Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Material Fabrication
3. Characterizations
4. Results and Discussion
4.1. FTIR Analysis
4.2. Microstructure Analysis
4.3. Surface Area Measurement and Pore Size Distribution
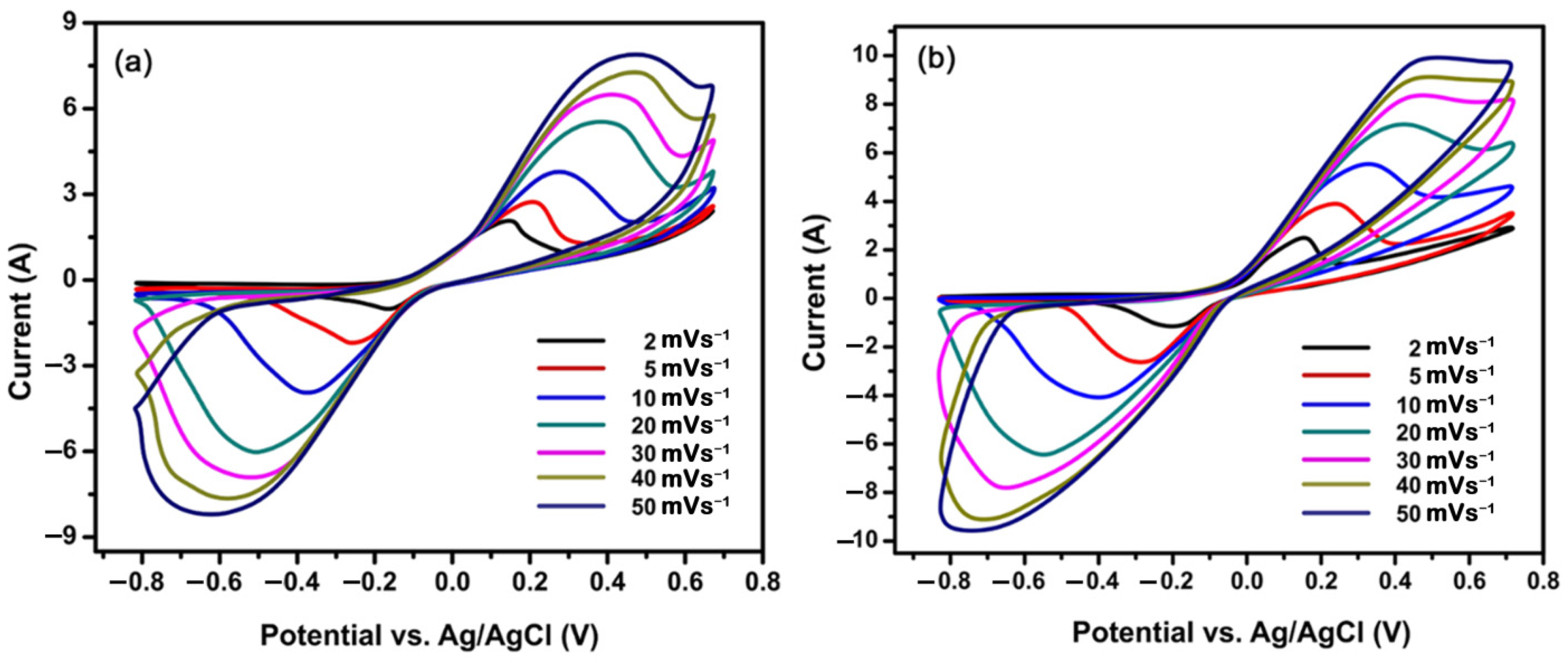
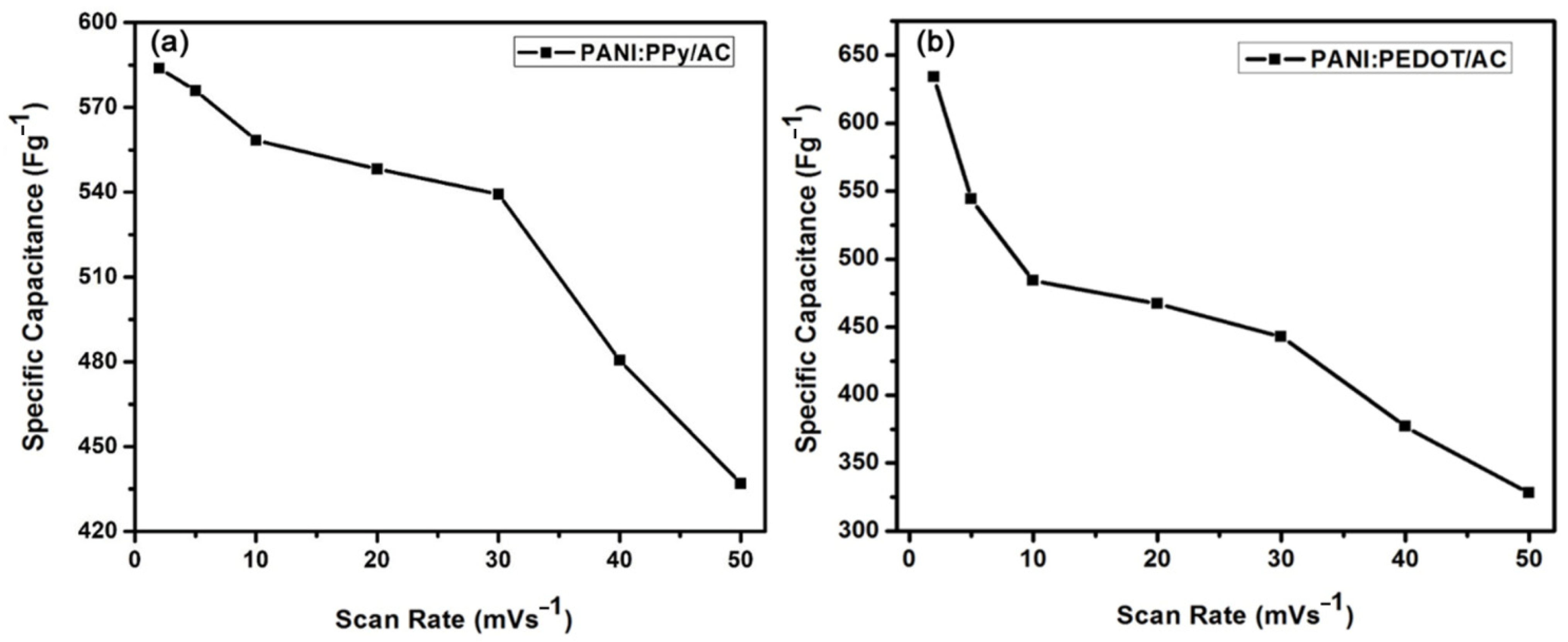
4.4. Cyclic Voltammetry
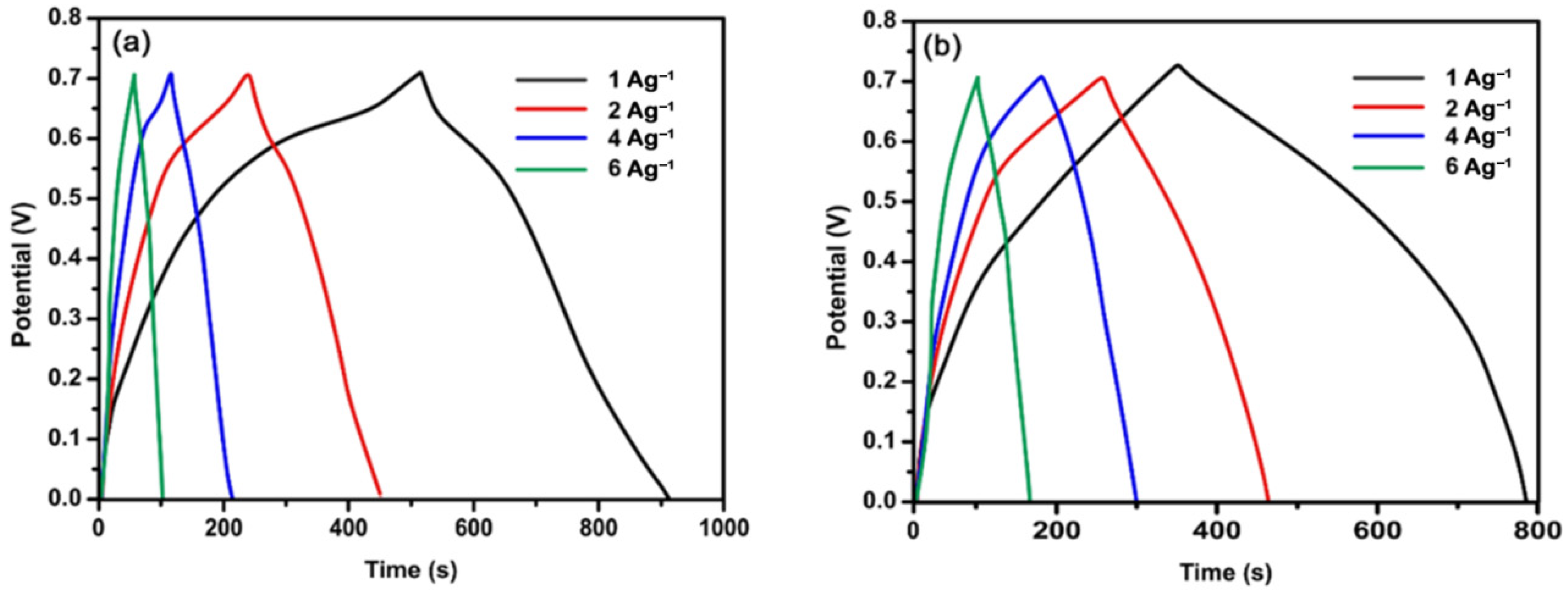
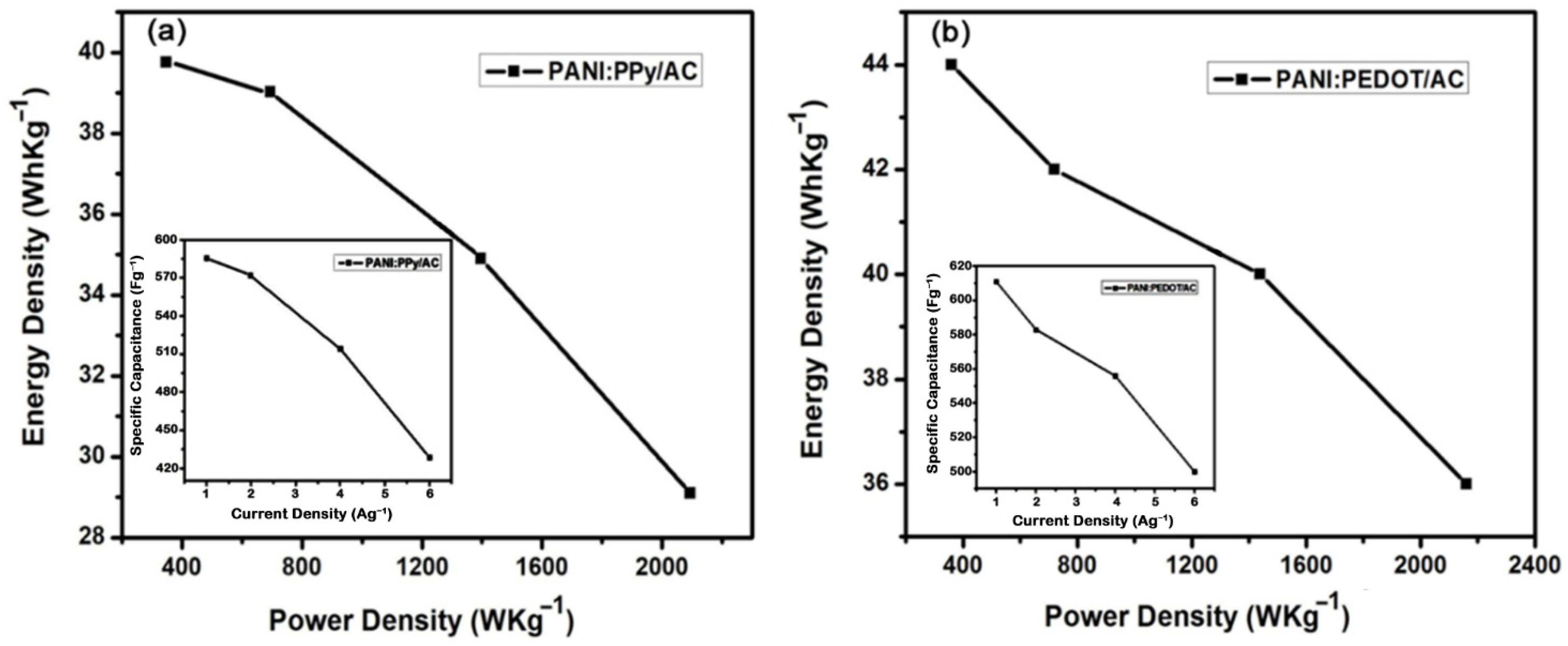
4.5. Galvanostatic Charge/Discharge
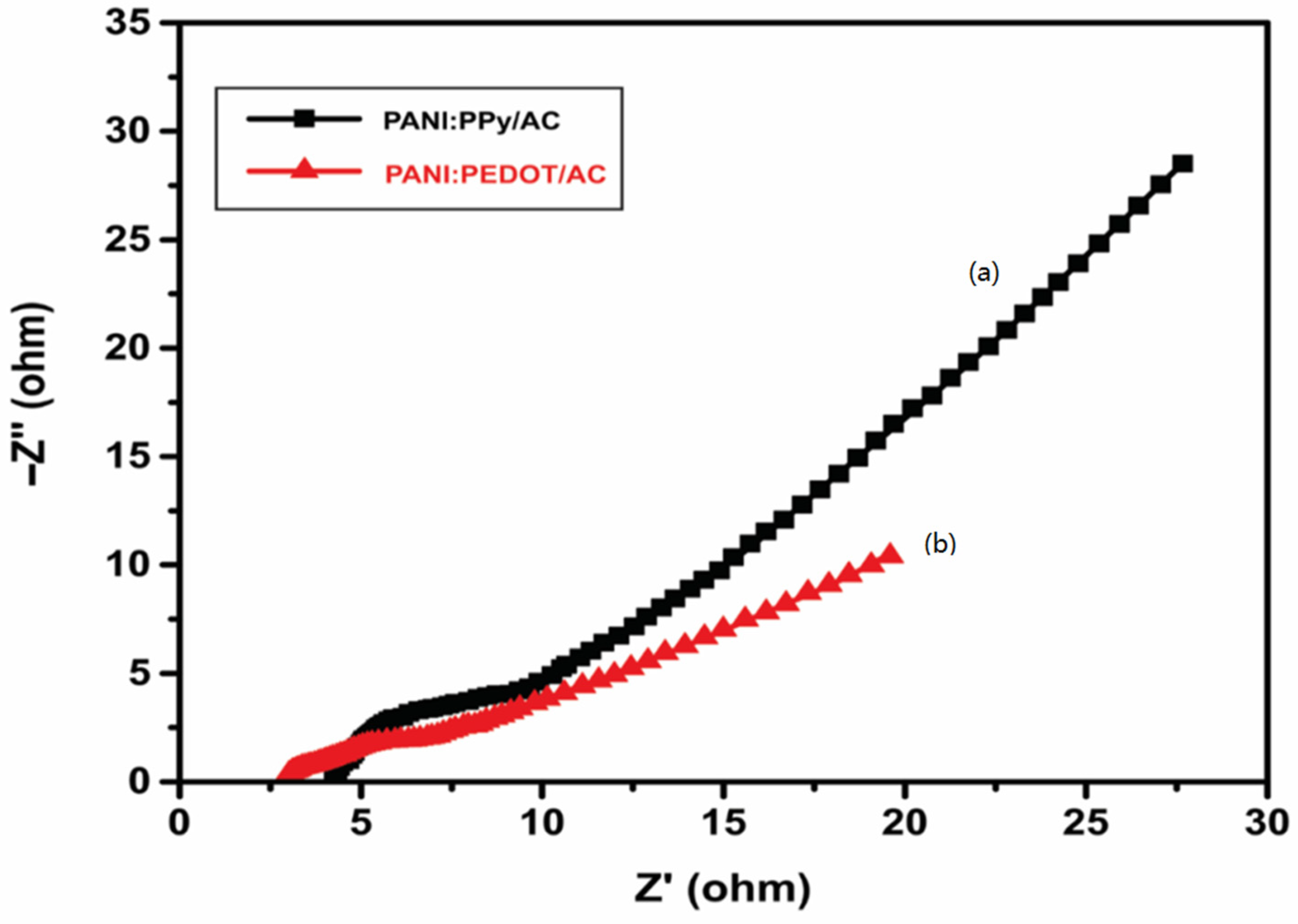
4.6. Electrochemical Impedance Spectroscopy
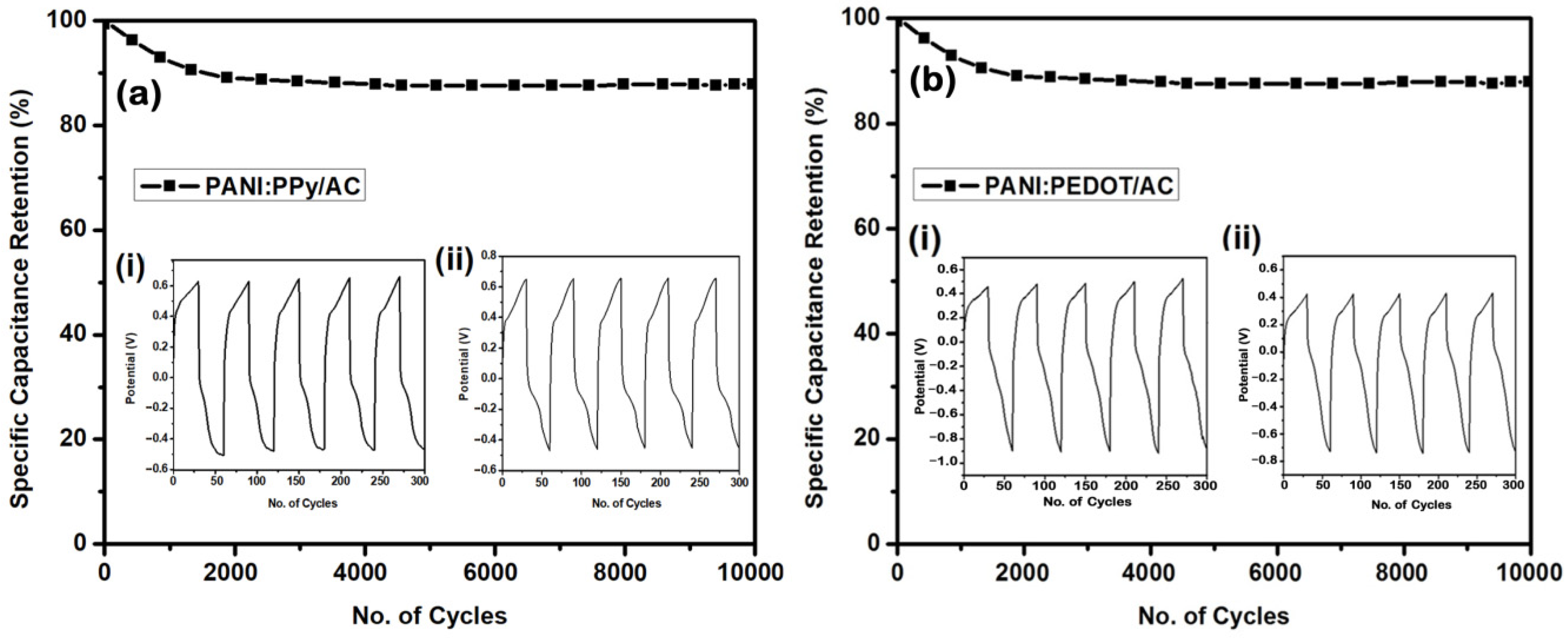
4.7. Cyclic Stability
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nishide, H.; Oyaizu, K. Toward flexible batteries. Science 2008, 319, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2008, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipomi, D.J.; Bao, Z. Stretchable, elastic materials and devices for solar energy conversion. Energy Environ. Sci. 2011, 4, 3314–3328. [Google Scholar] [CrossRef]
- Hussain, I.M.; Khalil AM, R.; Hussain, F. Computational Exploration of Structural, Electronic, and Optical Properties of Novel Combinations of Inorganic Ruddlesden–Popper Layered Perovskites Bi2XO4 (X = Be, Mg) using Tran and Blaha-Modified Becke–Johnson Approach for Optoelectronic Applications. Energy Technol. 2021, 9, 2001026. [Google Scholar] [CrossRef]
- Khan, S.U.D.; Almutairi, Z.A.; Al-Zaid, O.S. Development of low concentrated solar photovoltaic system with lead acid battery as storage device. Curr. Appl. Phys. 2020, 20, 582–588. [Google Scholar] [CrossRef]
- Jintao, Z.; Zhenhai, X.; Liming, D. Carbon-based electrocatalysts for advanced energy conversion and storage. Science 2015, 1, e1500564. [Google Scholar]
- Wu, Z.; Sun, Y.; Yuan-Zhi, T.Y.; Yang, S.; Feng, X.; Mullen, K. Three-Dimensional Graphene Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef]
- Shakoor, A.; Rizvi, T.Z.; Sulaiman, M.; Nasir, M.; Ishtiaq, M. Electronic properties of polyaniline doped with dodecylbenzenesulphonic acid (PANI-DBSA) and poly (methyl methacrylate)(PMMA) blends in the presence of hydroquinone. J. Mater. Sci. Mater. Electron. 2010, 21, 603–607. [Google Scholar] [CrossRef]
- Barbieri, O.; Hahn, M.; Foelske, A.; Kotz, R. Effect of Electronic Resistance and Water Content on the Performance of RuO2 for Supercapacitors. J. Electrochem. Soc. 2006, 153, A2049. [Google Scholar] [CrossRef]
