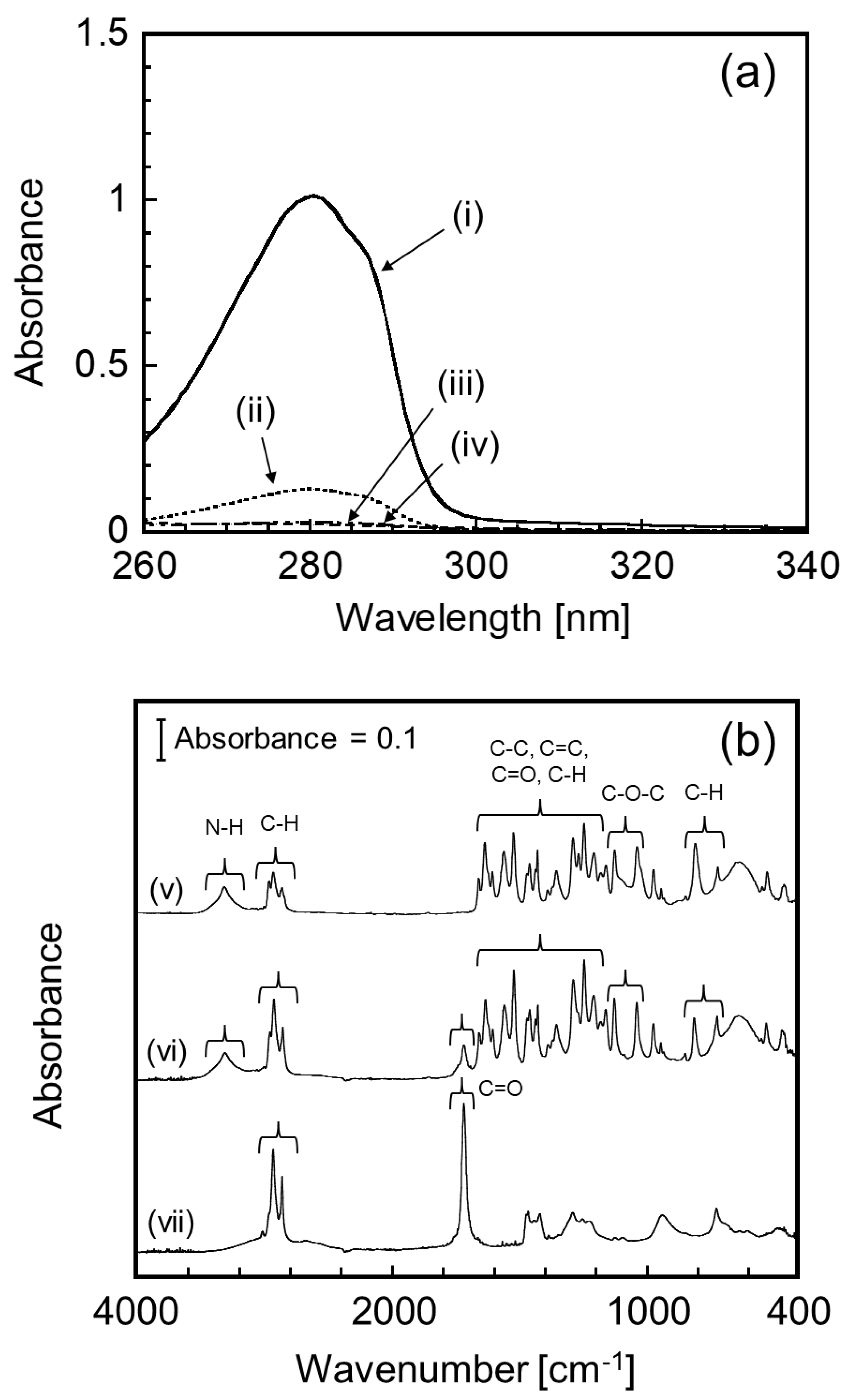
2.5. Fourier Transform Infrared Spectroscopic Analysis
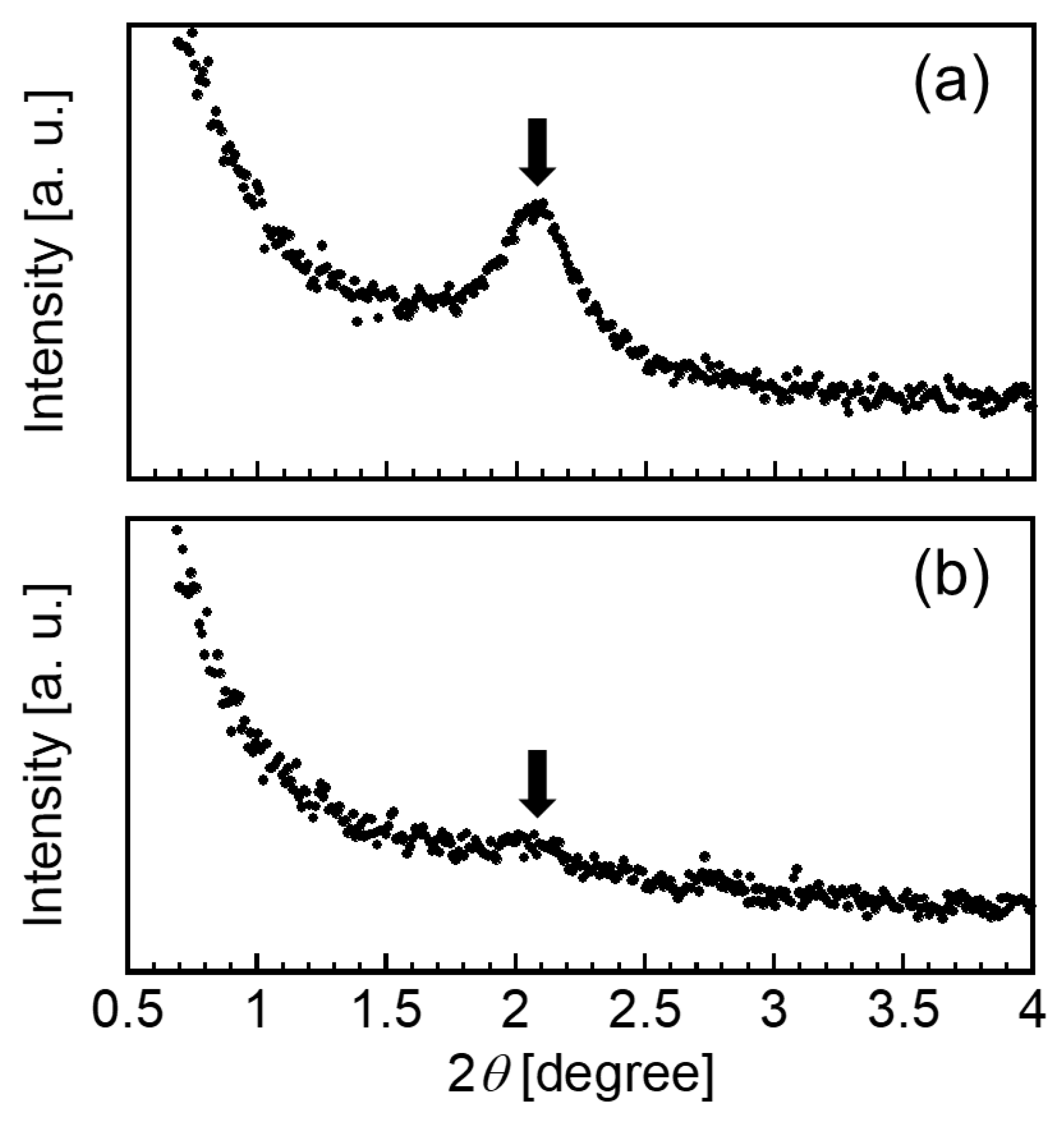
2.6. Small-Angle X-ray Scattering Measurements
2.7. Stability of CHI–OA–CAP Complex Particles
3. Results and Discussion
3.1. Preparation of the CAP-Loaded CHI–OA Complex Particle Suspension
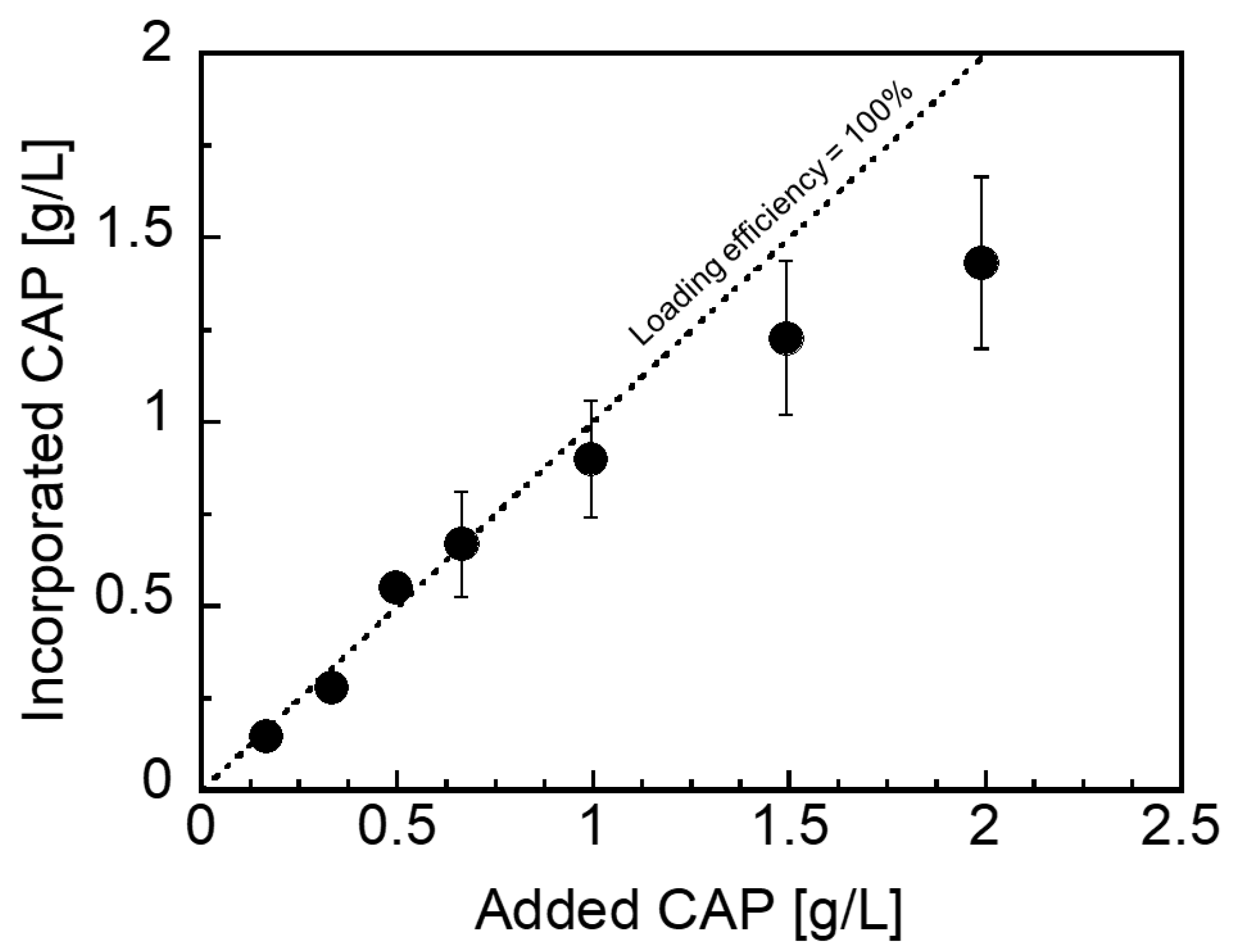
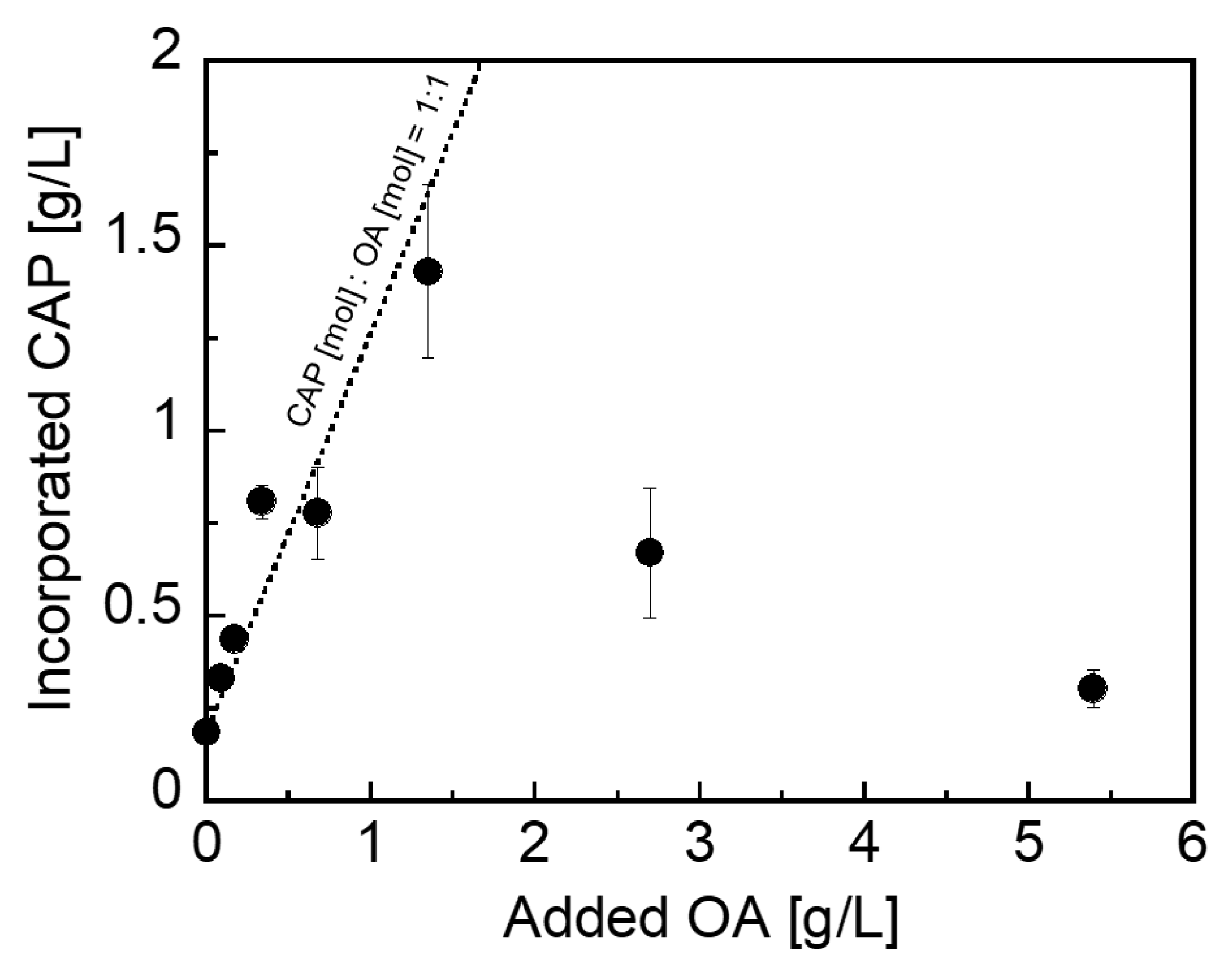
3.2. Loading Characteristics of CAP
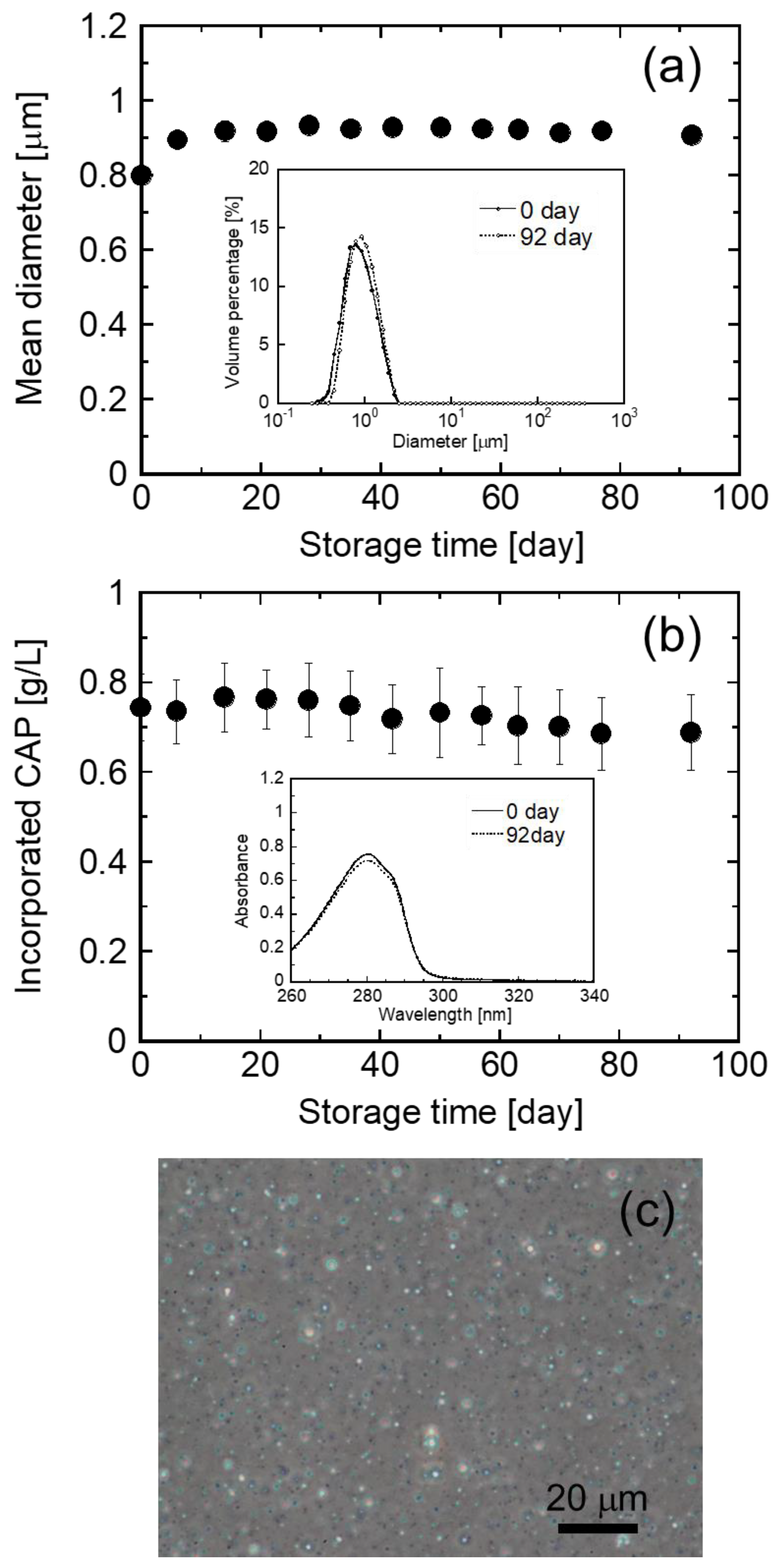
3.3. Evaluation of Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, K.; Sambaiah, K.; Chandrasekhara, N. Spices as beneficial hypolipidemic food adjuncts: A review. Food Rev. Int. 2004, 20, 187–220. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 194, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin—The major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2828–2860. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, A.; Hamishehkar, H.; Ehsani, A.; Ghasempour, Z.; Kia, E.M. Applications of capsaicin in food industry: Functionality, utilization, and stabilization. Crit. Rev. Food Sci. Nutri. 2021. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Studies on the in vitro absorption of spice principles—Curcumin, capsaicin and piperine in rat intestines. Food Chem. Toxicol. 2007, 45, 1437–1442. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zheng, Q.; Wang, M.; Deng, W.; Li, Q.; Firempong, C.K.; Wang, S.; Tong, S.; Xu, X.; et al. In vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. J. Sci. Food Agric. 2015, 95, 2678–2685. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.; Zhang, J.; Peng, W.; Firempong, C.K.; Deng, W.; Wang, Q.; Wang, S.; Shi, F.; Yu, J.; et al. Improved oral bioavailability of capsaicin via liposomal nanoformulation: Preparation, in vitro drug release and pharmacokinetics in rats. Arch. Pharm. Res. 2015, 38, 512–521. [Google Scholar] [CrossRef]
