Laser-Assisted Direct Grafting of Poly(ethyleneimine) on Poly(methyl methacrylate)
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Equipment
2.2. Preparation
2.3. Extraction and Reactions
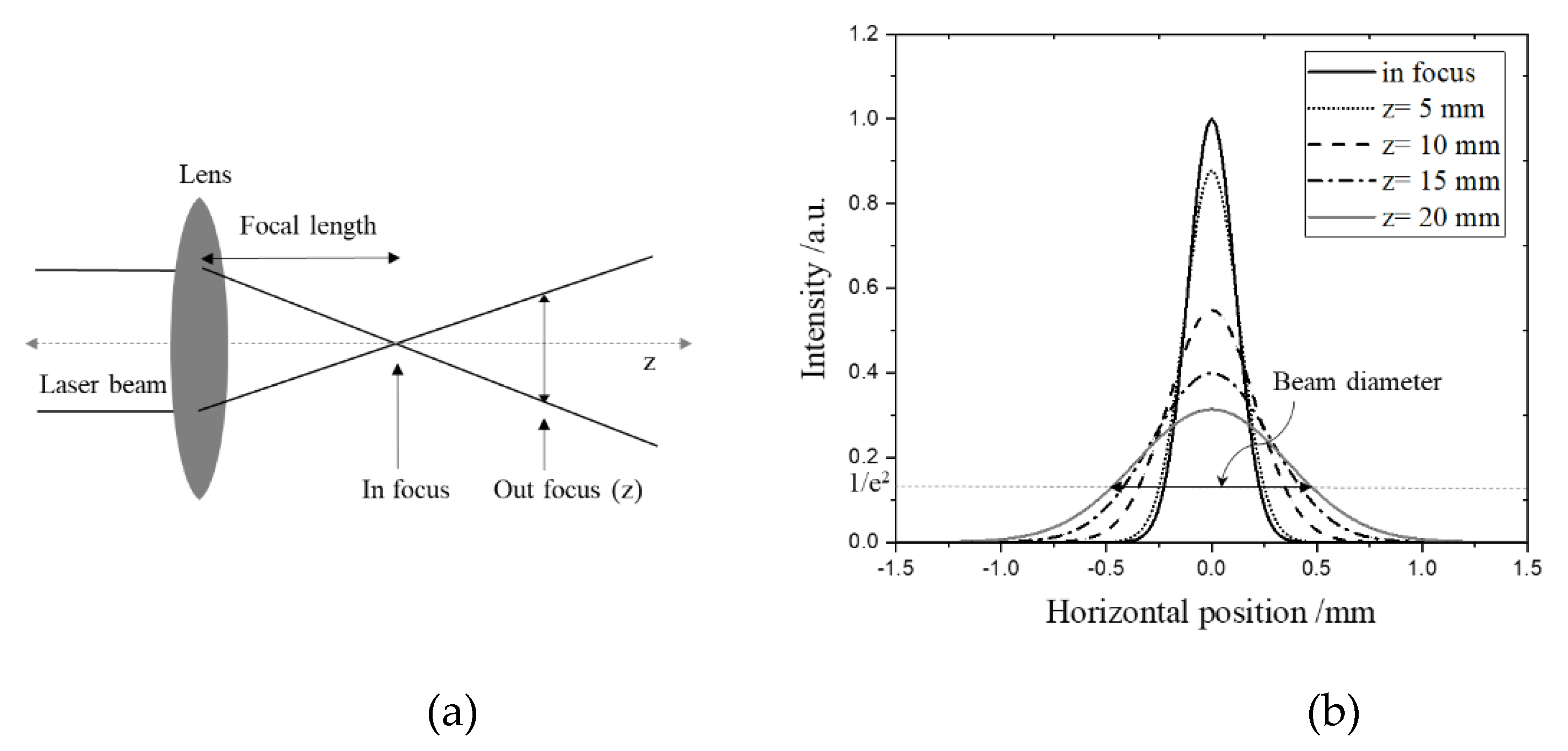
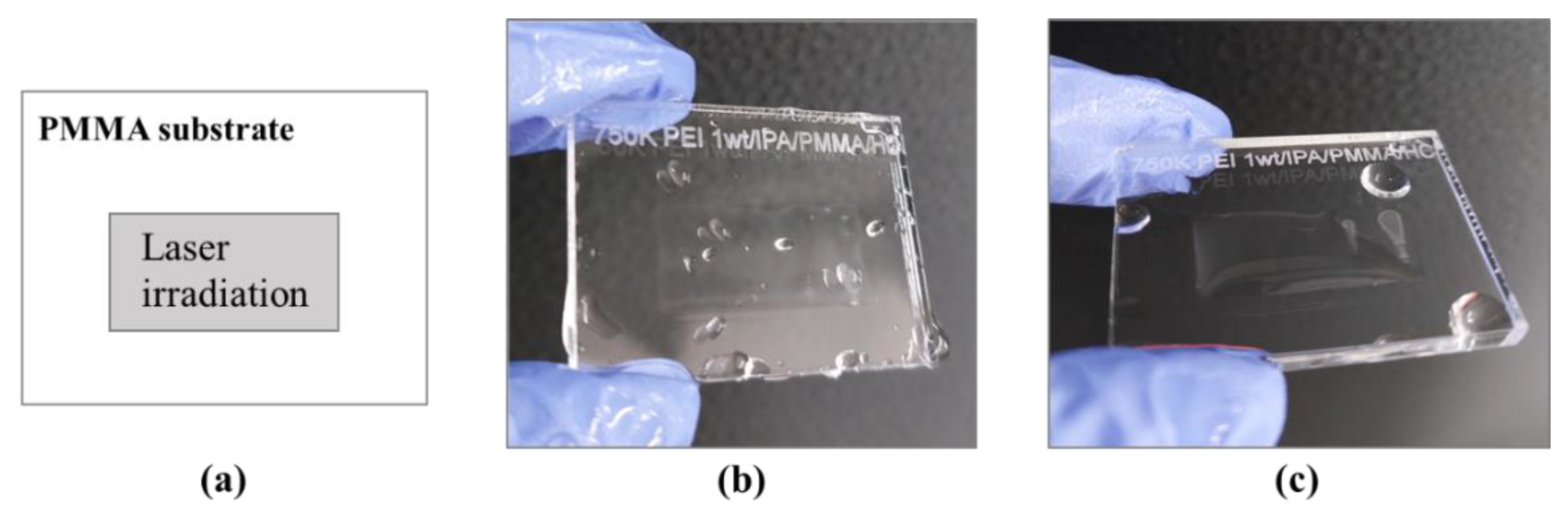
2.4. Laser Irradiation
2.5. Characterization
3. Results and Discussion
3.1. Effect of Energy Density for Grafting of PEI on PMMA
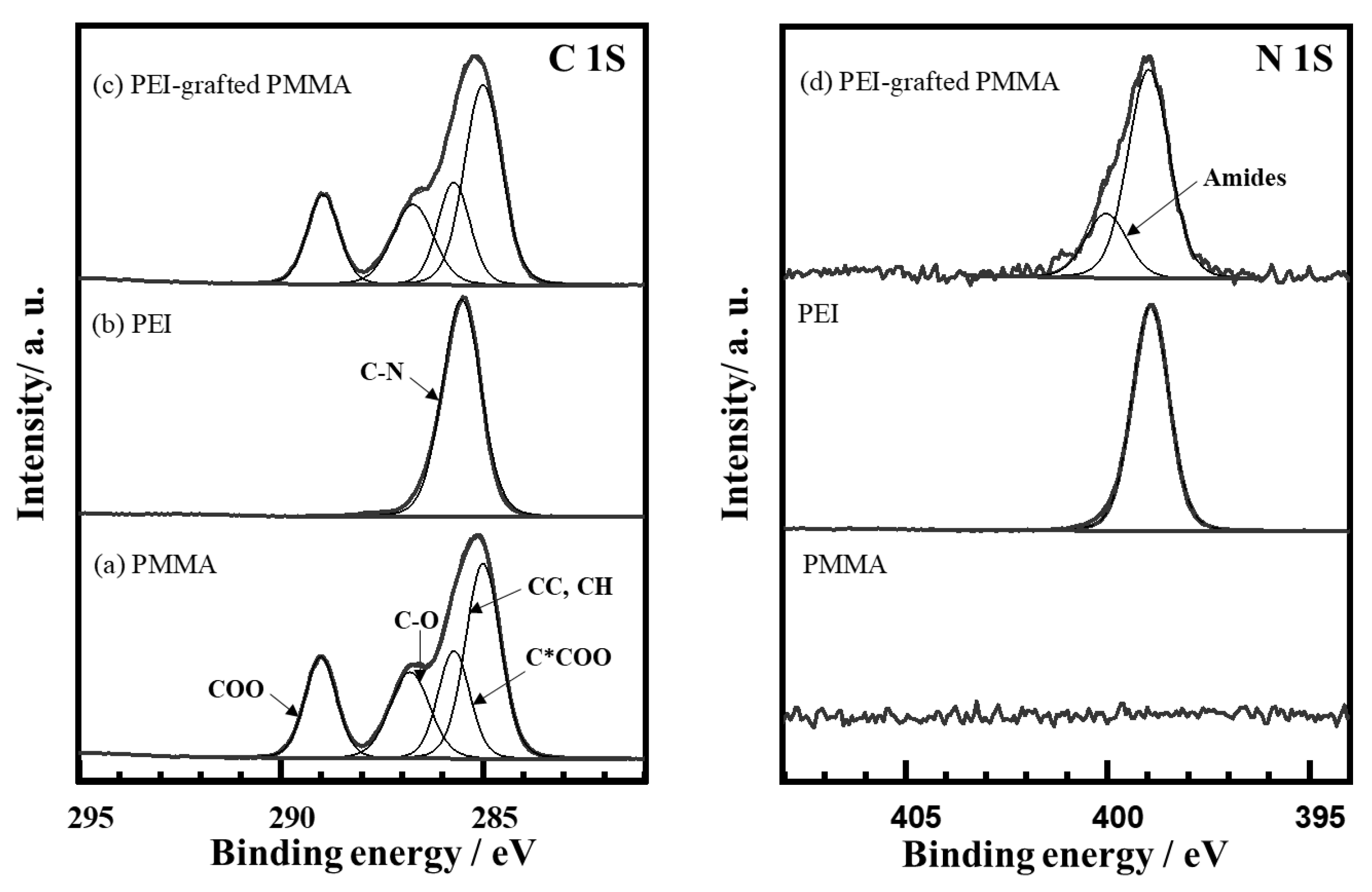
3.1.1. X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS)
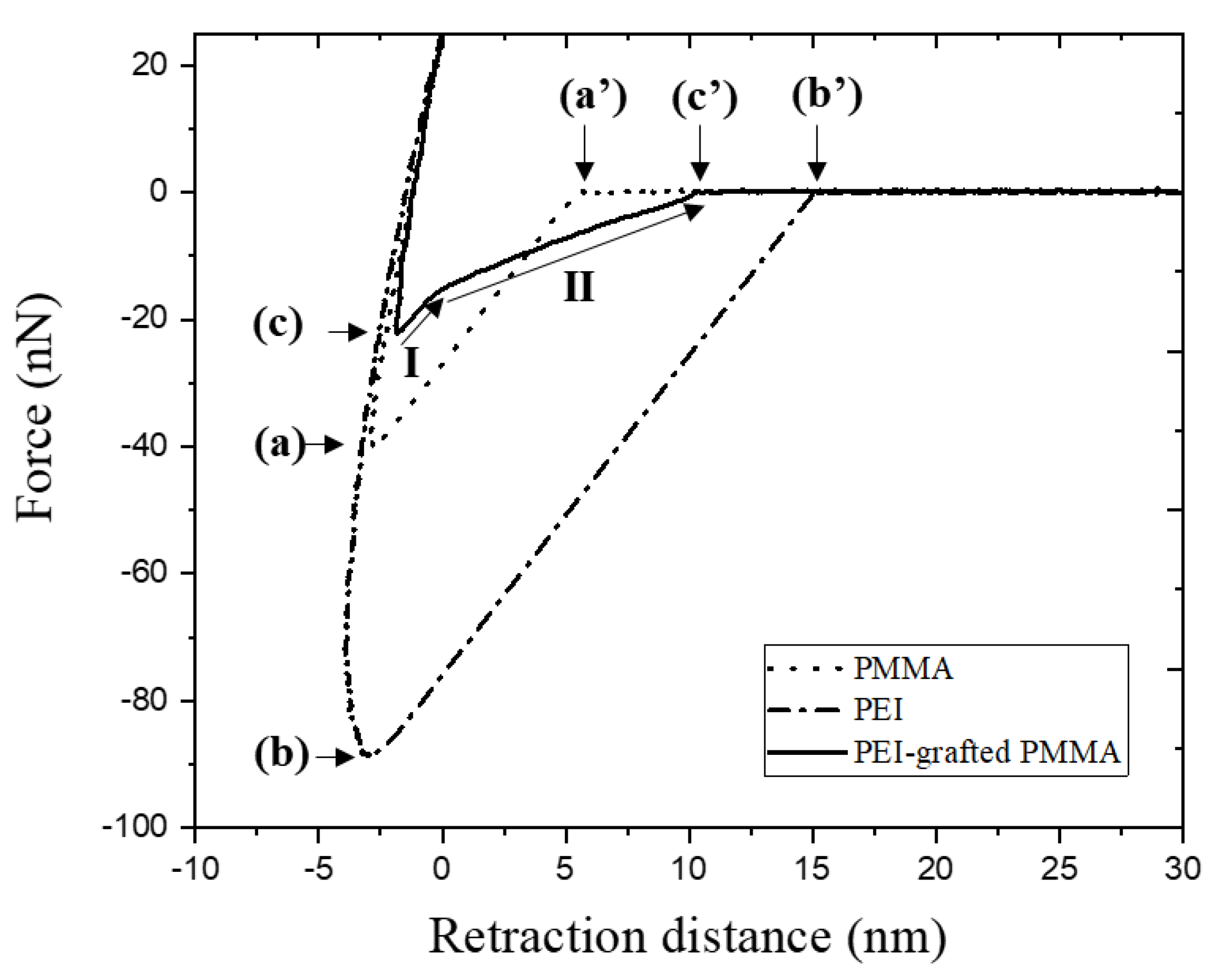
3.1.2. Adhesion Force, AFM
3.1.3. Contact Angle Measurements
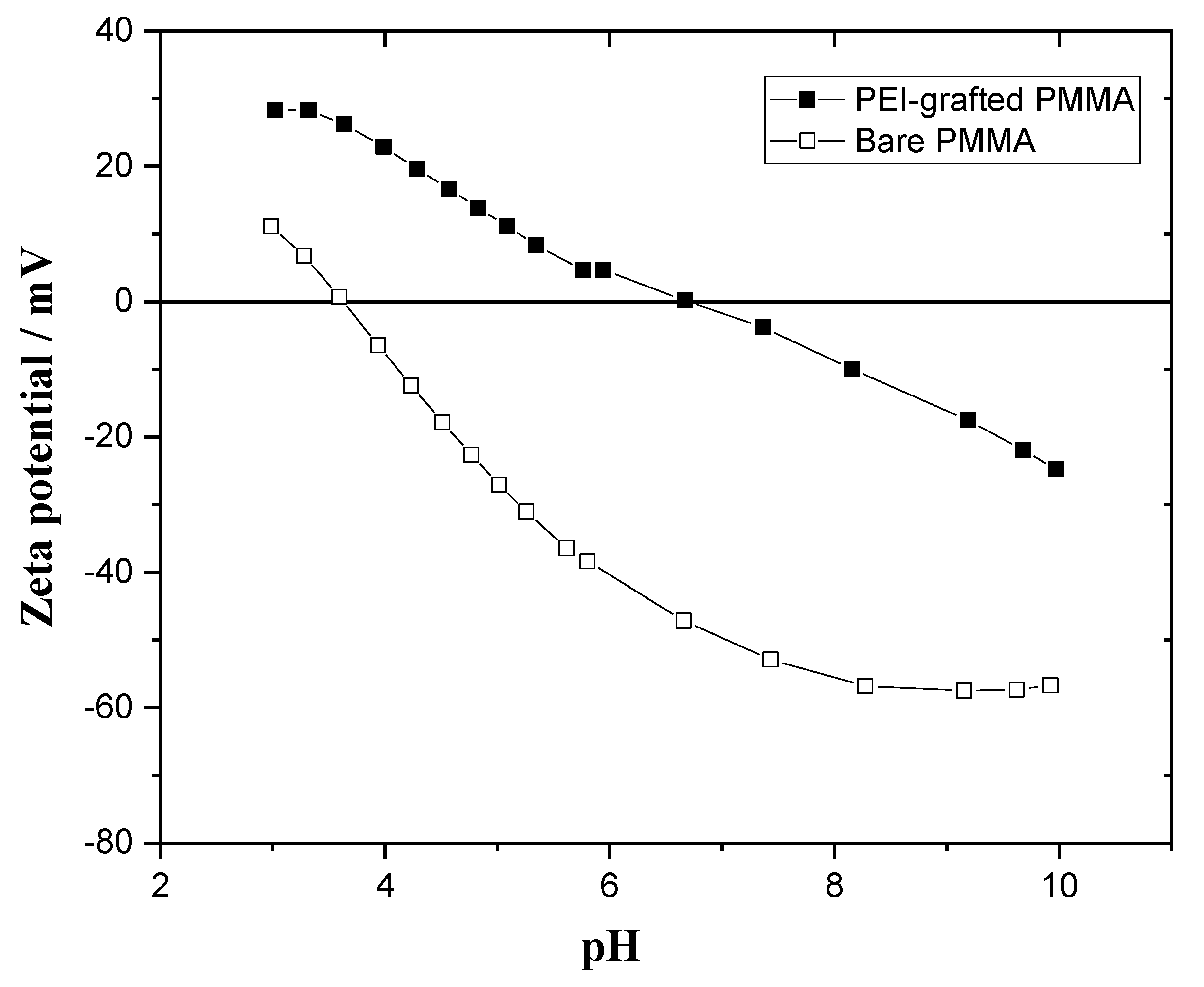
3.1.4. Zeta Potential Measurements
3.2. Reaction with Dye and Metal