- Hu, C.C.; Chen, C.W.; Chang, H.K. How to Achieve Maximum Utilization of Hydrous Ruthenium Oxide for Supercapacitors? J. Electrochem. Soc. 2004, 151, A281. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, B.K. Electrochemical Characterization of Hydrous Ruthenium Oxide Thin-Film Electrodes for Electrochemical Capacitor Applications. J. Electrochem. Soc. 2006, 153, A383. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portes, C.; Simon, P.P.L.; Taberna, L.P. Effect of pore size and surface area of carbide derived carbons on specific capacitance. Science 2006, 313, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the Nanoporous Texture of Activated Carbons and Their Capacitance Properties in Different Electrolytes. Carbon 2006, 44, 2498. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Zhan, J. Biological cell template synthesis of nitrogen-doped porous hollow carbon spheres/MnO2 composites for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 296, 907–915. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Shinya, N.; Qin, C.L. Polyaniline nano-cone coated graphene and carbon nanotube composite electrode for asymmetric supercapacitors with high energy density. J. Power Sources 2013, 241, 423–428. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. High performance electrochemical super capacitor from electrochemically synthesized nano-structured polyaniline. Mater. Lett. 2006, 60, 1466–1469. [Google Scholar] [CrossRef]
- Ryu, S.K.; Kim, M.K.; Park, G.N.; Park, J.Y.; Chang, H.S. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Le, T.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, N.R. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured Electrode Materials for Electrochemical Capacitor Applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.D.; Yoon, H. Recent advances in nanostructured conducting polymers: From synthesis to practical applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Anwar, H.; Rizvi, T.Z. Preparation, characterization and conductivity study of polypyrrole-pillared clay nanocomposites. J. Compos. Mater. 2008, 42, 2101–2109. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, H.; Luo, S.; Lu, G.; Wei, Z.W.; Kuang, Y. The effect of the polyaniline morphology on the performance of polyaniline supercapacitors. J. Solid State Electrochem. 2005, 9, 574. [Google Scholar] [CrossRef]
