Preparation of Rosin-Based Composite Membranes and Study of Their Dencichine Adsorption Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
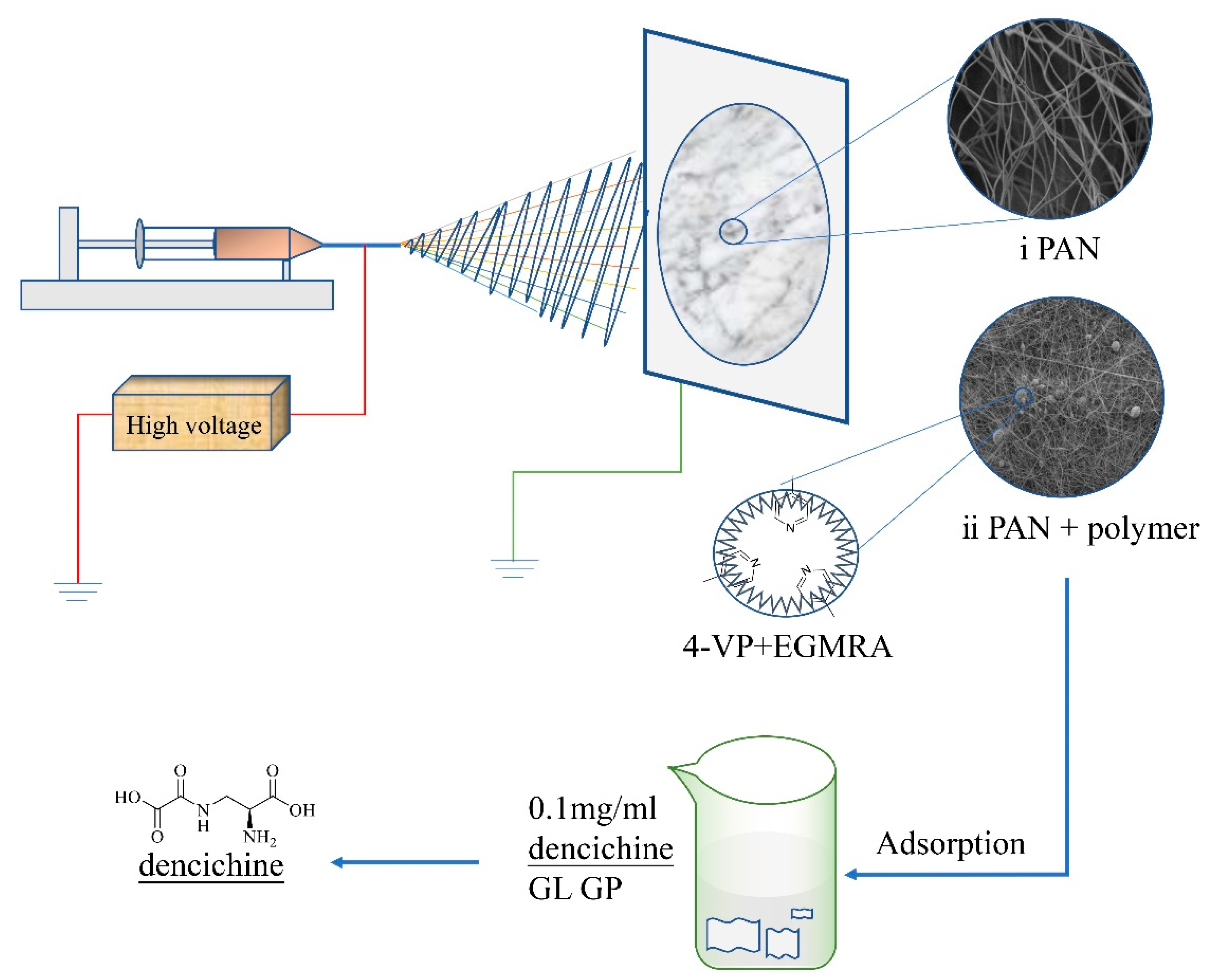
2.2. Preparation of RPMs
2.3. Preparation of RCMs
2.4. Characterization of RCMs and RPMs
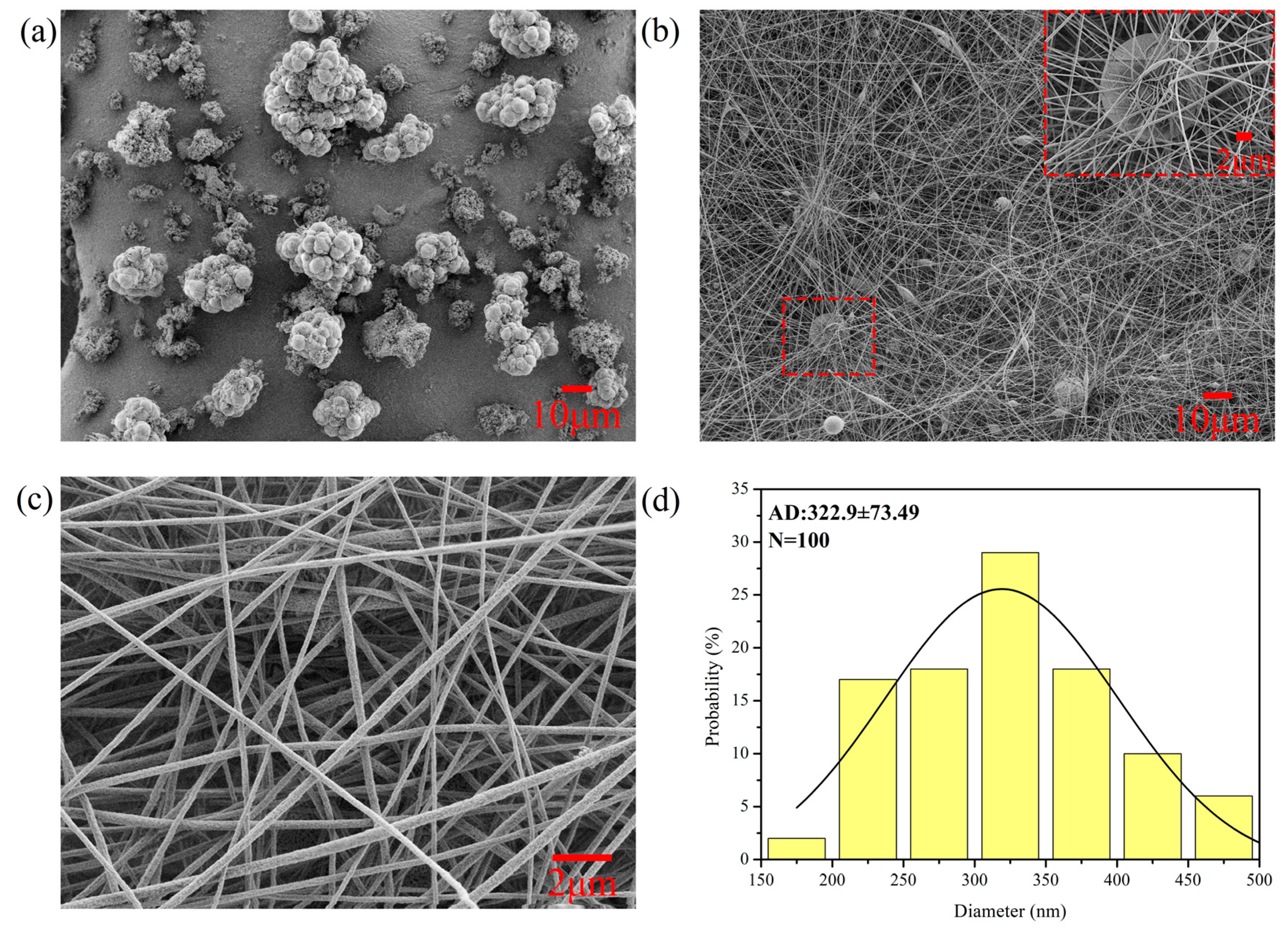
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Dynamic Mechanical Analyzer
2.5. HPLC Analysis
2.6. Static Adsorption
2.6.1. Standard Curve of Dencichine
2.6.2. Static Adsorption of Dencichine on RCMs and RPMs
2.6.3. Adsorption of Dencichine on Different Mass RCMs and RPMs
2.6.4. Adsorption Kinetics of Dencichine on RCMs and RPMs
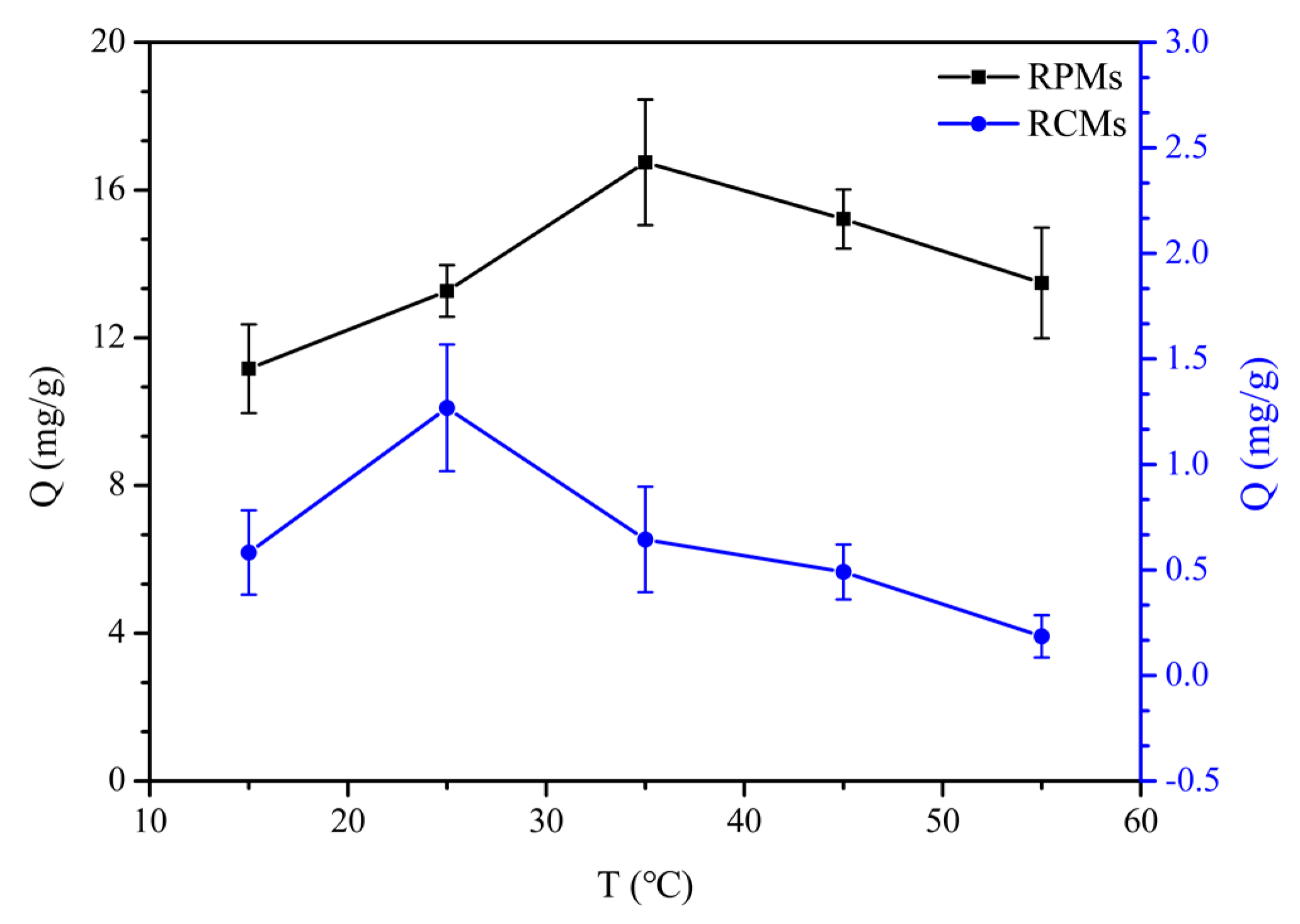
2.6.5. Adsorption Isotherm and Thermodynamics of Dencichine on the RCMs and RPMs
2.6.6. Adsorption of Dencichine on the RCMs and RPMs at Different PH
2.7. Selective Adsorption on the RCMs and RPMs
3. Results and Discussion
3.1. Characterization of the RCMs and RPMs
3.2. Optimization Preparation Conditions of the RCMs
3.3. Adsorption of Dencichine on Different Mass RCMs and RPMs
3.4. Adsorption Kinetics of Dencichine on the RCMs and RPMs
3.5. Adsorption Isotherm and Thermodynamics of Dencichine on the RCMs and RPMs
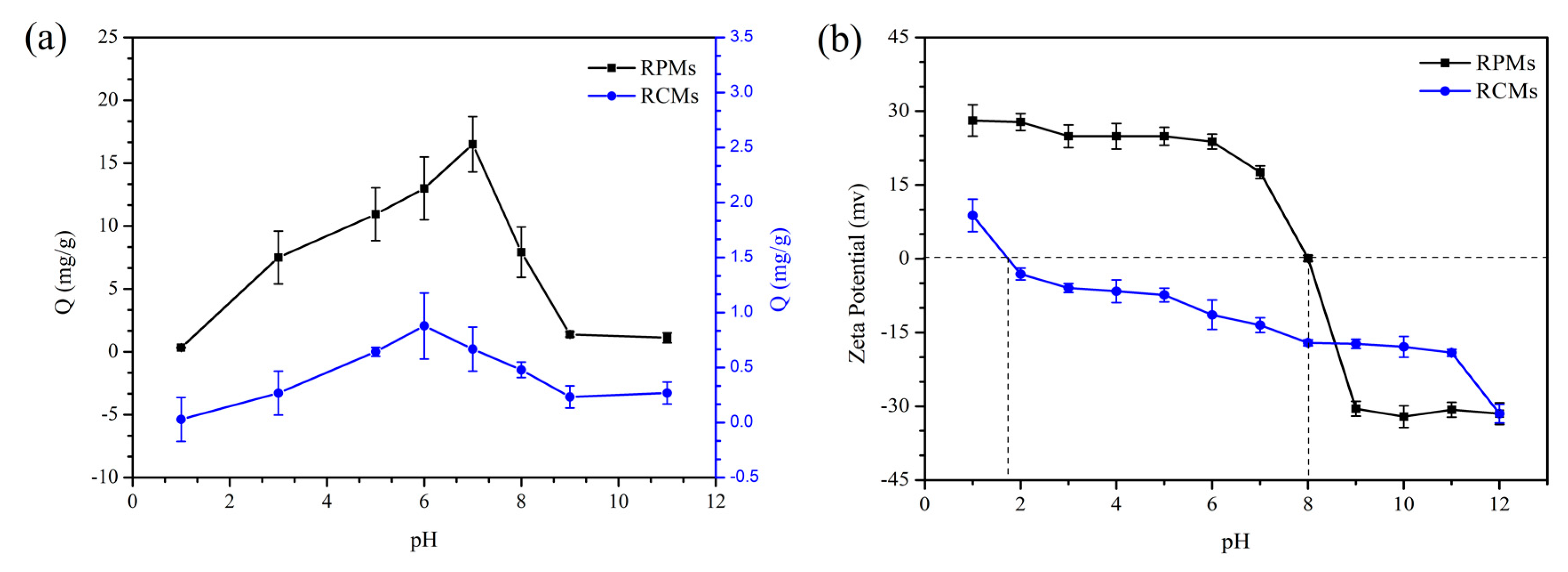
3.6. Adsorption of Dencichine on the RCMs and RPMs at Different PH Levels
3.7. Selective Adsorption on RCMs and RPMs
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chase, H.A. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat. Prod. Rep. 2010, 27, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cheng, L.; Feng, X.; Li, X.; Wang, L. Dencichine ameliorates renal injury by improving oxidative stress, apoptosis and fibrosis in diabetic rats. Life Sci. 2020, 258, 118146. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Ji, W.; Liu, D.; Liu, W.; Wang, D.; Lv, R.; Wang, X. Surface molecularly imprinted polymers with dummy templates for the separation of dencichine from Panax notoginseng. RSC Adv. 2015, 5, 48885–48892. [Google Scholar] [CrossRef]