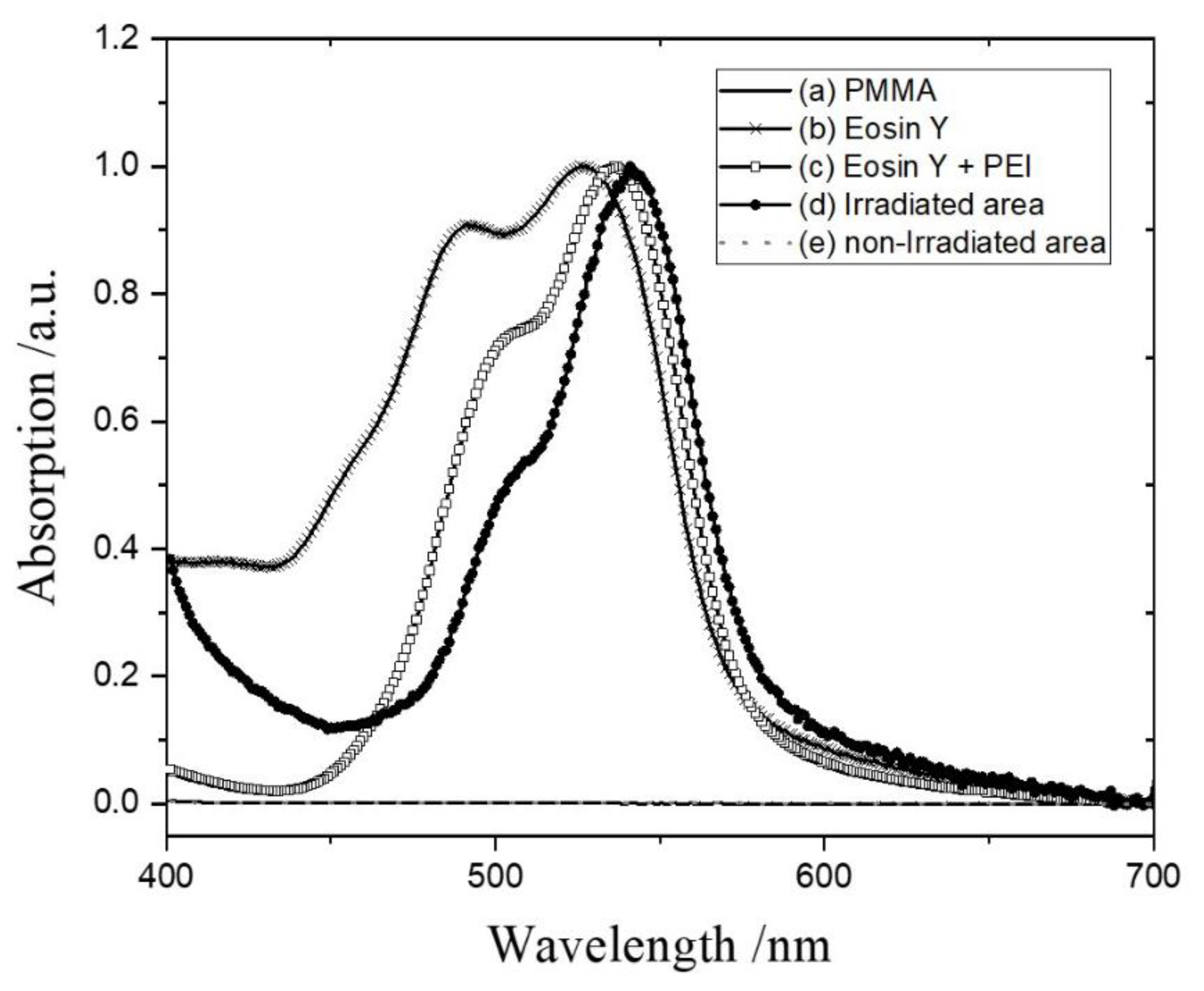
3.2.1. Eosin Y Binding
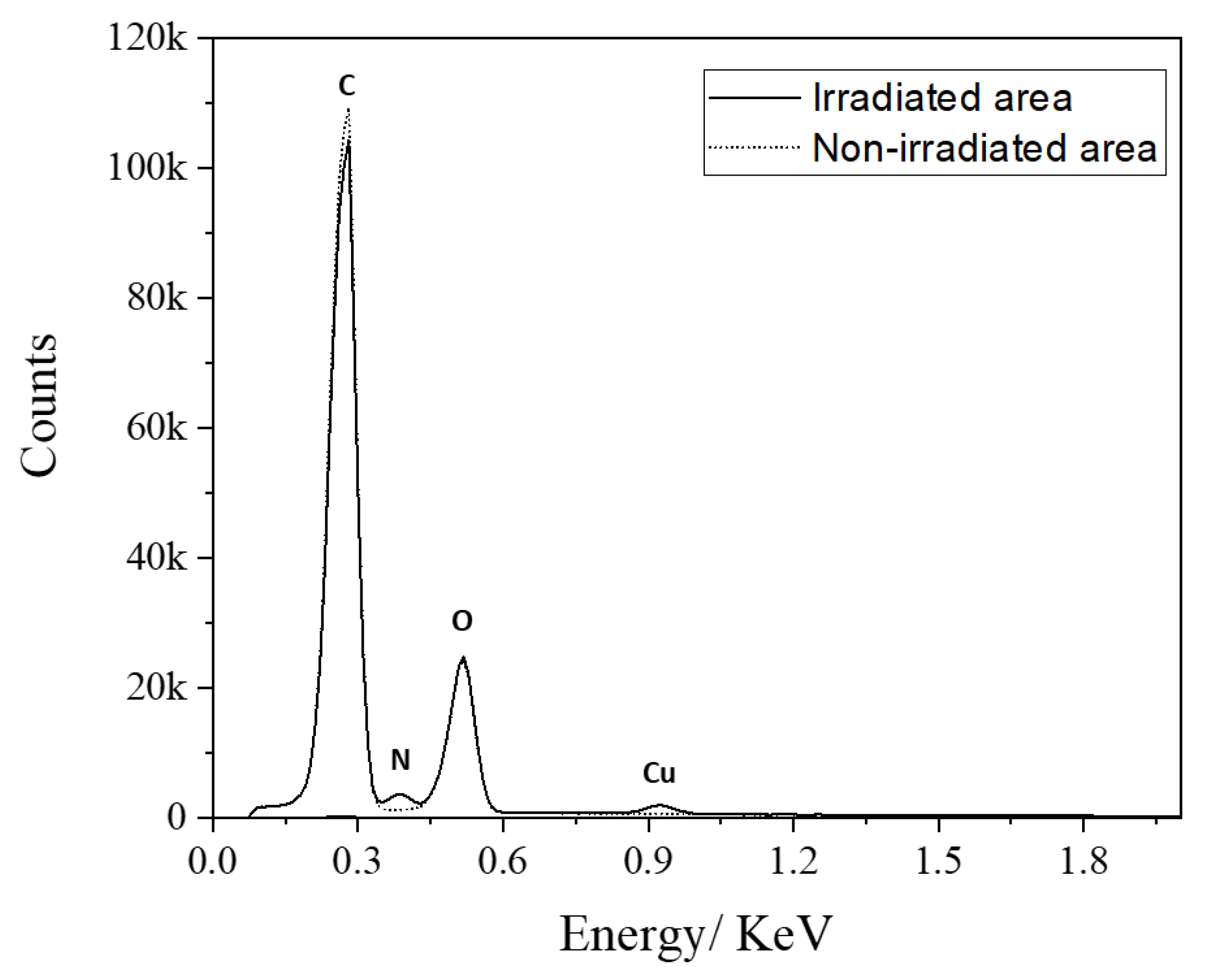
3.2.2. Metal Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wol, R. Plastic surface modification. In Surface Treatment and Adhesion; Carl Hanser Verlag: Munich, Germany, 2010; ISBN 9781569905975. [Google Scholar]
- Coleman, M. Fundamentals of Polymer Science: An Introductory Text; CRC Press: Boca Raton, FL, USA, 1998; ISBN 9781566765596. [Google Scholar]
- France, R.M.; Short, R.D. Plasma Treatment of Polymers: The Effects of Energy Transfer from an Argon Plasma on the Surface Chemistry of Polystyrene, and Polypropylene. A High-Energy Resolution X-ray Photoelectron Spectroscopy Study. Langmuir 1998, 14, 4827–4835. [Google Scholar] [CrossRef]