- Fusalba, F.; Gouerec, P.; Villers, D.; Belanger, D. Electrochemical Characterization of Polyaniline in Nonaqueous Electrolyte and Its Evaluation as Electrode Material for Electrochemical Supercapacitors. J. Electrochem. Soc. 2001, 148, A1. [Google Scholar] [CrossRef]
- Mondal, K.S.; Prasad, R.K.; Munichandraiah, N. Analysis of electrochemical impedance of polyaniline films prepared by galvanostatic, potentiostatic and potentiodynamic methods. Synthesis 2005, 148, 275. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, X.Z. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Dubal, P.D.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef]
- Qi, G.; Huang, L.; Wang, H. Highly conductive free standing polypyrrole films prepared by freezing interfacial polymerization. Chem. Commun. 2012, 48, 8246–8248. [Google Scholar] [CrossRef]
- Xiong, P.; Zhu, J.; Wang, X. Recent advances on multi-component hybrid nanostructures for electrochemical capacitors. J. Power Sources 2015, 294, 31–50. [Google Scholar] [CrossRef]
- Ramya, R.; Sivasubramanian, R.; Sangaranarayanan, V.M. Conducting polymers-based electrochemical supercapacitors-Progress and prospects. Electrochim. Acta 2013, 101, 109–129. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudo capacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sanchez, M.B.; Dobson, J.P.; Grant, S.P. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Dubal, P.D.; SHLee, H.S.; Kim, G.J.; Kim, B.W.; Lokhande, D.C. Porous polypyrrole clusters prepared by electro-polymerization for a high performance supercapacitor. J. Mater. Chem. 2012, 22, 3044–3052. [Google Scholar] [CrossRef]
- Wu, F.Q.; He, X.K.; Mi, Y.H.; Zhang, G.X. Electrochemical capacitance of polypyrrole nanowire prepared by using cetyltrimethylammonium bromide (CTAB) as soft template. Mater. Chem. Phys. 2007, 101, 367–371. [Google Scholar] [CrossRef]
- Anuradha, B.; Ravindra, N.; Prashant, R. Chemically synthesized 3D nanostructured polypyrrole electrode for high performance supercapacitor applications. J. Mater. Sci. Mater. Electron. 2018, 29, 15699–15707. [Google Scholar]
- Czardybon, A.; Lapkowski, M. Synthesis and electropolymerisation of 3,4-ethylenedioxythiophene functionalised with alkoxy groups. Synth. Met. 2001, 119, 161. [Google Scholar] [CrossRef]