- Ng, T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng). J. Pharm. Pharmacol. 2006, 58, 1007–1019. [Google Scholar] [CrossRef]
- Jie, L.; Pengcheng, Q.; Qiaoyan, H. Dencichine ameliorates kidney injury in induced type II diabetic nephropathy via the TGF-beta/Smad signalling pathway. Eur. J. Clin. Pharmacol. 2017, 812, 196–205. [Google Scholar] [CrossRef]
- Cang, D.; Zou, G.; Yang, C.; Shen, X.; Li, F.; Wu, Y.; Ji, B. Dencichine prevents ovariectomy-induced bone loss and inhibits osteoclastogenesis by inhibiting RANKL-associated NF-kappaB and MAPK signaling pathways. J. Pharmacol. Sci. 2021, 146, 206–215. [Google Scholar] [CrossRef]
- Milke, L.; Kallscheuer, N.; Kappelmann, J.; Marienhagen, J. Tailoring Corynebacterium glutamicum towards increased malonyl-CoA availability for efficient synthesis of the plant pentaketide noreugenin. Microb. Cell Factories 2019, 18, 71. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, Y.; Xu, S.; Gong, Q.; Zhao, C.; Cao, J.; Sun, C. Characterization of a Flavonoid 3’/5’/7-O-Methyltransferase from Citrus reticulata and Evaluation of the In Vitro Cytotoxicity of Its Methylated Products. Molecules 2020, 25, 858. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Su, K.; Wang, J.; Wang, J.; Xin, Z.; Li, F.; Fu, Y. Corynoline Exhibits Anti-inflammatory Effects in Lipopolysaccharide (LPS)-Stimulated Human Umbilical Vein Endothelial Cells through Activating Nrf2. Inflammation 2018, 41, 1640–1647. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, X.; Zheng, H.; Li, Z. Enantioselective determination of dencichine in rabbit plasma by high-performance liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2006, 840, 124–131. [Google Scholar] [CrossRef]
