Off-Stoichiometry Thiol–Enes Polymers Containing Silane Groups for Advanced Packaging Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
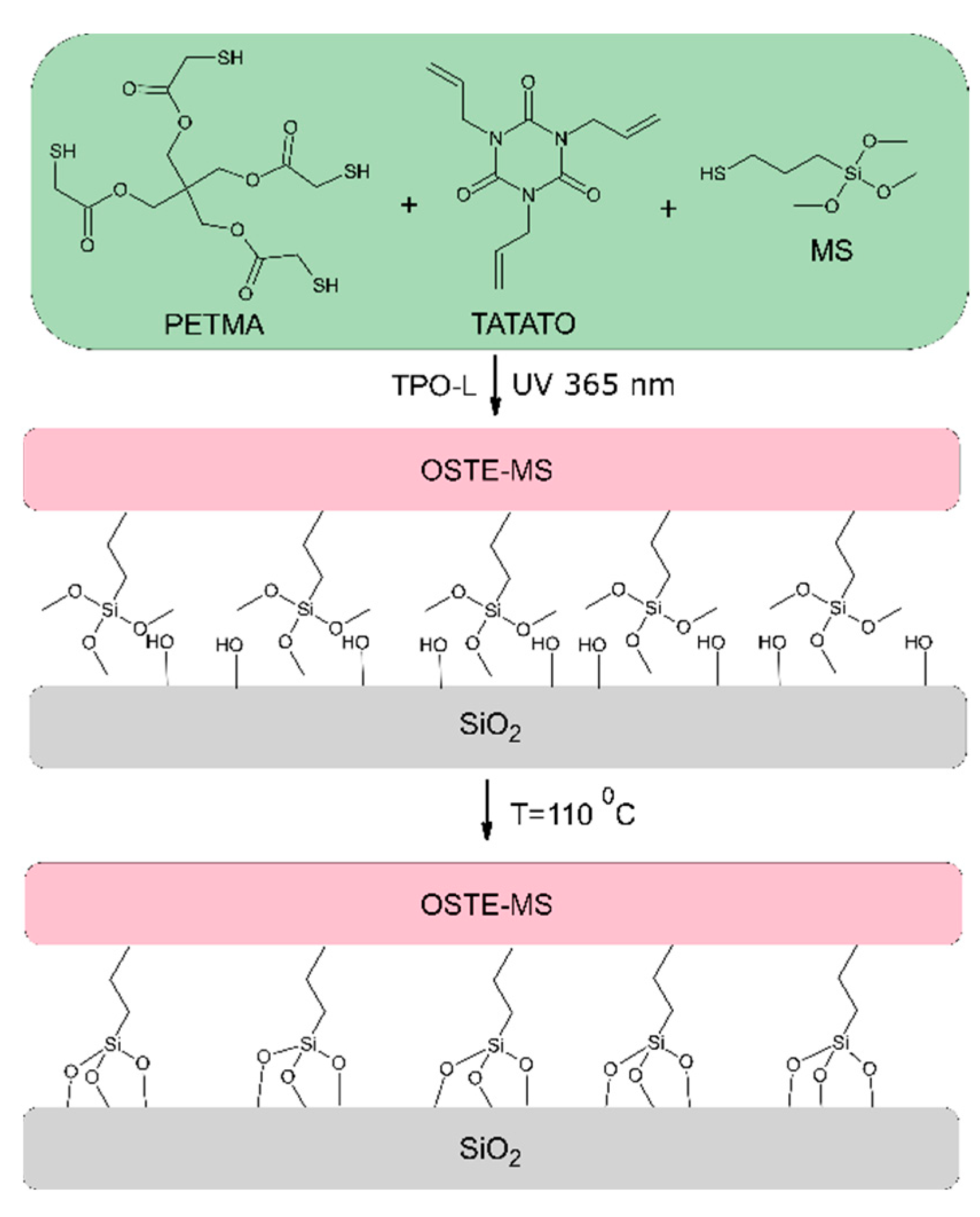
2.2. OSTE-MS Preparation
2.3. Characterization
2.4. Formation of Polymer Structures on a Chip
3. Results and Discussion
3.1. Preparation of OSTE-MS Polymers
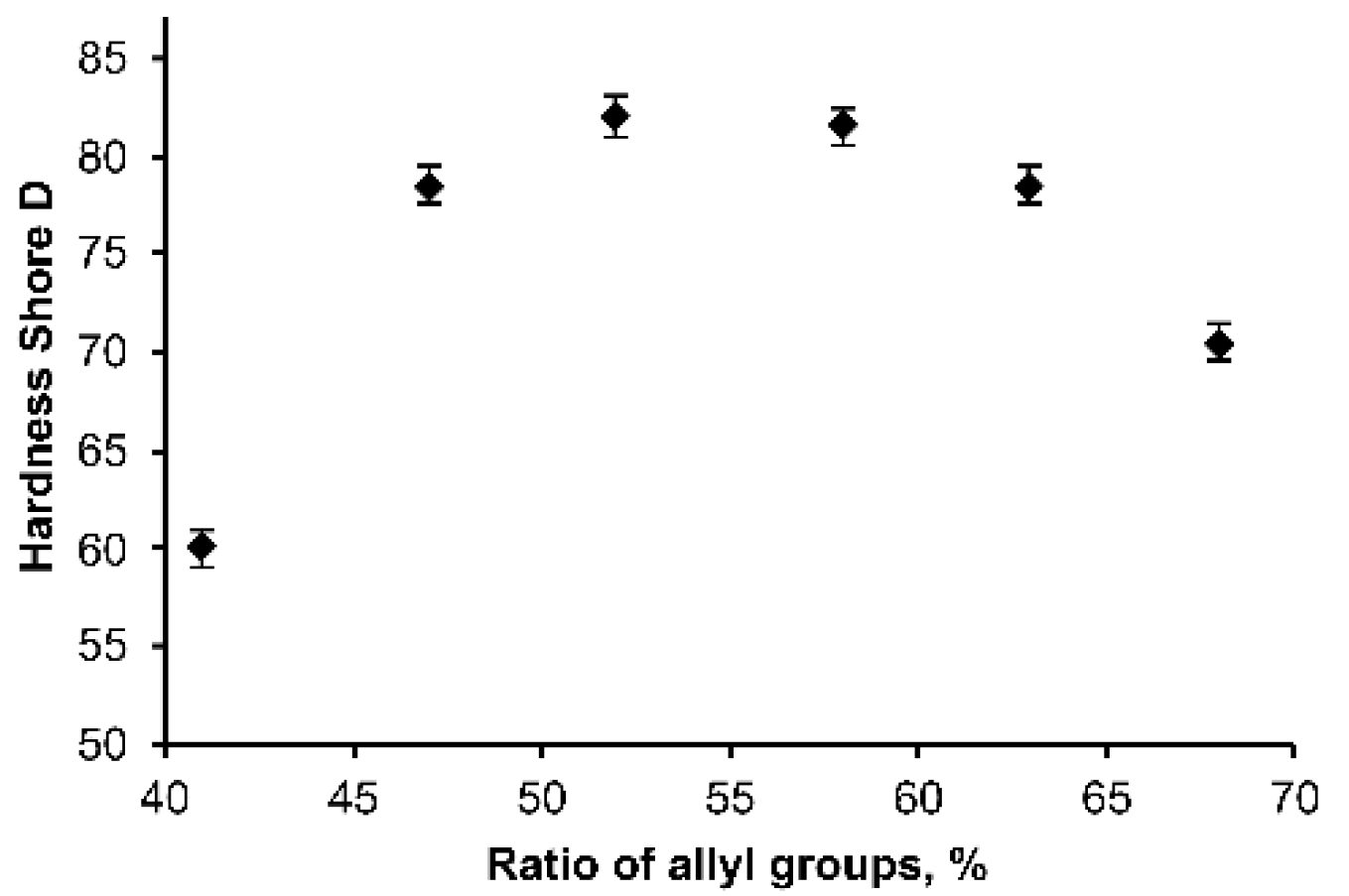
3.2. Optimization of Polymer Composition
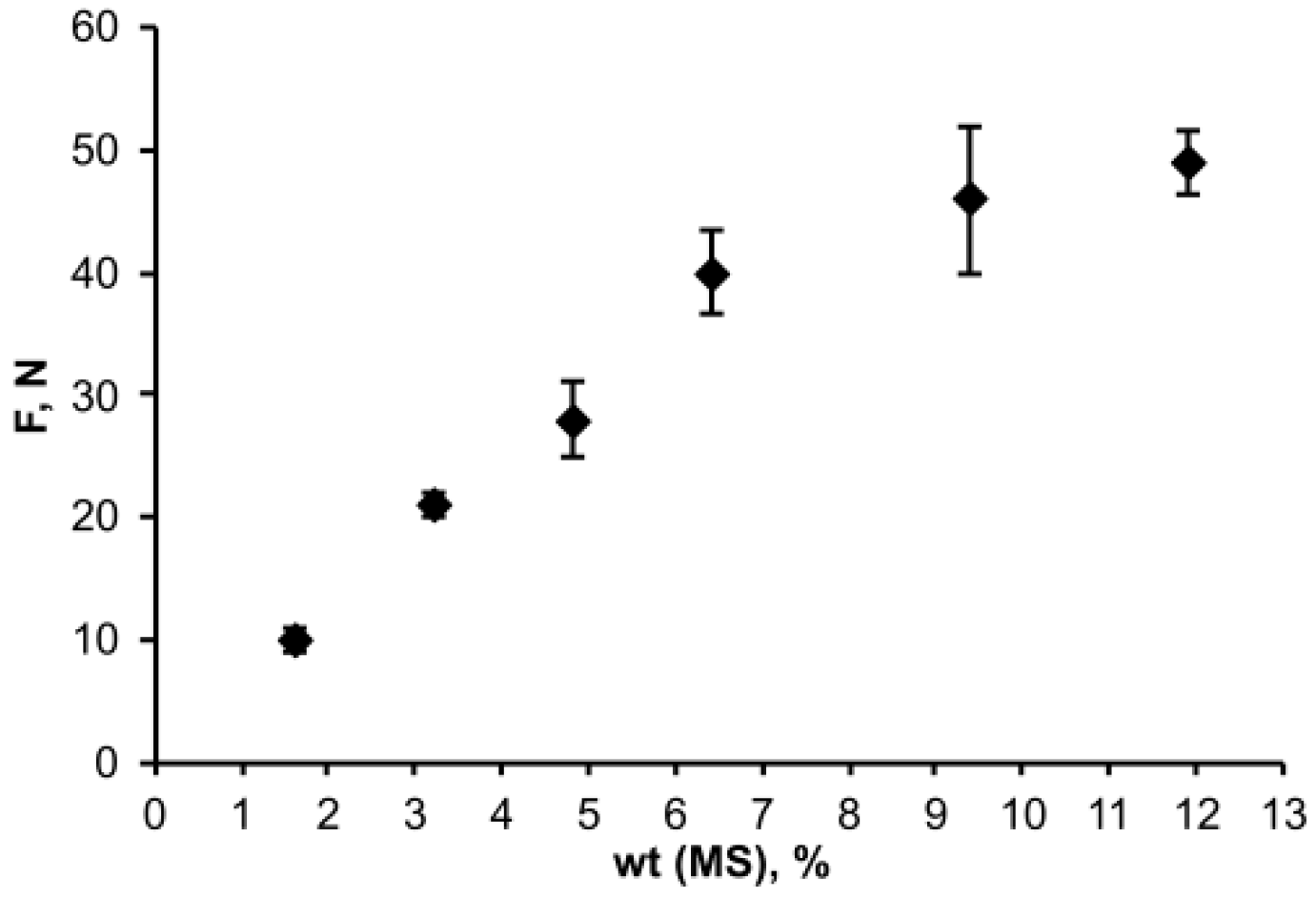
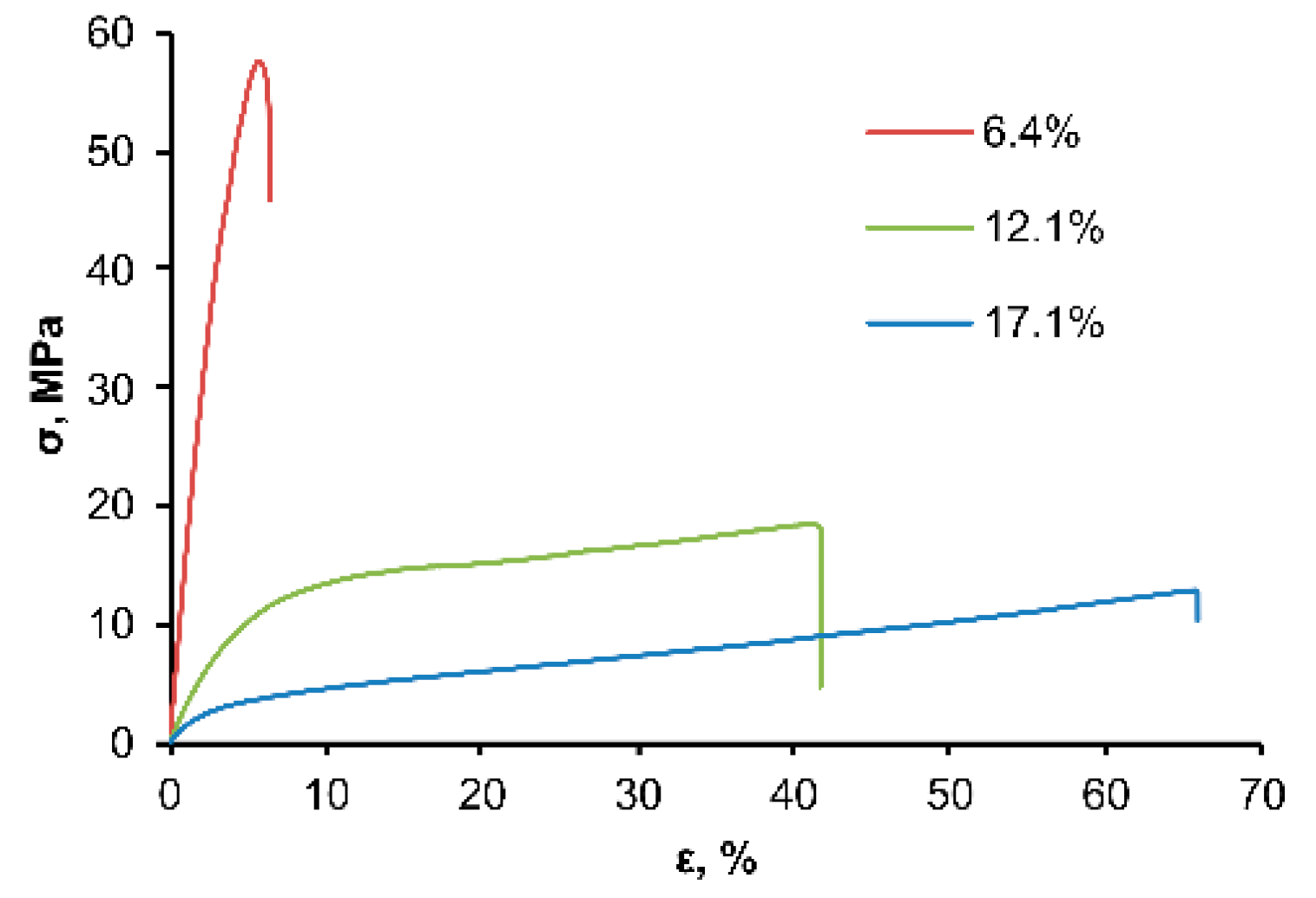
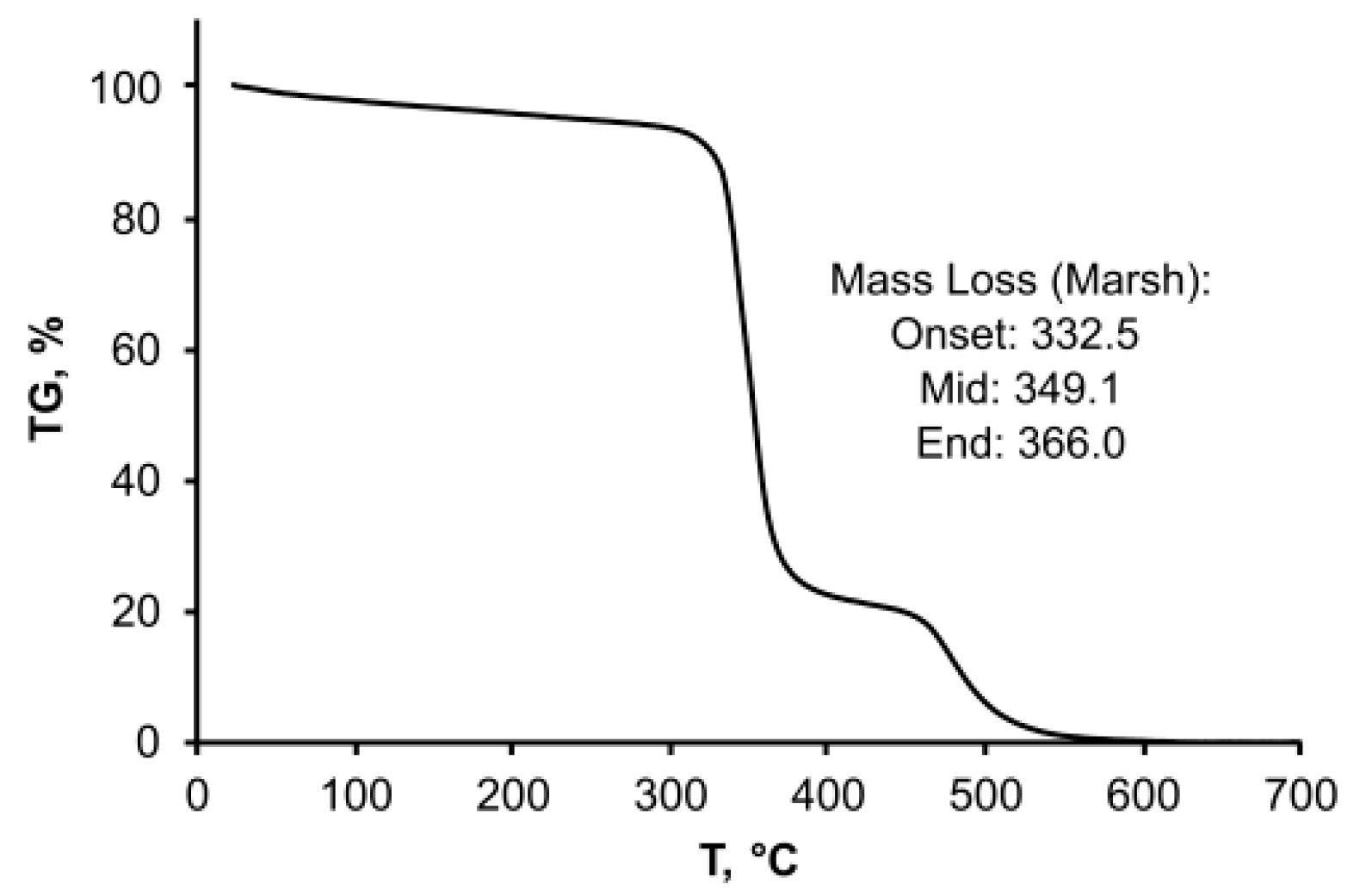
3.3. Physicochemical Properties of OSTE-MS Polymer
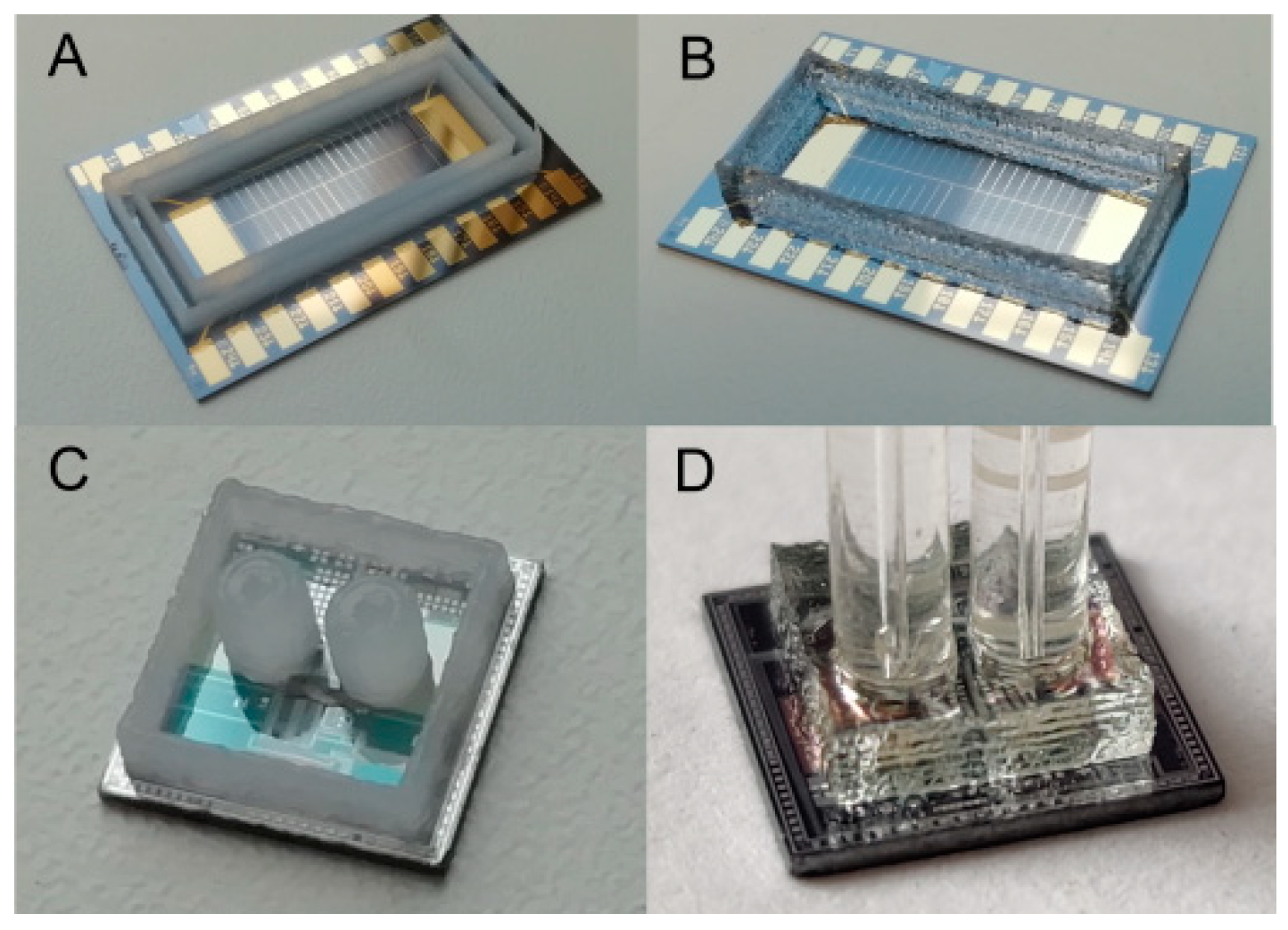
3.4. Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlborg, C.F.; Haraldsson, T.; Oberg, K.; Malkoch, M.; van der Wijngaart, W. Beyond PDMS: Off-stoichiometry thiol-ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip 2011, 11, 3136–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandström, N.; Shafagh, R.Z.; Carlborg, C.F.; Haraldsson, T.; Stemme, G.; van der Wijngaart, W. One step integration of gold coated sensors with OSTE polymer cartridges by low temperature dry bonding. TRANSDUCERS’11 2011, 5969534, 2778–2781. [Google Scholar] [CrossRef] [Green Version]