- Groenendaal, L.B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater 2000, 12, 481. [Google Scholar] [CrossRef]
- Dietrich, M.; Heinze, J.; Heywang, G.; Jonas, F. Electrochemical and spectroscopic characterization of polyalkylenedioxythiophenes. J. Electroanal. Chem. 1994, 369, 87. [Google Scholar] [CrossRef]
- Patra, A.; Bendikov, M. Chand S Poly(3,4-ethylenedioxyselenophene) and its derivatives: Novel organic electronic materials. Acc. Chem. Res. 2014, 47, 1465–1474. [Google Scholar] [CrossRef]
- Pettersson, L.A.; Carlsson, F.; Inganas, O.; Arwin, H. Spectroscopic ellipsometry studies of the optical properties of doped poly(3,4-ethylenedioxythiophene) an anisotropic metal. Thin Solid Film. 1998, 313, 356. [Google Scholar] [CrossRef]
- Groenendaal, L.B.; Zotti, G.; Aubert, P.H.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of Poly(3,4-alkylenedioxythiophene) Derivatives. Adv. Mater 2003, 15, 855. [Google Scholar] [CrossRef]
- Jurewicz, K.; Delpeux, S.; Bertagna, V.; Beguin, F.; Frackowiak, E. Supercapacitors from nanotubes/polypyrrole composites. J. Chem. Phys. Lett. 2001, 347, 36–40. [Google Scholar] [CrossRef]
- An, H.K.; Jeon, K.K.; Heo, K.J.; Lim, C.S.; Bae, J.D.; Lee, H.Y. High-Capacitance Supercapacitor Using a Nanocomposite Electrode of Single-Walled Carbon Nanotube and Polypyrrole. J. Electrochem. Soc. 2002, 149, A1058. [Google Scholar] [CrossRef]
- Park, K.J.; Ko, M.J.; Park, O.O.; Kim, W.D. Capacitance properties of graphite/polypyrrole composite electrode prepared by chemical polymerization of pyrrole on graphite fiber. J. Power Sources 2002, 105, 20. [Google Scholar] [CrossRef]
- Ali, N.I.; Tan HT, D.; Madden, D.J. Towards High Power Polypyrrole/Carbon Capacitors. Synth. Met. 2005, 152, 129. [Google Scholar]
- Han, G.; Yuan, J.; Shi, G.; Wei, F. Electrodeposition of polypyrrole/multiwalled carbon nanotube composite films. Thin Solid Film. 2005, 474, 64. [Google Scholar] [CrossRef]
- Ham, T.H.; Choi, S.Y.; Jeong, N.; Chung, J.I. Singlewall carbon nanotubes covered with polypyrrole nanoparticles by the miniemulsion polymerization. Fluid Phase Equilib. 2005, 234, 6308. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Beguin, F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations. Electrochim. Acta 2005, 50, 2499. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295. [Google Scholar] [CrossRef]