- Giri, T.K.; Mukherjee, P.; Barman, T.K.; Maity, S. Nano-encapsulation of capsaicin on lipid vesicle and evaluation of their hepatocellular protective effect. Int. J. Biol. Macromol. 2016, 88, 236–243. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.S.; Azzam, H.; Aburjai, T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Dev. Ther. 2015, 9, 4209–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.R.; Gao, S.Q.; Niu, X.Q.; Li, L.J.; Ying, X.Y.; Hu, Z.J.; Gao, J.Q. Capsaicin-loaded nanolipoidal carriers for topical application: Design, characterization, and in vitro/in vivo evaluation. Int. J. Nanomed. 2017, 12, 3881–3898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Zhang, S.; Liu, X.; Xiao, C. Fabrication of capsaicin emulsions: Improving the stability of the system and relieving the irritation to the gastrointestinal tract of rats. J. Sci. Food Agric. 2020, 100, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Teng, F.; Tian, T.; Sami, R.; Wu, C.; Zhu, Y.; Zheng, L.; Jiang, L.; Wang, Z.; Li, Y. The impact of soy protein isolate-dextran conjugation on capsicum oleoresin (Capsicum annuum L.) nanoemulsions. Food Hydrocoll. 2020, 108, 105818. [Google Scholar] [CrossRef]
- Wu, X.; Xu, N.; Cheng, C.; McClements, J.D.; Chen, X.; Zou, L.; Liu, W. Encapsulation of hydrophobic capsaicin within the aqueous phase of water-in-oil high internal phase emulsions: Controlled release, reduced irritation, and enhanced bioaccessibility. Food Hydrocoll. 2022, 123, 107184. [Google Scholar] [CrossRef]