- Saharil, F.; Carlborg, C.F.; Haraldsson, T.; van der Wijngaart, W. Biocompatible ‘‘click’’ wafer bonding for microfluidic devices. Lab Chip 2012, 12, 3032–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharil, F.; Forsberg, F.; Liu, Y.; Kumar, N.; Niklaus, F.; Haraldsson, T.; van der Wijngaart, W.; Gylfason, K.B. Dry adhesive bonding of nanoporous inorganic membranes to microfluidic devices using the OSTE(+) dual-cure polymer. J. Micromechanics Microengineering 2013, 23, 25021. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, Y.; Ding, X. Cell adhesion pattern created by OSTE polymers. Biofabrication 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Urucu, O.A.; Aracier, E.D.; Çakmakçi, E. Allylimidazole containing OSTE based photocured materials for selective and efficient removal of gold from aqueous media. Microchem. J. 2019, 146, 997–1003. [Google Scholar] [CrossRef]
- Pardon, G.; Saharil, F.; Karlsson, J.M.; Supekar, O.; Carlborg, C.F.; van der Wijngaart, W.; Haraldsson, T. Rapid mold-free manufacturing of microfluidic devices with robust and spatially directed surface modifications. Microfluid. Nanofluidics 2014, 17, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Pinelo, M.; Woodley, J.M.; Daugaard, A.E. Development of a thiol-ene based screening platform for enzyme immobilization demonstrated using horseradish peroxidase. Biotechnol. Prog. 2017, 33, 1267–1277. [Google Scholar] [CrossRef]