- Ko, M.J.; Song, R.Y.; Yu, H.J.; Yoon, J.W.; Min, B.G.; Kim, D.W. Capacitive performance of the composite electrodes consisted of polyaniline and activated carbons powder in a solid-like acid gel electrolyte. Electrochim. Acta 2004, 50, 873. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Omastova, M.; Pionteck, J.; Trchová, M. Properties and morphology of polypyrrole containing a surfactant. Synth. Met. 2003, 135, 437–438. [Google Scholar] [CrossRef]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.K.; Samanta, S.B.; Chand, S. Application of electrochemically prepared polypyrrole–polyvinyl sulphonate films to DNA biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivalu, S.; Zainal, Z.; Sulaiman, Y. Influence of monomer concentration on the morphologies and electrochemical properties of PEDOT, PANI, and PPy prepared from aqueous solution. Int. J. Polym. Sci. 2016, 2016, 8518293. [Google Scholar] [CrossRef] [Green Version]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Kulandaivalsulu, S.; Zainal, Z.; Sulaiman, Y. A New Approach for Electrodeposition of (PEDOT/PANI) Copolymer. Int. J. Electrochem. Sci. 2015, 10, 8926–8940. [Google Scholar]
- Chafidz, A.; Astuti, W.; Augustia, V.; Novira, D.T.; Rofiah, N. Removal of methyl violet dye via adsorption using activated carbon prepared from Randu sawdust (Ceiba pentandra). IOP Conf. Ser. Earth Environ. Sci. 2018, 167, 012013. [Google Scholar] [CrossRef]
- Patil, S.S.; Koiry, S.P.; Veerender, P.; Aswal, D.K.; Gupta, S.K.; Joag, D.S.; More, M.A. Synthesis of vertically aligned polyaniline (PANI) nanofibers, nanotubes on APTMS monolayer and their field emission characteristics. RSC Adv. 2012, 2, 5822–5827. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Chauhan, N.; Dahiya, T.; Pundir, C.S. Construction of amperometric uric acid biosensor based on uricase immobilized on PBNPs/cMWCNT/PANI/Au composite. Int. J. Biol. Macromol. 2012, 50, 112–118. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Chemical synthesis of PEDOT–Au nanocomposite. Nanoscale Res. Lett. 2007, 2, 546. [Google Scholar] [CrossRef] [Green Version]