- Xing, F.; Cheng, G.; Yang, B.; Ma, L. Microencapsulation of capsaicin by the complex coacervation of gelatin, acacia and tannins. J. Appl. Polym. Sci. 2004, 91, 2669–2675. [Google Scholar] [CrossRef]
- Xing, F.; Cheng, G.; Yi, K.; Ma, L. Nanoencapsulation of capsaicin by complex coacervation of gelatin, acacia, and tannins. J. Appl. Polym. Sci. 2005, 96, 2225–2229. [Google Scholar] [CrossRef]
- Wang, J.C.; Chen, S.H.; Xu, Z.C. Synthesis and properties research on the nanocapsulated capsaicin by simple coacervation method. J. Dispers. Sci. Technol. 2008, 29, 687–695. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Chen, S. Preparation and properties of nanocapsulated capsaicin by complex coacervation method. Chem. Eng. Commun. 2010, 197, 919–933. [Google Scholar] [CrossRef]
- De Freitas, G.B.L.; De Almeida, D.J.; Carraro, E.; Kerppers, I.I.; Martins, G.A.G.; Mainardes, R.M.; Khalil, N.M.; Messias-Reason, I.J.T. Formulation, characterization, and in vitro/in vivo studies of capsaicin-loaded albumin nanoparticles. Mater. Sci. Eng. C 2018, 93, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S. Preparation and characterization of microcapsules containing capsaicin. J. Appl. Polym. Sci. 2010, 116, 2234–2241. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Chen, S.; Lou, J. Microencapsulation of capsaicin by solvent evaporation method and thermal stability study of microcapsules. Colloid J. 2013, 75, 26–33. [Google Scholar] [CrossRef]