- Hoffmann, C.; Grey, C.; Pinelo, M.; Woodley, J.M.; Daugaard, A.E.; Adlercreutz, P. Improved alkyl glycoside synthesis by trans-glycosylation through tailored microenvironments of immobilized β-glucosidase. ChemPlusChem 2020, 85, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Bourg, S.; d’Orlyé, F.; Griveau, S.; Bedioui, F.; da Silva, J.A.F.; Varenne, A. Multiple zones modification of open off-stoichiometry thiol-ene microchannel by aptamers: A methodological study & a proof of concept. Chemosensors 2020, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Çakmakçi, E.; Yuce-Dursun, B.; Demir, S. Maleic anhydride functionalization of OSTE based coatings via thiol-ene “Click” reaction for the covalent immobilization of xylanase. React. Funct. Polym. 2017, 111, 38–43. [Google Scholar] [CrossRef]
- Hoffmann, C.; Pereira Rosinha Grundtvig, I.; Thrane, J.; Garg, N.; Gernaey, K.V.; Pinelo, M.; Woodley, J.M.; Krühne, U.; Daugaard, A.E. Experimental and computational evaluation of area selectively immobilized horseradish peroxidase in a microfluidic device. Chem. Eng. J. 2018, 332, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Tähkä, S.; Sarfraz, J.; Urvas, L.; Provenzani, R.; Wiedmer, S.K.; Peltonen, J.; Jokinen, V.; Sikanen, T. Immobilization of proteolytic enzymes on replica-molded thiol-ene micropillar reactors via thiol-gold interaction. Anal. Bioanal. Chem. 2019, 411, 2339–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zverina, L.; Pinelo, M.; Woodley, J.M.; Daugaard, A.E. Monolithic flow reactor for enzymatic oxidations. J. Chem. Technol. Biotechnol. 2021, 96, 2488–2495. [Google Scholar] [CrossRef]
- Kemas, A.M.; Youhanna, S.; Zandi Shafagh, R.; Lauschke, V.M. Insulin-dependent glucose consumption dynamics in 3D primary human liver cultures measured by a sensitive and specific glucose sensor with nanoliter input volume. FASEB J. 2021, 35, e21305. [Google Scholar] [CrossRef] [PubMed]
- Vastesson, A.; Guo, M.; Haraldsson, T.; van der Wijngaart, W. Polymer nanoliter well arrays for liquid storage and rapid on-demand electrochemical release. Sens. Actuators B 2018, 267, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Breukers, J.; Horta, S.; Struyfs, C.; Spasic, D.; Feys, H.F.; Geukens, N.; Thevissen, K.; Cammue, B.P.A.; Vanhoorelbeke, K.; Lammertyn, J. Tuning the surface interactions between single cells and an OSTE+ Microwell array for enhanced single cell manipulation. ACS Appl. Mater. Interfaces 2021, 13, 2316–2326. [Google Scholar] [CrossRef]