- Xie, G.X.; Qiu, Y.P.; Qiu, M.F.; Gao, X.F.; Liu, Y.M.; Jia, W. Analysis of dencichine in Panax notoginseng by gas chromatography-mass spectrometry with ethyl chloroformate derivatization. J. Pharm. Biomed. Anal. 2007, 43, 920–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, X.; Li, X.; Zhong, D. Development of a liquid chromatographic–tandem mass spectrometric method with precolumn derivatization for the determination of dencichine in rat plasma. Anal. Chim. Acta 2006, 566, 200–206. [Google Scholar] [CrossRef]
- Dong, J.; Yin, Z.; Su, L.; Yu, M.; Wang, M.; Li, L.; Mao, C.; Lu, T. Comparative pharmacokinetic analysis of raw and steamed Panax notoginseng roots in rats by UPLC-MS/MS for simultaneously quantifying seven saponins. Pharm. Biol. 2021, 59, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Liu, F.; Zhong, Z.F.; Wan, J.B. Comparative study on chemical components and anti-inflammatory effects of Panax notoginseng flower extracted by water and methanol. J. Sep. Sci. 2017, 40, 4730–4739. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhou, C.; Zeng, Y.; Zhang, H.; Hossen, A.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Structures, physicochemical and bioactive properties of polysaccharides extracted from Panax notoginseng using ultrasonic/microwave-assisted extraction. LWT—Food Sci. Technol. 2022, 154, 112446. [Google Scholar] [CrossRef]
- Qin, S.; Jin, F.; Gao, L.; Su, L.; Li, Y.; Han, S.; Wang, P. Determination of sulfamerazine in aquatic products by molecularly imprinted capillary electrochromatography. R. Soc. Open Sci. 2019, 6, 190119. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Qin, L.; Wang, T.; Dai, L.; Li, H.; Jiang, J.; Zhou, J.; Li, H.; Cheng, X.; Lei, F. Preparation and adsorption characteristics of rosin-based polymer microspheres for berberine hydrochloride and separation of total alkaloids from coptidis rhizoma. Chem. Eng. J. 2019, 392, 123707. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Zhang, Y.; Wang, L.; Lv, L.; Ma, X.; Zeng, S.; Wang, H. Biodegradable functional chitosan membrane for enhancement of artemisinin purification. Carbohydr. Polym. 2020, 246, 116590. [Google Scholar] [CrossRef]
