New Antioxidant Active Packaging Films Based on Yeast Cell Wall and Naphtho-γ-Pyrone Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Yeast Cell Wall by High-Pressure Homogenization (YCW-H)
2.2. Preparation of NGP Extract
2.3. Preparation of Bioactive Films
2.4. Bioactive Films Characterization
2.4.1. Thickness and Density Measurements
2.4.2. Thermal Properties
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.4. Mechanical Uniaxial Tensile Tests
2.4.5. Colour Determination (CIELab Coordinates)
2.4.6. Measurement of Antioxidant Activity by the ABTS Method
2.4.7. Specific Migration Test in Fatty Food Simulant
2.4.8. Water Sorption Isotherms
2.4.9. Water Vapour Permeability Measurements
2.4.10. Determination of Effective Water Solubility and Effective Water Diffusion
2.4.11. Statistical Analyses
3. Results and Discussion
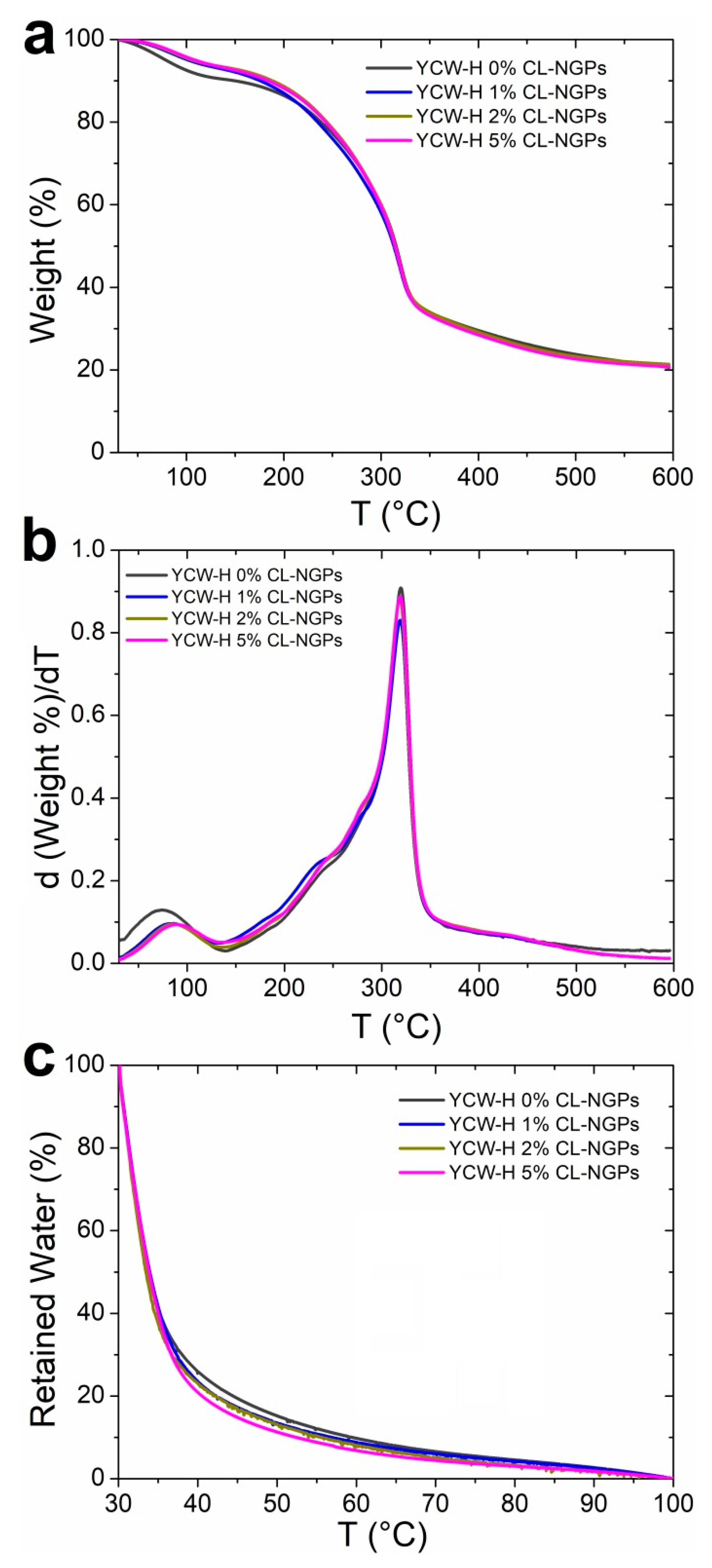
3.1. Thermal Properties
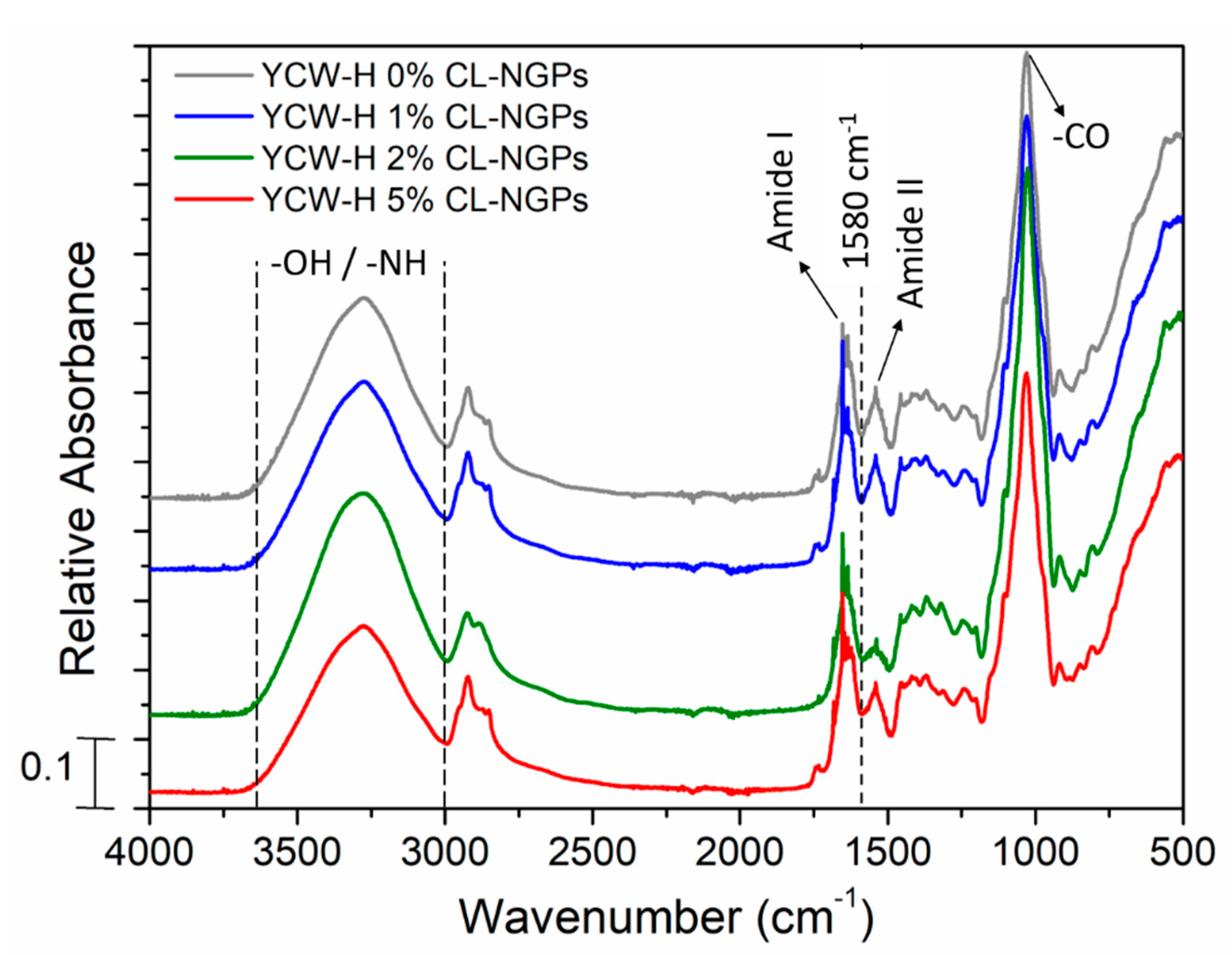
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Mechanical Uniaxial Tensile Tests
3.4. Colour Determination (CIELab Coordinates)
3.5. Measurement of Antioxidant Activity of the CL-NGP Extract by the ABTS Method
3.6. Determination of Antioxidant Capacity of Bioactive Films
3.7. Specific Migration Test in Fatty Food Simulant
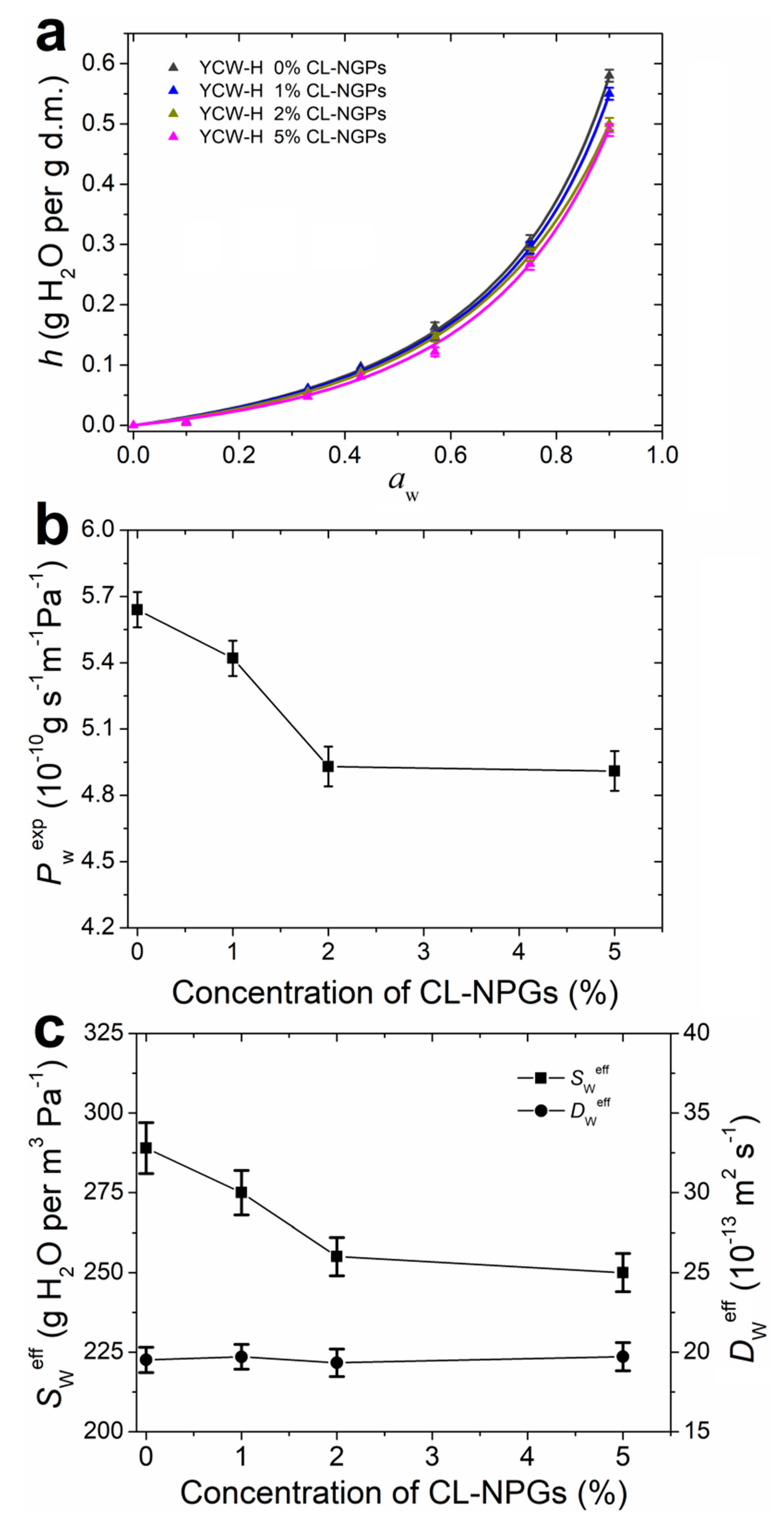
3.8. Hydration and Water Vapour Transport Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lopez de Dicastillo, C.; Nerin, C.; Alfaro, P.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly(lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltzer, M.; Wagner, J.; Jimenez, A. Migration study of carvacrol as a natural antioxidant in high-density polyethylene for active packaging. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA-PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; Wagner, J.R. Use of edible films and coatings for functional foods developments: A review. In Functional Foods Sources, Health Effects and Future Perspectives; Nelson, D.L., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2017. [Google Scholar]