- Guo, W.; Vilaplana, L.; Hansson, J.; Marco, M.-P.; van der Wijngaart, W. Immunoassays on thiol-ene synthetic paper generate a superior fluorescence signal. Biosens. Bioelectron. 2020, 163, 112279. [Google Scholar] [CrossRef]
- Xiao, Z.; Sun, L.; Yang, Y.; Feng, Z.; Dai, S.; Yang, H.; Zhang, X.; Sheu, C.-L.; Guo, W. High-performance passive plasma separation on OSTE pillar forest. Biosensors 2021, 11, 355. [Google Scholar] [CrossRef]
- Svejdal, R.R.; Sticker, D.; Sønderby, C.; Kutter, J.P.; Rand, K.D. Thiol-ene microfluidic chip for fast on-chip sample clean-up, separation and ESI mass spectrometry of peptides and proteins. Anal. Chim. Acta 2020, 1140, 168–179. [Google Scholar] [CrossRef]
- Lu, N.; Sticker, D.; Kretschmann, A.; Petersen, N.J.; Kutter, J.P. A thiol-ene microfluidic device enabling continuous enzymatic digestion and electrophoretic separation as front-end to mass spectrometric peptide analysis. Anal. Bioanal. Chem. 2020, 412, 3559–3571. [Google Scholar] [CrossRef] [PubMed]
- Kuusisto, E.; Heikkinen, J.J.; Järvinen, P.; Sikanen, T.; Franssila, S.; Jokinen, V. Inkjet-printed flexible silver electrodes on thiol-enes. Sens. Actuators B Chem. 2021, 336, 129727. [Google Scholar] [CrossRef]