- Chan, C.M.; Ko, T.M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Lim, H.; Lee, Y.; Han, S.; Cho, J.; Kim, K. Surface treatment and characterization of PMMA, PHEMA, and PHPMA. Vac. Sci. Technol. A 2001, 19, 1490–1496. [Google Scholar] [CrossRef]
- Muck, A.; Svatoˇs, A. Chemical modification of polymeric microchip devices. Talanta 2007, 74, 333–341. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Wesaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Banik, S.; Bandyopadhyau, S.; Ganguly, S. Bioeffects of microwave-a brief review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green. Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free synthesis of heterocyclic compounds using microwaves. J. Heterocycl. Chem. 1999, 36, 1565–1571. [Google Scholar] [CrossRef]
- Shao, T.; Liu, F.; Hai, B.; Ma, Y.; Wang, R.; Ren, C. Surface Modification of Epoxy Using an Atmospheric Pressure Dielectric Barrier Discharge to Accelerate Surface Charge Dissipation. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1557–1565. [Google Scholar] [CrossRef]
- Alekhina, A.P.; Boleikoa, G.M.; Gudkovaa, S.A.; Markeeva, A.M.; Sigareva, A.A.; Toknovaa, V.F.; Kirilenkob, A.G.; Lapshinb, R.V.; Kozlovc, E.N.; Tetyukhin, D.V. Synthesis of Biocompatible Surfaces by Nanotechnology Methods. Nanotechnol. Russ. 2010, 5, 696–708. [Google Scholar] [CrossRef]