- Losada-Perez, P.; Polat, O.; Parikh, A.N.; Seker, E.; Renner, F.U. Engineering the interface between lipid membranes and nanoporous gold: A study by quartz crystal microbalance with dissipation monitoring. Biointerphases 2018, 13, 011002. [Google Scholar] [CrossRef]
- McClements, J.; Seumo Tchekwagep, P.M.; Vilela Strapazon, A.L. Immobilization of Molecularly Imprinted Polymer Nanoparticles onto Surfaces Using Different Strategies: Evaluating the Influence of the Functionalized Interface on the Performance of a Thermal Assay for the Detection of the Cardiac Biomarker Troponin I. ACS Appl. Mater. Interfaces 2021, 13, 27868–27879. [Google Scholar] [CrossRef]
- Wackers, G.; Vandenryt, T.; Cornelis, P. Array formatting of the heat-transfer method (HTM) for the detection of small organic molecules by molecularly imprinted polymers. Sensors 2014, 14, 11016–11030. [Google Scholar] [CrossRef] [Green Version]
- Mousa, H.M.; Alenezi, J.F.; Mohamed, I.M.A.; Yasin, A.S.; Hashem, A.-F.M.; Abdal-hay, A. Synthesis of TiO2@ZnO heterojunction for dye photodegradation and wastewater treatment. J. Alloys Compd. 2021, 886, 161169. [Google Scholar] [CrossRef]
- Mousa, H.M.; Fahmy, H.S.; Abouzeid, R.; Abdel-Jaber, G.T.; Ali, W.Y. Polyvinylidene fluoride-cellulose nanocrystals hybrid nanofiber membrane for energy harvesting and oil-water separation applications. Mater. Lett. 2022, 306, 130965. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alfadhel, H.; Ateia, M.; Abdel-Jaber, G.T. Polysulfone-iron acetate/polyamide nanocomposite membrane for oil-water separation. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100314. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alfadhel, H.; Nasr, E.A. Engineering and Characterization of Antibacterial Coaxial Nanofiber Membranes for Oil/Water Separation. Polymers 2020, 12, 2597. [Google Scholar] [CrossRef]