- Ko, J.M.; Kang, Y.H.; Lee, C.; Cho, S.Y. Electrically and thermally stable gate dielectrics from thiol-ene cross-linked systems for use in organic thin-film transistors. J. Mater. Chem. C 2013, 1, 3091–3097. [Google Scholar] [CrossRef]
- Al-Azawi, A.; Cenev, Z.; Tupasela, T.; Bo Peng, B.; Ikkala, O.; Zhou, Q.; Jokinen, V.; Franssila, S.; Ras, R.H.A. Tunable and Magnetic thiol–ene micropillar arrays. Macromol. Rapid Commun. 2020, 41, 1900522. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, L.; Huang, S.; Zhang, P.; Linnros, J.; Zhong, H.; Sychugov, I. Photodegradation of organometal hybrid perovskite nanocrystals: Clarifying the role of oxygen by single-dot photoluminescence. J. Phys. Chem. Lett. 2019, 10, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shu, J.; Zhang, X.; Zhao, A.; Liu, Y.; Li, R.; Di, Y.; Xu, H.; Gan, Z. Encapsulation of colloid perovskite nanocrystals into solid polymer matrices: Impact on electronic transition and photoluminescence. J. Lumin. 2020, 219, 116938. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Haraldsson, T.; Clemments, A.; Fujii, M.; Sugimoto, H.; Xu, B.; Sychugov, I. Triplex glass laminates with silicon quantum dots for luminescent solar concentrators. Sol. RRL 2020, 4, 2000195. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Aswal, D.K.; Lenfant, S.; Guerin, D.; Yakhmi, J.V.; Vuillaume, D. Self-assembled monolayers on silicon for molecular electronics. Anal. Chim. Acta 2005, 568, 84–108. [Google Scholar] [CrossRef]
- Sandström, N.; Shafagh, R.Z.; Vastesson, A.; Carlborg, C.F.; van der Wijngaart, W.; Haraldsson, T. Reaction injection molding and direct covalent bonding of OSTE+ polymer microfluidic devices. J. Micromechanics Microengineering 2015, 25, 75002. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Vastesson, A.; Liu, Y.; van der Wijngaart, W.; Johansson, M.; Haraldsson, T. Functional off-stoichiometry thiol-ene-epoxy thermosets featuring temporally controlled curing stages via an UV/UV dual cure process. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2604–2615. [Google Scholar] [CrossRef]

| Solvent | Chemical Resistance 1 |
|---|---|
| Methanol | Good |
| Ethanol | Good |
| Hexane | Good |
| Decane | Good |
| 2-Propanol | Good |
| Benzene | Good |
| Limonene | Good |
| Toluene | Good |
| Tetrachloromethane | Good |
| Ethyl acetate | Normal |
| 2-Butanone | Normal |
| Acetic acid | Normal |
| Dimethyl sulfoxide | Satisfactory |