- Almeida, M.A.; Nadal, J.M.; Grassiolli, S.; Paludo, K.S.; Zawadzki, S.F.; Cruz, L.; Paula, J.P.; Farago, P.V. Enhanced gastric tolerability and improved anti-obesity effect of capsaicinoids-loaded PCL microparticles. Mater. Sci. Eng. C 2014, 40, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Günel, Z.; Varhan, E.; Koç, M.; Topuz, A.; Sahin-Nadeem, H. Production of pungency-suppressed capsaicin microcapsules by spray chilling. Food Biosci. 2021, 40, 100918. [Google Scholar] [CrossRef]
- Hudita, A.; Galateanu, B.; Costache, M.; Negrei, C.; Ion, R.M.; Iancu, L.; Ginghina, O. In vitro cytotoxic protective effect of alginate-encapsulated capsaicin might improve skin side effects associated with the topical application of capsaicin. Molecules 2021, 26, 1455. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Valle-Gallego, A.; Stefani, R.; Menchicchi, B.; David, L.; Rochas, C.; Santander-Ortega, M.J.; Alonso, M.J. Chitosan-based nanocapsules: Physical characterization, stability in biological media and capsaicin encapsulation. Colloid Polym. Sci. 2012, 290, 1423–1434. [Google Scholar] [CrossRef]
- Kaiser, M.; Kirsch, B.; Hauser, H.; Schneider, D.; Seuß-Baum, I.; Goycoolea, F.M. In vitro and sensory evaluation of capsaicin-loaded nanoformulations. PLoS ONE 2015, 10, e0141017. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, M.; Pereira, S.; Pohl, L.; Ketelhut, S.; Kemper, B.; Gorzelanny, C.; Galla, H.J.; Moerschbacher, B.M.; Goycoolea, F.M. Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci. Rep. 2015, 5, 10048. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sun, C.; Shi, F.; Firempong, C.K.; Yu, J.; Xu, X.; Zhang, W. Preparation, characterization, and pharmacokinetics study of capsaicin via hydroxypropyl-beta-cyclodextrin encapsulation. Pharm. Biol. 2016, 54, 130–138. [Google Scholar] [CrossRef]