- Fan, J.-P.; Cheng, Y.-T.; Zhang, X.-H.; Xiao, Z.-P.; Liao, D.-D.; Chen, H.-P.; Huang, K.; Peng, H.-L. Preparation of a novel mixed non-covalent and semi-covalent molecularly imprinted membrane with hierarchical pores for separation of genistein in Radix Puerariae Lobatae. React. Funct. Polym. 2019, 146, 104439. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zheng, D.; Cao, S.; Chen, L.; Huang, L.; Xiao, H. Bio-inspired construction of cellulose-based molecular imprinting membrane with selective recognition surface for paclitaxel separation. Appl. Surf. Sci. 2018, 466, 244–253. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- Saljoughi, E.; Mousavi, S. Preparation and characterization of novel polysulfone nanofiltration membranes for removal of cadmium from contaminated water. Sep. Purif. Technol. 2012, 90, 22–30. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Moghareh Abed, M.R.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Y.; Yu, D.; Yang, T.; Wu, M.; Yang, L.; Petru, M. Porous Film Coating Enabled by Polyvinyl Pyrrolidone (PVP) for Enhanced Air Permeability of Fabrics: The Effect of PVP Molecule Weight and Dosage. Polymers 2020, 12, 2961. [Google Scholar] [CrossRef]
- Li, H.; Mu, P.; Li, J.; Wang, Q. Inverse desert beetle-like ZIF-8/PAN composite nanofibrous membrane for highly efficient separation of oil-in-water emulsions. J. Mater. Chem. A 2021, 9, 4167–4175. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Yang, Q.; Song, X.; Qin, C.; Wang, S.; Li, K. Effects of residual lignin on composition, structure and properties of mechanically defibrillated cellulose fibrils and films. Cellulose 2019, 26, 1577–1593. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Qin, C.; Yang, S.; Song, X.; Wang, S.; Li, K. Enzyme-assisted mechanical grinding for cellulose nanofibers from bagasse: Energy consumption and nanofiber characteristics. Cellulose 2018, 25, 7065–7078. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Wang, L.; Song, X.; Qin, C.; Wang, S. Tuning of size and properties of cellulose nanofibers isolated from sugarcane bagasse by endoglucanase-assisted mechanical grinding. Ind. Crops Prod. 2020, 146, 112201. [Google Scholar] [CrossRef]