| Tetrahydrofuran | Satisfactory |
| Acetone | Satisfactory |
| Acetonitrile | Satisfactory |
| Dimethylformamide | Satisfactory |
| Dichloromethane | Unsatisfactory |
| Chloroform | Unsatisfactory |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchnin, K.; Ryazantsev, D.; Grudtsov, V.; Golubev, Y.; Kuznetsov, A. Off-Stoichiometry Thiol–Enes Polymers Containing Silane Groups for Advanced Packaging Technologies. Polymers 2022, 14, 1988. https://doi.org/10.3390/polym14101988
Puchnin K, Ryazantsev D, Grudtsov V, Golubev Y, Kuznetsov A. Off-Stoichiometry Thiol–Enes Polymers Containing Silane Groups for Advanced Packaging Technologies. Polymers. 2022; 14(10):1988. https://doi.org/10.3390/polym14101988
Chicago/Turabian StylePuchnin, Kirill, Dmitriy Ryazantsev, Vitaliy Grudtsov, Yaroslav Golubev, and Alexander Kuznetsov. 2022. "Off-Stoichiometry Thiol–Enes Polymers Containing Silane Groups for Advanced Packaging Technologies" Polymers 14, no. 10: 1988. https://doi.org/10.3390/polym14101988
APA StylePuchnin, K., Ryazantsev, D., Grudtsov, V., Golubev, Y., & Kuznetsov, A. (2022). Off-Stoichiometry Thiol–Enes Polymers Containing Silane Groups for Advanced Packaging Technologies. Polymers, 14(10), 1988. https://doi.org/10.3390/polym14101988