- Sánchez-Segura, L.; Ochoa-Alejo, N.; Carriles, R.; Zavala-Garciá, L.E. Development of bovine serum albumin–capsaicin nanoparticles for biotechnological applications. Appl. Nanosci. 2018, 8, 1877–1886. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Bonaccorso, A.; Musumeci, T.; Ruozi, B.; Pignatello, R.; Carbone, C.; Parenti, C.; Chiechio, S. Lipid nanoparticle inclusion prevents capsaicin-induced TRPV1 defunctionalization. Pharmaceutics 2020, 12, 339. [Google Scholar] [CrossRef]
- Isaschar-Ovdat, S.; Shani-Levi, C.; Lesmes, U. Capsaicin stability and bio-accessibility affected by complexation with high-amylose corn starch (HACS). Food Funct. 2021, 12, 6992–7000. [Google Scholar] [CrossRef]
- Romano, A.; Lajterer, C.; Shpigelman, A.; Lesmes, U. Bovine alpha-lactalbumin assemblies with capsaicin: Formation, interactions, loading and physiochemical characterization. Food Chem. 2021, 352, 129306. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Engelberg, Y.; Shani-Levi, C.; Lesmes, U. Bovine alpha-lactalbumin particulates for controlled delivery: Impact of dietary fibers on stability, digestibility, and gastro-intestinal release of capsaicin. Food Hydrocoll. 2022, 128, 107536. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.; Liang, S.; Lu, Y.; Zheng, J.; Zhang, J.; Zhang, G.; Li, W.; Jiang, H. Encapsulation of capsaicin in whey protein and OSA-modified starch using spray-drying: Physicochemical properties and its stability. Foods 2022, 11, 612. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kobayashi, I.; Chuah, A.M.; Nakajima, M.; Ichikawa, S. Formulation and stabilization of nano-/microdispersion systems using naturally occurring edible polyelectrolytes by electrostatic deposition and complexation. Adv. Colloid Interface Sci. 2015, 226, 86–100. [Google Scholar] [CrossRef]