- Song, X.; Yang, S.; Liu, X.; Wu, M.; Li, Y.; Wang, S. Transparent and Water-Resistant Composites Prepared from Acrylic Resins ABPE-10 and Acetylated Nanofibrillated Cellulose as Flexible Organic Light-Emitting Device Substrate. Nanomaterials 2018, 8, 648. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Liu, H.; Shang, S.; Song, J.; Liao, S.; Wang, D.; Song, Z. Preparation and properties of maleopimaric acid-based polyester polyol dispersion for two-component waterborne polyurethane coating. Prog. Org. Coat. 2016, 90, 309–316. [Google Scholar] [CrossRef]
- Liu, B.; Nie, J.; He, Y. From rosin to high adhesive polyurethane acrylate: Synthesis and properties. Int. J. Adhes. Adhes. 2016, 66, 99–103. [Google Scholar] [CrossRef]
- Choi, S.J.; Yim, T.; Cho, W.; Mun, J.; Jo, Y.N.; Kim, K.J.; Jeong, G.; Kim, T.-H.; Kim, Y.-J. Rosin-Embedded Poly(acrylic acid) Binder for Silicon/Graphite Negative Electrode. ACS Sustain. Chem. Eng. 2016, 4, 6362–6370. [Google Scholar] [CrossRef]
- Liu, S.; Ding, Y.; Li, P.; Diao, K.; Tan, X.; Lei, F.; Zhan, Y.; Li, Q.; Huang, B.; Huang, Z. Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N,N-dimethyl dehydroabietylamine oxide. Chem. Eng. J. 2014, 248, 135–144. [Google Scholar] [CrossRef]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2015, 31, 111–126. [Google Scholar] [CrossRef]
- Chen, P.; Wang, X.; Kong, J.; Hu, X. A Facile Route to Fabricate CS/GO Composite Film for the Application of Therapeutic Contact Lenses. Adv. Mater. Sci. Eng. 2020, 2020, 8476025. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Yu, Q.; Fan, Z.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. Fabricating high thermal conductivity rGO/polyimide nanocomposite films via a freeze-drying approach. RSC Adv. 2018, 8, 22169–22176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Ma, J.; Zhu, Q.; Qin, J. Fluorescent and mechanical properties of UiO-66/PA composite membrane. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127083. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Li, X.; Zhang, Q. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018, 257, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Scherer, G.W. Evaluation of drying methods by nitrogen adsorption. Cem. Concr. Res. 2019, 120, 13–26. [Google Scholar] [CrossRef]
- Arami, M.; Limaee, N.; Mahmoodi, N. Evaluation of the adsorption kinetics and equilibrium for the potential removal of acid dyes using a biosorbent. Chem. Eng. J. 2008, 139, 2–10. [Google Scholar] [CrossRef]
- Chang, Q.; Lin, W.; Ying, W.C. Impacts of amount of impregnated iron in granular activated carbon on arsenate adsorption capacities and kinetics. Water Environ. Res. 2012, 84, 514–520. [Google Scholar] [CrossRef]
- Guo, L.; Li, G.; Liu, J.; Meng, Y.; Xing, G. Nonlinear Analysis of the Kinetics and Equilibrium for Adsorptive Removal of Cd(II) by Starch Phosphate. J. Dispers. Sci. Technol. 2012, 33, 403–409. [Google Scholar] [CrossRef]
- Din, M.I.; Mirza, M.L.; Ata, S.; Athar, M.; Mohsin, I.U. Thermodynamics of Biosorption for Removal of Co(II) Ions by an Efficient and Ecofriendly Biosorbent (Saccharum bengalense): Kinetics and Isotherm Modeling. J. Chem. 2012, 2013, 528542. [Google Scholar]
- Abukhadra, M.R.; El-Meligy, M.A.; El-Sherbeeny, A.M. Evaluation and characterization of Egyptian ferruginous kaolinite as adsorbent and heterogeneous catalyst for effective removal of safranin-O cationic dye from water. Arab. J. Geosci. 2020, 13, 169. [Google Scholar] [CrossRef]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Moon, H.G.; Jung, Y.; Shin, B.; Song, Y.G.; Kim, J.H.; Lee, T.; Lee, S.; Jun, S.C.; Kaner, R.B.; Kang, C.; et al. On-Chip Chemiresistive Sensor Array for On-Road NO x Monitoring with Quantification. Adv. Sci. 2020, 7, 2002014. [Google Scholar] [CrossRef]