- Lapshin, R.V.; Alekhin, A.P.; Kirilenkoa, A.G.; Odintsova, S.L.; Krotkov, V.A. Vacuum Ultraviolet Smoothing of Nanometer-Scale Asperities of Poly(methyl methacrylate) Surface. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2010, 4, 1–11. [Google Scholar] [CrossRef]
- Bertazzo, S.; Zambuzzi, W.F.; da SilvaH, A.; Ferreira, C.V.; Bertran, C.A. Bioactivation of alumina by surfacemodification: A possibility forimproving the applicability of aluminain bone and oral repair. Clin. Oral Impl. Res. 2009, 20, 288–293. [Google Scholar] [CrossRef]
- London, G.; Chen, K.; Carroll, G.T.; Feringa, B.L. Towards Dynamic Control of Wettability by Using Functionalized Altitudinal Molecular Motors on Solid Surfaces. Chemistry 2013, 19, 10690–10697. [Google Scholar] [CrossRef]
- Ravi-Kumar, S.; Lies, B.; Zhang, X.; Lyu, H.; Qin, H. Laser Ablation of Polymers: A Review. Polym. Int. 2019, 68, 1391–1401. [Google Scholar] [CrossRef]
- Lippert, T. Interaction of Photons with Polymers: From Surface Modification to Ablation. Plasma Processes Polym. 2005, 2, 525–546. [Google Scholar] [CrossRef]
- Naundorf, H.G. Wisbrock, Leiterbahnstrukturen auf Einem Nichtleitenden Trägermaterial, Insbesondere Feine Leiterbahnstrukturen, und Verfahren zu Ihrer Herstellung. Patent WO 1999005895 A1, 16 July 1998. [Google Scholar]
- Graham, B.; David, B. XPS of Polymers Database; Surface Spectra: Manchester, UK, 2000. [Google Scholar]
- CasaXPS, version 2.3.9; Casa Software Ltd.: Teighnmouth, UK, 2003.