- Choque, E.; Rezzani, G.; Salvay, A.G.; Mathieu, F.; Peltzer, M.A. Impact of fungal extracts on the physical and antioxidant properties of bioactive films based on enzymatically hydrolyzed yeast cell wall. J. Polym. Environ. 2021, 29, 1954–1962. [Google Scholar] [CrossRef]
- Ernst-Russell, M.A.; Chai, C.L.; Wardlaw, J.H.; Elix, J.A. Euplectin and coneuplectin, new naphthopyrones from the Lichen Flavoparmelia euplecta. J. Nat. Prod. 2000, 63, 129–131. [Google Scholar] [CrossRef]
- Lee, G.Y.; Jang, D.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Naphthopyrone Glucosides from the seeds of cassia tora with inhibitory activity on advanced glycation end products (AGEs) formation. Arch. Pharm. Res. 2006, 29, 587–590. [Google Scholar] [CrossRef]
- Bokesch, H.R.; Cartner, L.K.; Fuller, R.W.; Wilson, J.A.; Henrich, C.J.; Kelley, J.A.; Gustafson, K.R.; McMahon, J.B.; McKee, T.C. Inhibition of ABCG2-mediated drug efflux by naphthopyrones from marine crinoids. Bioorg. Med. Chem. Lett. 2010, 20, 3848–3850. [Google Scholar] [CrossRef] [Green Version]
- Barrow, R.A.; McCulloch, M.W.B. Linear naphtho-gamma-pyrones: A naturally occurring scaffold of biological importance. Mini Rev. Med. Chem. 2009, 9, 273–292. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef]
- Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-naphtho-γ-pyrones from fungi and their bioactivities. Molecules 2014, 19, 7169–7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choque, E.; Klopp, C.; Valiere, S.; Raynal, J.; Mathieu, F. Whole-genome sequencing of Aspergillus tubingensis G131 and overview of its secondary metabolism potential. BMC Genom. 2018, 19, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choque, E.; El Rayess, Y.; Raynal, J.; Mathieu, F. Fungal naphtho-γ-pyrones--secondary metabolites of industrial interest. Appl. Microbiol. Biotechnol. 2015, 99, 1081–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaika, L.L.; Smith, J.L. Antioxidants and pigments of Aspergillus niger. J. Sci. Food Agric. 1975, 26, 1357–1369. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased materials from microbial biomass and its derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef] [Green Version]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of residual yeast cell wall for new biobased materials production: Effect of plasticization on film properties. Food Bioproc. Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. Β-glucan, a Promising polysaccharide for bio-based films developments for food contact materials and medical applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Dong, P.; Luo, Y.; Cheng, F. Extraction of polysaccharides from Saccharomyces cerevisiae and its immune enhancement activity. Int. J. Pharmacol. 2013, 9, 288–296. [Google Scholar] [CrossRef]
- ASTM D882-97; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: Philadelphia, PA, USA, 1997.
- Ramírez Tapias, Y.A.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial cellulose films production by kombucha symbiotic community cultured on different herbal infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, 135, 3–11. [Google Scholar]
- Coma, M.E.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Water kefir grains as an innovative source of materials: Study of plasticiser content on film properties. Eur. Polym. J. 2019, 120, 109234. [Google Scholar] [CrossRef]
- Guggenheim, E.A. Applications of Statistical Mechanics; Oxford University Press: London, UK, 1966. [Google Scholar]
- Salvay, A.G.; Colombo, M.F.; Raúl Grigera, J. Hydration effects on the structural properties and haem–haem interaction in haemoglobin. Phys. Chem. Chem. Phys. 2003, 5, 192–197. [Google Scholar] [CrossRef]
- ASTM-E96; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Roy, S.; Gennadios, A.; Weller, C.L.; Testin, R.F. Water vapor transport parameters of a cast wheat gluten film. Ind. Crops Prod. 2000, 11, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. Water vapour transport in biopolymeric materials: Effects of thickness and water vapour pressure gradient on yeast biomass-based films. J. Polym. Environ. 2022, 1–14. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.D.