- HunBok Jung, M.; YanZheng, A.C. A field, laboratory and modeling study of reactive transport of groundwater arsenic in a coastal aquifer. Environ. Sci. Technol. 2009, 43, 5333–5338. [Google Scholar] [CrossRef] [Green Version]



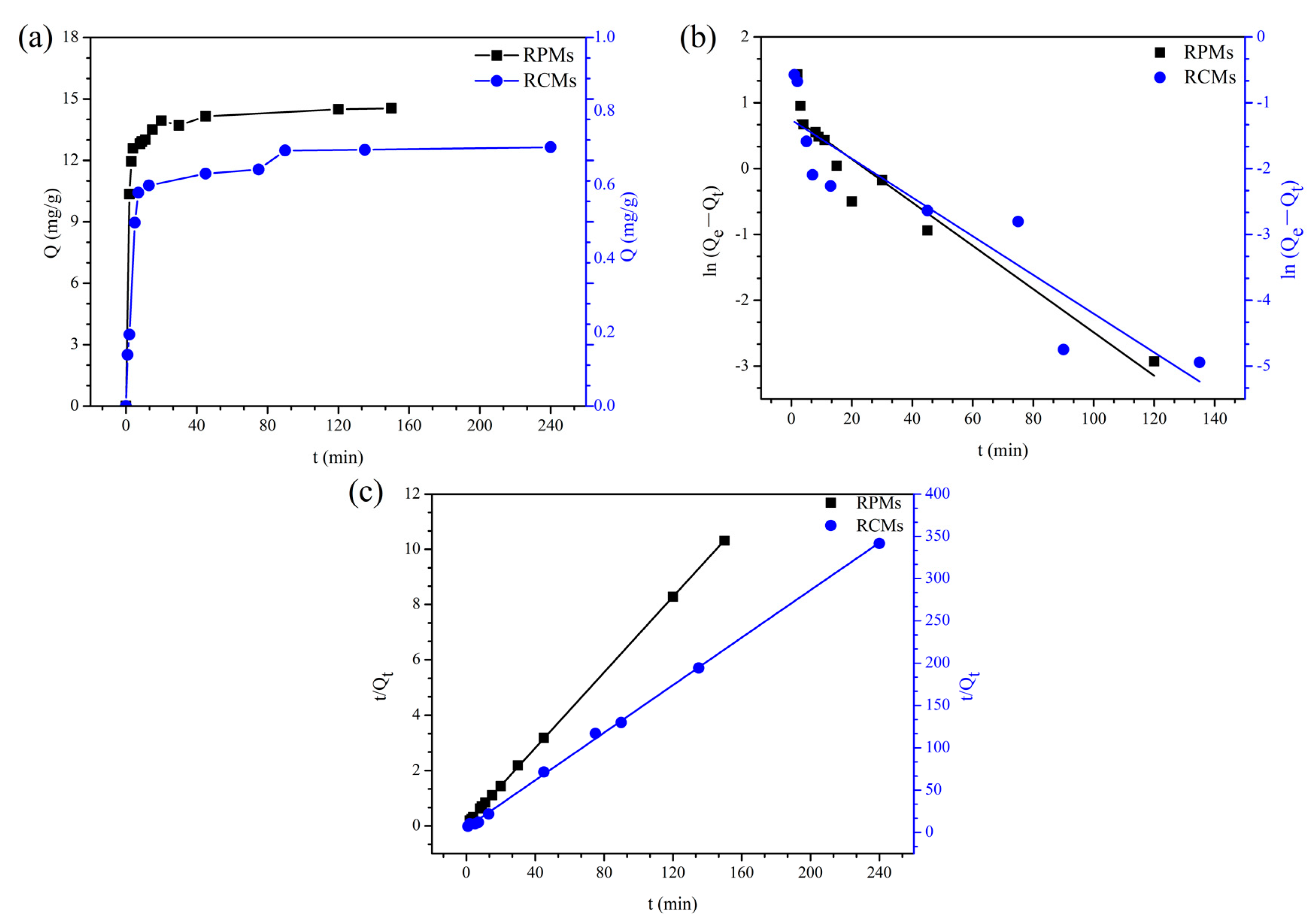

| Samples | PFO Kinetic | PSO Kinetic | ||
|---|---|---|---|---|
| K1 (min−1) | R2 | K2 (g mg−1 min−1) | R2 | |
| RCMs | 0.0678 | 0.8235 | 0.3662 | 0.9992 |
| RPMs | 0.0758 | 0.9082 | 0.0603 | 0.9999 |
| Samples | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| K3 (mL·mg−1) | R2 | Qm (mg·g−1) | K4 (mL·mg−1) | R2 | 1/n | |
| RCMs | −4.990 | 0.9570 | 0.6864 | 84.19 | 0.9842 | 2.062 |
| RPMs | 2.511 | 0.9968 | 85.11 | 106.8 | 0.9976 | 0.7988 |
| Material | The Single Adsorption (mg/g) | The Compound Adsorption (mg/g) | ||||
|---|---|---|---|---|---|---|
| Dencichine | GL | GP | Dencichine | GL | GP | |
| RPMs | 15.57 | 1.148 | 0.5734 | 13.79 | 1.261 | 0.3456 |
| PCMs | 1.056 | 0.0260 | 0.1841 | 0.8625 | 0.2930 | 0.4494 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, X.; Li, L.; Wei, S.; Huang, Q. Preparation of Rosin-Based Composite Membranes and Study of Their Dencichine Adsorption Properties. Polymers 2022, 14, 2161. https://doi.org/10.3390/polym14112161
Li L, Liu X, Li L, Wei S, Huang Q. Preparation of Rosin-Based Composite Membranes and Study of Their Dencichine Adsorption Properties. Polymers. 2022; 14(11):2161. https://doi.org/10.3390/polym14112161
Chicago/Turabian StyleLi, Long, Xiuyu Liu, Lanfu Li, Sentao Wei, and Qin Huang. 2022. "Preparation of Rosin-Based Composite Membranes and Study of Their Dencichine Adsorption Properties" Polymers 14, no. 11: 2161. https://doi.org/10.3390/polym14112161
APA StyleLi, L., Liu, X., Li, L., Wei, S., & Huang, Q. (2022). Preparation of Rosin-Based Composite Membranes and Study of Their Dencichine Adsorption Properties. Polymers, 14(11), 2161. https://doi.org/10.3390/polym14112161