- Pavlinec, J.; Lazar, M. Cross-Linking of Poly(methy1 methacrylate) by Aminolysis of Ester Functions with Diamines. J. Appl. Polym. Sci. 1995, 55, 39–45. [Google Scholar] [CrossRef]
- Jacobasch, H.J.; Simon, F.; Werner, C.; Bellmann, C. Determination of the zeta potential from Streaming potential and Streaming currenl measurements. Techn. Messen Sensoren Geräte Syst. 1996, 63, 447–452. [Google Scholar]
- Schwarz, S.; Eichhorn, K.-J.; Wischerhoff, E.; Laschewsky, A. Polyelectrolyte adsorption onto planar surfaces: A study by streaming potential and ellipsometry measurement. Colloids Surf. A. 1999, 159, 491–501. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Zhao, Z.; Yan, X. Amino-functionalized polymethylmethacrylate-co- polyethyleneimine (PMMA-co-PEI) as a template to fabricate nano-silica. Mater. Res. Express. 2020, 7, 7025010. [Google Scholar] [CrossRef]
- D’Couto, G.C.; Babu, S.V. Heat transfer and material removal in pulsed excimer-laser-induced ablation: Pulsewidth dependence. J. Appl. Phys. 1994, 76, 3052–3058. [Google Scholar] [CrossRef]
- Cain, S.R. A photothermal model for polymer ablation: Chemical modification. J. Phys. Chem. 1993, 97, 7572–7577. [Google Scholar] [CrossRef]
- Mahan, G.; Cole, H.; Liu, Y.; Philipp, H. Theory of polymer ablation. Appl. Phys. Lett. 1988, 53, 2377–2379. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Katz, J. Optimum focusing of Gaussian laser beams: Beam waist shift in spot size minimization. Opt. Eng. 1994, 33, 1152–1155. [Google Scholar]
- Hill, D. How to Convert FWHM Measurements to 1/e-Squared Halfwidths Radiant. Zemax Knowledge Base. 2016. Available online: https://support.zemax.com/hc/en-us/articles/1500005488161-How-to-convert-FWHM-measurements-to-1-e-2-halfwidths (accessed on 15 November 2021).
- Amor, B.; Baud, G.; Jacquet, M.; Nanse, G.; Fioux, P.; Nardin, M. XPS characterisation of plasma-treated and alumina-coated PMMA. Appl. Surf. Sci. 2000, 153, 172–183. [Google Scholar] [CrossRef]
- Vanzetti, L.; Pasquardini, L.; Potrich, C.; Vaghi, V.; Battista, E.; Causadand, F.; Pederzolli, C. XPS analysis of genomic DNA adsorbed onPEI-modified surfaces. Surf. Interface Anal. 2016, 48, 611–615. [Google Scholar] [CrossRef]
- Vickerman, B. ToF-SIMS: Surface Analysis by Mass Spectrometry; IM Publications LLP: Chichester, UK, 2001; ISBN 1901019039. [Google Scholar]
- Thewes, N.; Loskill, P.; Spengler, C.; Hümbert, S.; Bischoff, M.; Jacobs, K. A detailed guideline for the fabrication of single bacterial probes used for atomic force spectroscopy. Eur. Phys. J. E 2015, 38, 140. [Google Scholar] [CrossRef]
- Iturri, J.; Weber, A.; María, d.M.V.; Toca-Herrera, J. Single-Cell Probe Force Studies to Identify Sox2 Overexpression-Promoted Cell Adhesion in MCF7 Breast Cancer Cells. Cells 2020, 9, 935. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, W.; Zhang, W. Quantifying the Interactions between PEI and Double-Stranded DNA: Toward the Understanding of the Role of PEI in Gene Delivery. Appl. Mater. Interfaces 2016, 8, 21055–21062. [Google Scholar] [CrossRef]
- Müller, C.; Lüders, A.; Hoth-Hannig, W.; Hannig, M.; Ziegler, C. Initial Bioadhesion on Dental Materials as a Function of Contact Time, pH, Surface Wettability, and Isoelectric Point. Langmuir 2010, 26, 4136–4141. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Leung, A.; Lam, C.N.C.; Li, A.; Wu, R.; Neumann, A.W. Low-Rate Dynamic Contact Angles on Poly(methyl methacrylate) and the Determination of Solid Surface Tensions. J. Colloid Interface Sci. 1998, 206, 44–51. [Google Scholar] [CrossRef]
- Chibowski, E.; Ontiveros-Ortega, A.; Perea-Carpio, R. On the interpretation of contact angle hysteresis. J. Adhesion Sci. Technol. 2002, 16, 1367–1404. [Google Scholar] [CrossRef]
- Erbil, H.Y.; McHale, G.; Rowan, S.M.; Newton, M.I. Determination of the Receding Contact Angle of Sessile Drops on Polymer Surfaces by Evaporation. Langmuir 1999, 15, 7378–7385. [Google Scholar] [CrossRef]