- Becker, A.L.; Johnson, A.P.R.; Caruso, F. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small 2010, 6, 1836–1852. [Google Scholar] [CrossRef]
- Pan, H.M.; Yu, H.; Guigas, G.; Fery, A.; Weiss, M.; Patzel, V.; Trau, D. Engineering and design of polymeric shells: Inwards interweaving polymers as multilayer nanofilm, immobilization matrix, or chromatography resins. ACS Appl. Mater. Interfaces 2017, 9, 5447–5456. [Google Scholar] [CrossRef]
- Ichikawa, S.; Iwamoto, S.; Watanabe, J. Formation of biocompatible nanoparticles by self-assembly of enzymatic hydrolysates of chitosan and carboxymethyl cellulose. Biosci. Biotechnol. Biochem. 2005, 69, 1637–1642. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, J.; Iwamoto, S.; Ichikawa, S. Entrapment of some compounds into biocompatible nano-sized particles and their releasing properties. Colloids Surf. B Biointerface 2005, 42, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Kawauchi, Y.; Moriyoshi, R.; Shino, H.; Suzuki, T.; Ichikawa, S.; Kobayashi, I.; Uemura, K.; Kanazawa, A. Biocompatible homogeneous particle formation via the self-complexation of chitosan with oleic acid and its application as an encapsulation material for a water-insoluble compound. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126808. [Google Scholar] [CrossRef]
- Claesson, P.M.; Ninham, B.W. pH-dependent interactions between adsorbed chitosan layers. Langmuir 1992, 8, 1406–1412. [Google Scholar] [CrossRef]