- Abac, U.; Guney, H.Y.; Kadiroglu, U. Morphological and electrochemical properties of PPy, PAni bilayer films and enhanced stability of their electrochromic devices (PPy/PAni–PEDOT, PAni/PPy–PEDOT). Electrochim. Acta 2013, 96, 214–224. [Google Scholar] [CrossRef]
- Wen, P.; Fan, M.; Yang, D.; Wang, Y.; Cheng, H.; Wang, J. An asymmetric supercapacitor with ultrahigh energy density based on nickle cobalt sulfide nanocluster anchoring multi-wall carbon nanotubes hybrid. J. Power Sources 2016, 320, 28–36. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, K.G.; Radhakrishnan, S.; Kim, J.S. One pot hydrothermal growth of hierarchical nanostructured Ni3S2 on Ni foam for super capacitor application. Chem. Eng. J. 2014, 251, 116–122. [Google Scholar] [CrossRef]
- Huang, J.K.; Zhang, Z.J.; Cai, L.J. Preparation of porous layered molybdenum selenide-graphene composites on Ni foam for high-performance super capacitor and electrochemical sensing. Electrochim. Acta 2015, 180, 770–777. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, A.S. Nanosized rambutan-like nickel oxides as electrochemical sensor and pseudo capacitor. Sens. Actu. B Chem. 2014, 193, 644–652. [Google Scholar] [CrossRef]
- María, J.; Martínez, B.; Peng, C.; Zhang, S.; George, Z.C.; Morallón, E.; Amorós, C.D. Electrochemical Methods to Enhance the Capacitance in Activated Carbon/Polyaniline Composites. J. Electrochem. Soc. 2008, 155, A672. [Google Scholar]
- Kujundziski, P.A.; Chamovska, D.; Grchev, T. Capacitive properties of polypyrrole/activated carbon composite. Hem. Ind. 2014, 68, 709–719. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, F.; Dai, Y.; Zhang, H.; Lei, J. Electrosynthesis and Performance of Poly(aniline/pyrrole) Copolymer. Int. J. Electrochem. Sci. 2016, 11, 4000–4006. [Google Scholar] [CrossRef]
- Huang, J.K.; Zhang, Z.J.; Liu, Y.; Liu, M.Y. Synthesis of reduced graphene oxide wrapped-copper sulfide hollow spheres as electrode material for supercapacitor. Inter. J. Hydrogen Energy 2015, 40, 10158–10167. [Google Scholar] [CrossRef]
- Veeraraghavan, B.; Paul, J.; Haran, B.; Popov, B. Study of polypyrrole graphite composite as anode material for secondary lithium-ion batteries. J. Power Sources 2002, 109, 377–387. [Google Scholar] [CrossRef]
- Grchev, T.; Cvetkovska, M.; Obradovic, T. Redox properties of an electrochemically activated (oxidized) carbon fibre electrode. J. Serb. Chem. Soc. 1997, 62, 157–164. [Google Scholar]