- Inphonlek, S.; Pimpha, N.; Sunintaboon, P. Synthesis of poly (methyl methacrylate) core/chitosan-mixed-polyethyleneimine shell nanoparticles and their antibacterial property. Colloids Surf. B Biointerfaces 2010, 77, 219–226. [Google Scholar] [CrossRef]
- Falahati, H.; Wong, L.; Davarpanah, L.; Garg, A.; Schmitz, P.; Barz, D.P.J. The zeta potential of PMMA in contact withelectrolytes of various conditions: Theoretical and experimental investigation. Electrophoresis 2014, 35, 870–882. [Google Scholar] [CrossRef]
- Yoshida, T.; Minoura, H. Electrochemical Self-Assembly of Dye-Modified Zinc Oxide Thin Films. Adv. Mater. 2000, 12, 1219–1222. [Google Scholar] [CrossRef]
- Gan, X.; Li, X.; Gao, X.; He, X.; Zhuge, F. Deposition potential dependence of ZnO–eosin Y hybrid thin films prepared by electrochemical deposition and their photoelectrochemical properties. Mater. Chem. Phys. 2009, 114, 920–925. [Google Scholar] [CrossRef]
- Liang, J.; Deng, X.; Tan, K. An eosin Y-based “turn-on” fluorescent sensor for detection of perfluorooctane sulfonate. Spectrochim. Acta Part A 2015, 150, 772–777. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Shen, T.; Xu, H.J. Photoinduced interaction between eosine and viologen. J. Photochem. Photobiol. A 1990, 52, 47–53. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hiroishi, K.; Tokunoh, M.; Saegusa, T. Chelating Properties of Linear and Branched Poly(ethy1enimines). Macromolecules 1987, 20, 1496–1500. [Google Scholar] [CrossRef]
- Amara, M.; Kerdjoudj, H. Modification of the cation exchange resin properties by impregnation in polyethyleneimine solutions Application to the separation of metallic ions. Talanta 2003, 60, 991–1001. [Google Scholar] [CrossRef]
- Utsuno, K.; Uludag, H. Thermodynamics of Polyethylenimine-DNA Binding and DNA Condensation. Biophys. J. 2010, 99, 201–207. [Google Scholar] [CrossRef]



| Sample | C | O | N |
|---|---|---|---|
| PMMA | 79.4 | 20.6 | - |
| PEI-coated PMMA | 72.6 | 1.8 | 25.5 |
| PEI-grafted PMMA | 79.4 | 17.5 | 2.9 |
| Fragments | Mass (u) | PEI-Coated PMMA | PEI-Grafted PMMA |
|---|---|---|---|
| CN− | 26.01 | 6.49 × 10−2 | 8.97 × 10−3 |
| CNO− | 42.01 | 2.55 × 10−2 | 3.98 × 10−3 |
| NH− | 15.01 | 3.68 × 10−3 | 4.58 × 10−4 |
| Samples | Adhesion Force, nN | Rupture Length, nm |
|---|---|---|
| PMMA | (a) 40.8 ± 4.8 | (a’) 6.1 ± 0.4 |
| PEI-coated PMMA | (b) 87.9 ± 6.1 | (b’) 15.1± 1.4 |
| PEI-grafted PMMA | (c) 23.9 ± 3.3 | (c’) 9.7 ± 1.3 |
| Samples | with H2O | with 0.1 M HCl | ||||
|---|---|---|---|---|---|---|
| θa | θr | ∆θ | θa | θr | ∆θ | |
| Laser-treated bare PMMA | 81.7 | 50.4 | 31.3 | 80.8 | 55.1 | 25.7 |
| PEI-grafted PMMA | 57.1 | 27.3 | 29.8 | 53.4 | 19.9 | 33.5 |
| Cu–PEI Complex | ||||
|---|---|---|---|---|
| on the Irradiated Area | on the Non-Irradiated Area | |||
| Element | At % | Wt % | At % | Wt % |
| C | 69.7 | 63.1 | 77.8 | 72.6 |
| N | 9.8 | 10.3 | 1.4 | 1.5 |
| O | 20.1 | 24.2 | 20.9 | 25.9 |
| Cu | 0.5 | 2.4 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Wiesing, M.; Zimmermann, P.; Janke, A.; Schwarz, S.; Nagel, J. Laser-Assisted Direct Grafting of Poly(ethyleneimine) on Poly(methyl methacrylate). Polymers 2022, 14, 2041. https://doi.org/10.3390/polym14102041
Park H, Wiesing M, Zimmermann P, Janke A, Schwarz S, Nagel J. Laser-Assisted Direct Grafting of Poly(ethyleneimine) on Poly(methyl methacrylate). Polymers. 2022; 14(10):2041. https://doi.org/10.3390/polym14102041
Chicago/Turabian StylePark, Hyeyoung, Martin Wiesing, Philipp Zimmermann, Andreas Janke, Simona Schwarz, and Jürgen Nagel. 2022. "Laser-Assisted Direct Grafting of Poly(ethyleneimine) on Poly(methyl methacrylate)" Polymers 14, no. 10: 2041. https://doi.org/10.3390/polym14102041
APA StylePark, H., Wiesing, M., Zimmermann, P., Janke, A., Schwarz, S., & Nagel, J. (2022). Laser-Assisted Direct Grafting of Poly(ethyleneimine) on Poly(methyl methacrylate). Polymers, 14(10), 2041. https://doi.org/10.3390/polym14102041