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Lin, J.; Niu, S.; Liu, S.; Liu, L. Pestalotiones A–D: Four New secondary metabolites from the plant endophytic fungus Pestalotiopsis theae. Molecules 2020, 25, 470. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Jędra, F.; Mizielińska, M. New poly(lactic acid) active packaging composite films incorporated with fungal melanin. Polymers 2018, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Caseinate films modified with tung oil. Food Hydrocoll. 2010, 24, 800–808. [Google Scholar] [CrossRef]
- Bravin, B.; Peressini, D.; Sensidoni, A. Influence of emulsifier type and content on functional properties of polysaccharide lipid-based edible films. J. Agric. Food Chem. 2004, 52, 6448–6455. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martínez-Bustos, F.; Grosso, C.R.F. Effect of surfactants on the functional properties of gelatin-based edible films. J. Food Eng. 2011, 103, 129–136. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [Green Version]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum Basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar]
- Putri, A.R.; Salni, S.; Widjajanti, H. Antioxidant activity of the secondary metabolites produced by endophytic fungi isolated from jeruju (Acanthus ilicifolius L.) plant. Biovalentia Biol. Res. J. 2019, 5, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Yildiztekin, F.; Nadeem, S.; Erol, E.; Yildiztekin, M.; Tuna, A.L.; Ozturk, M. Antioxidant, anticholinesterase and tyrosinase inhibition activities, and fatty acids of Crocus mathewii—A forgotten endemic angiosperm of Turkey. Pharm. Biol. 2016, 54, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Ramírez Tapias, Y.A.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Kombucha tea by-product as source of novel materials: Formulation and characterization of films. Food Bioproc. Technol. 2020, 13, 1166–1180. [Google Scholar] [CrossRef]


| Samples | E (MPa) | ε (%) | TS (MPa) |
|---|---|---|---|
| YCW-H 0% CL-NGPs | 37 ± 3 a | 32 ± 3 a | 4.5 ± 0.7 a |