- Cistola, P.; Hamilton, J.A.; Jackson, D.; Small, D.M. Ionization and phase behavior of fatty acids in water: Application of the Gibbs phase rule. Biochemistry 1988, 27, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Goto, A.; Yoshioka, H.; Goto, R.; Morigaki, K.; Walde, P. Electron spin resonance study of the pH-induced transformation of micelles to vesicles in an aqueous oleic acid/oleate system. Langmuir 2001, 17, 4223–4231. [Google Scholar] [CrossRef]
- Wang, W.; Bo, S.; Li, S.; Qin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef]
- Shimizu, N.; Mori, T.; Igarashi, N.; Ohta, H.; Nagatani, Y.; Kosuge, T.; Ito, K. Refurbishing of small-angle X-ray scattering beamline, BL-6A at the photon factory. J. Phys. Conf. Ser. 2013, 425, 202008. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Igarashi, N.; Mori, T.; Saijo, S.; Ohta, H.; Nagatani, Y.; Kosuge, T.; Shimizu, N. Upgrade of small angle X-ray scattering beamline BL-6A at the photon factory. AIP Conf. Proc. 2016, 1741, 030018. [Google Scholar] [CrossRef] [Green Version]
- PF Small-Angle X-ray Scattering Beamline Home Page. Available online: http://pfwww.kek.jp/saxs/SAngler.html (accessed on 4 April 2022).
- Zhang, R.; Chen, G.; Yang, B.; Wu, Y.; Du, M.; Kan, J. Insights into the stability of carotenoids and capsaicinoids in water-based or oil-based chili systems at different processing treatments. Food Chem. 2021, 342, 128308. [Google Scholar] [CrossRef]