- Ates, M. Review study of electrochemical impedance spectroscopy and equivalent electrical circuits of conducting polymers on carbon surfaces. Prog. Org. Coat. 2011, 71, 1–10. [Google Scholar] [CrossRef]
- Jovanovic, M.S.; Stankovic, R.; Laninovic, V.; Nestorovic, G; Popovic, M.; Vidic, B.; Pavlovic, O.; Krstajic, N.; Grgur, B.; Vojnovic, M.; et al. Synthesis and electrochemical properties of polypyrrole, polyaniline and poly-3-methyl thiophene. Hem. Ind. 2000, 54, 417–427. [Google Scholar]
- Ho, C.; Raistrick, I.D.; Huggins, R.A. Application of A-C Techniques to the Study of Lithium Diffusion in Tungsten Trioxide Thin Films. J. Electrochem. Soc. 1980, 127, 343–350. [Google Scholar] [CrossRef]
- Song, Z.; Li, L.; Zhu, D.; Miao, L.; Duan, H.; Wang, Z.; Xiong, W.; Lv, Y.; Liu, M.; Gan, L. Synergistic design of a N, O co-doped honeycomb carbon electrode and an ionogel electrolyte enabling all-solid-state supercapacitors with an ultrahigh energy density. J. Mater. Chem. A 2019, 7, 816–826. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Prakash, A.; Bahadur, D. The role of ionic electrolytes on capacitive performance of ZnO-reduced graphene oxide nanohybrids with thermally tunable morphologies. ACS Appl. Mater. Inter. 2014, 6, 1394–1405. [Google Scholar] [CrossRef]
- Tuken, T.; Ozyılmaz, A.T.; Erbil, M. Electrochemical synthesis of polyaniline on mild steel in acetonitrile-LiClO4 and corrosion performance. Appl. Surf. Sci. 2004, 1, 292–305. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Zhang, Y.; Wang, L.Z.; Li, X.F. Electro-synthesis and capacitive performance of polyaniline–Polypyrrole composite. Polym. Compos. 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Inhibition of corrosion of mild steel by homopolymer and bilayer coatings of polyaniline and polypyrrole. Prog. Org. Coat. 2007, 59, 297–303. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, S.; An, Y.; Wu, S.; Meng, C. Bio-inspired high performance electrochemical supercapacitors based on conducting polymer modified coral-like monolithic carbon. J. Mater. Chem. A 2013, 1, 8876–8887. [Google Scholar] [CrossRef]