| YCW-H 1% CL-NGPs | 24 ± 3 b | 42 ± 2 b | 4.4 ± 0.3 a |
| YCW-H 2% CL-NGPs | 40 ± 2 a | 45 ± 3 b | 5.6 ± 0.1 b |
| YCW-H 5% CL-NGPs | 24 ± 2 b | 29 ± 1 a | 3.5 ± 0.3 c |
| Samples | L* | a* | b* | ΔE |
|---|---|---|---|---|
| YCW-H 0% CL-NGPs | 90 ± 1 a | −0.2 ± 0.1 a | 10 ± 1 a | - |
| YCW-H 1% CL-NGPs | 86 ± 1 b | −2.8 ± 0.1 b | 26 ± 1 b | 16 ± 1 a |
| YCW-H 2% CL-NGPs | 83 ± 1 c | −4.3 ± 0.1 c | 36 ± 2 c | 27 ± 2 b |
| YCW-H 5% CL-NGPs | 79 ± 2 d | −4.6 ± 0.9 c | 52 ± 2 d | 45 ± 7 c |
| Extract | % of NGPs | VCEAC (mM) | VCEAC (mg vit C/g NGPs) | IC50 (mg/L) |
|---|---|---|---|---|
| CL-NGPs | 90 | 13.47 | 130.98 | 17.78 |
| Vit C | N.C. | N.C. | N.C. | 2.50 |
| Samples | N (g/g) | c | k | h90%r.h. (g/g) | R2 |
|---|---|---|---|---|---|
| YCW-H 0% CL-NGPs | 0.23 ± 0.01 | 0.8 ± 0.1 | 0.84 ± 0.02 | 0.58 ± 0.01 | 0.999 |
| YCW-H 1% CL-NGPs | 0.22 ± 0.01 | 0.7 ± 0.1 | 0.82 ± 0.01 | 0.55 ± 0.01 | 0.999 |
| YCW-H 2% CL-NGPs | 0.20 ± 0.01 | 0.6 ± 0.1 | 0.78 ± 0.2 | 0.50 ± 0.01 | 0.999 |
| YCW-H 5% CL-NGPs | 0.20 ± 0.01 | 0.6 ± 0.1 | 0.77 ± 0.1 | 0.49 ± 0.01 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezzani, G.D.; Choque, E.; Salvay, A.G.; Mathieu, F.; Peltzer, M.A. New Antioxidant Active Packaging Films Based on Yeast Cell Wall and Naphtho-γ-Pyrone Extract. Polymers 2022, 14, 2066. https://doi.org/10.3390/polym14102066
Rezzani GD, Choque E, Salvay AG, Mathieu F, Peltzer MA. New Antioxidant Active Packaging Films Based on Yeast Cell Wall and Naphtho-γ-Pyrone Extract. Polymers. 2022; 14(10):2066. https://doi.org/10.3390/polym14102066
Chicago/Turabian StyleRezzani, Guillermo D., Elodie Choque, Andrés G. Salvay, Florence Mathieu, and Mercedes A. Peltzer. 2022. "New Antioxidant Active Packaging Films Based on Yeast Cell Wall and Naphtho-γ-Pyrone Extract" Polymers 14, no. 10: 2066. https://doi.org/10.3390/polym14102066
APA StyleRezzani, G. D., Choque, E., Salvay, A. G., Mathieu, F., & Peltzer, M. A. (2022). New Antioxidant Active Packaging Films Based on Yeast Cell Wall and Naphtho-γ-Pyrone Extract. Polymers, 14(10), 2066. https://doi.org/10.3390/polym14102066