- Jia, L.; Yu, L.; Li, R.; Yan, X.; Zhang, Z. Synthesis and solution behavior of hydrophobically associating polyacrylamide containing capsaicin-like moieties. J. Appl. Polym. Sci. 2013, 130, 1794–1804. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Yan, Y.; Hu, J.; Wang, H.; Cai, Y.; Qu, J. Preparation and characterization of a novel antibacterial acrylate polymer composite modified with capsaicin. Chin. J. Chem. Eng. 2019, 27, 3043–3052. [Google Scholar] [CrossRef]
- Kogure, K.; Goto, S.; Nishimura, M.; Yasumoto, M.; Abe, K.; Ohiwa, C.; Sassa, H.; Kusumi, T.; Terada, H. Mechanism of potent antiperoxidative effect of capsaicin. Biochim. Biophys. Acta 2002, 1573, 84–92. [Google Scholar] [CrossRef]
- Okada, Y.; Tanaka, K.; Sato, E.; Okajima, H. Kinetics and antioxidative sites of capsaicin in homogeneous solution. J. Am. Oil Chem. Soc. 2010, 87, 1397–1405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroiwa, T.; Higuchi, Y. One-Step Encapsulation of Capsaicin into Chitosan–Oleic Acid Complex Particles: Evaluation of Encapsulation Ability and Stability. Polymers 2022, 14, 2163. https://doi.org/10.3390/polym14112163
Kuroiwa T, Higuchi Y. One-Step Encapsulation of Capsaicin into Chitosan–Oleic Acid Complex Particles: Evaluation of Encapsulation Ability and Stability. Polymers. 2022; 14(11):2163. https://doi.org/10.3390/polym14112163
Chicago/Turabian StyleKuroiwa, Takashi, and Yoshiki Higuchi. 2022. "One-Step Encapsulation of Capsaicin into Chitosan–Oleic Acid Complex Particles: Evaluation of Encapsulation Ability and Stability" Polymers 14, no. 11: 2163. https://doi.org/10.3390/polym14112163
APA StyleKuroiwa, T., & Higuchi, Y. (2022). One-Step Encapsulation of Capsaicin into Chitosan–Oleic Acid Complex Particles: Evaluation of Encapsulation Ability and Stability. Polymers, 14(11), 2163. https://doi.org/10.3390/polym14112163