- Fu, H.; Du, Z.; Zou, W.; Lia, H.; Zhang, C. Carbon nanotube reinforced polypyrrole nanowire network as a high-performance super capacitor electrode. J. Mater. Chem. A 2013, 1, 14943–14950. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.X. Conducting Polymers Directly Coated on Reduced Graphene Oxide Sheets as High-Performance Supercapacitor Electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Khan, S.; Majid, A.; Raza, R. Synthesis of PEDOT:PPy/AC composite as an electrode for supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 13597–13609. [Google Scholar] [CrossRef]
- Chen, W.C.; Wen, T.C.; Teng, H. Polyaniline-deposited porous carbon electrode for supercapacitor. Electrochim. Acta 2003, 48, 641–649. [Google Scholar] [CrossRef]
- Kai, Z.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Graphene oxide doped polyaniline for supercapacitors. Electrochem. Commun. 2009, 11, 1158–1161. [Google Scholar] [CrossRef]
- Ates, M.; Serin, M.A.; Ekmen, I.; Ertas, Y.N. Supercapacitor behaviors of polyaniline/CuO, polypyrrole/CuO and PEDOT/CuO nanocomposites. Polym. Bull. 2015, 72, 2573–2589. [Google Scholar] [CrossRef]
- Giri, S.; Ghosh, D.; Das, C.K. In situ synthesis of cobalt doped polyaniline modified graphene composites for high performance supercapacitor electrode materials. J. Electroanal. Chem. 2013, 697, 32–45. [Google Scholar] [CrossRef]
- Prasankumar, T.; Wiston, B.R.; Gautam, C.R.; Ilangovan, R.; Jose, S.P. Synthesis and enhanced electrochemical performance of PANI/Fe3O4 nanocomposite as supercapacitor electrode. J. Alloys Compd. 2018, 757, 466–475. [Google Scholar] [CrossRef]
- Lu, W.; Chen, L.; Yan, B.; Wang, C.; Zhu, F.; Jiang, X.; Chao, Y.; Yang, G. In situ preparation of SnO 2@ polyaniline nanocomposites and their synergetic structure for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 8334–8341. [Google Scholar]
- Rajesh, M.; Raj, J.; Manikandan, R.; Kim, C.B.; Park, Y.S.; Yu, H.K. A high performance PEDOT/PEDOT symmetric supercapacitor by facile in-situ hydrothermal polymerization of PEDOT nanostructures on flexible carbon fiber cloth electrodes. Mater. Today Energy 2017, 6, 96–104. [Google Scholar] [CrossRef]
- Dan, W.; Xie, X.; Ma, Y.; Zhang, J.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Morphology controlled hierarchical NiS/carbon hexahedrons derived from nitrilotriacetic acid-assembly strategy for high-performance hybrid supercapacitors. Chem. Eng. J. 2022, 433, 133673. [Google Scholar]
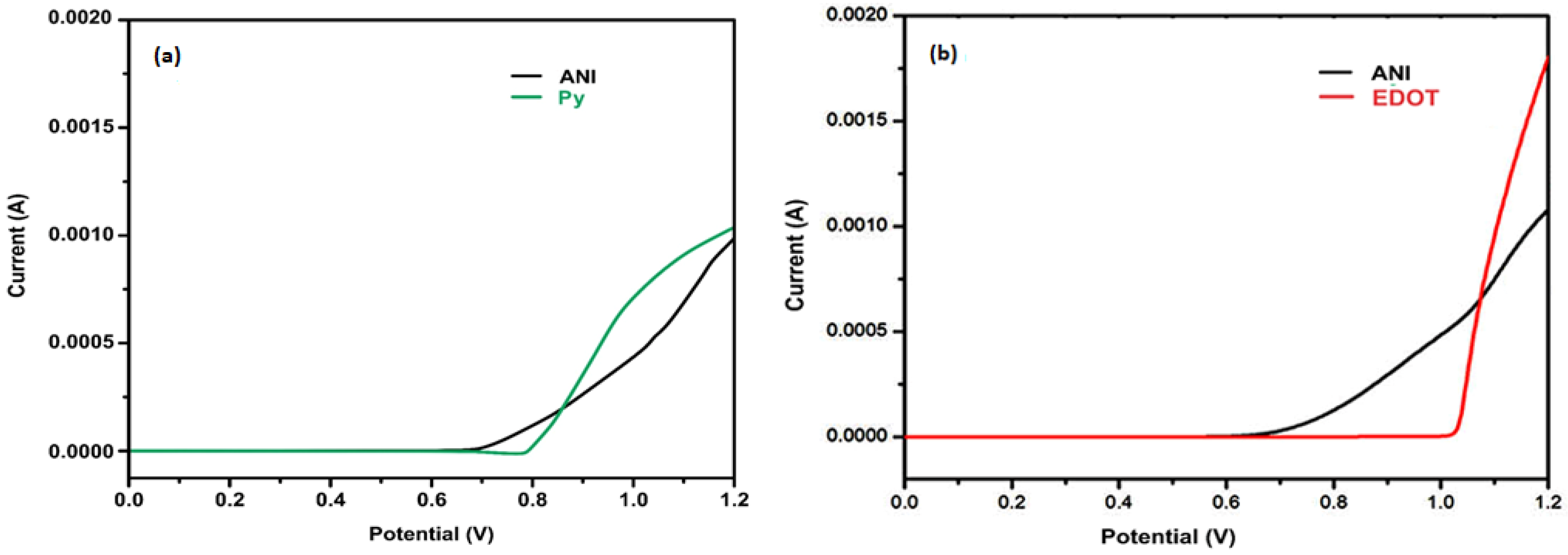
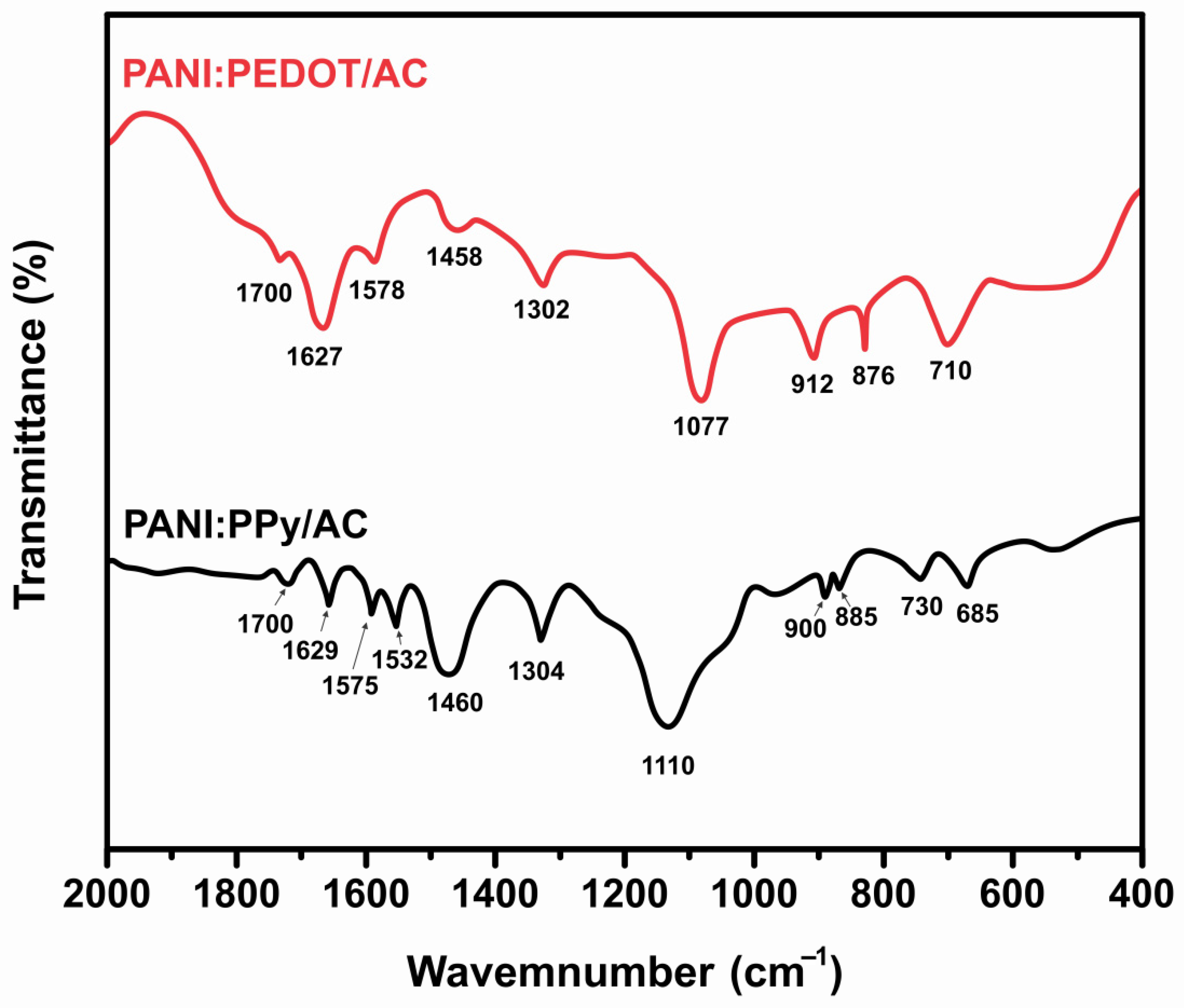
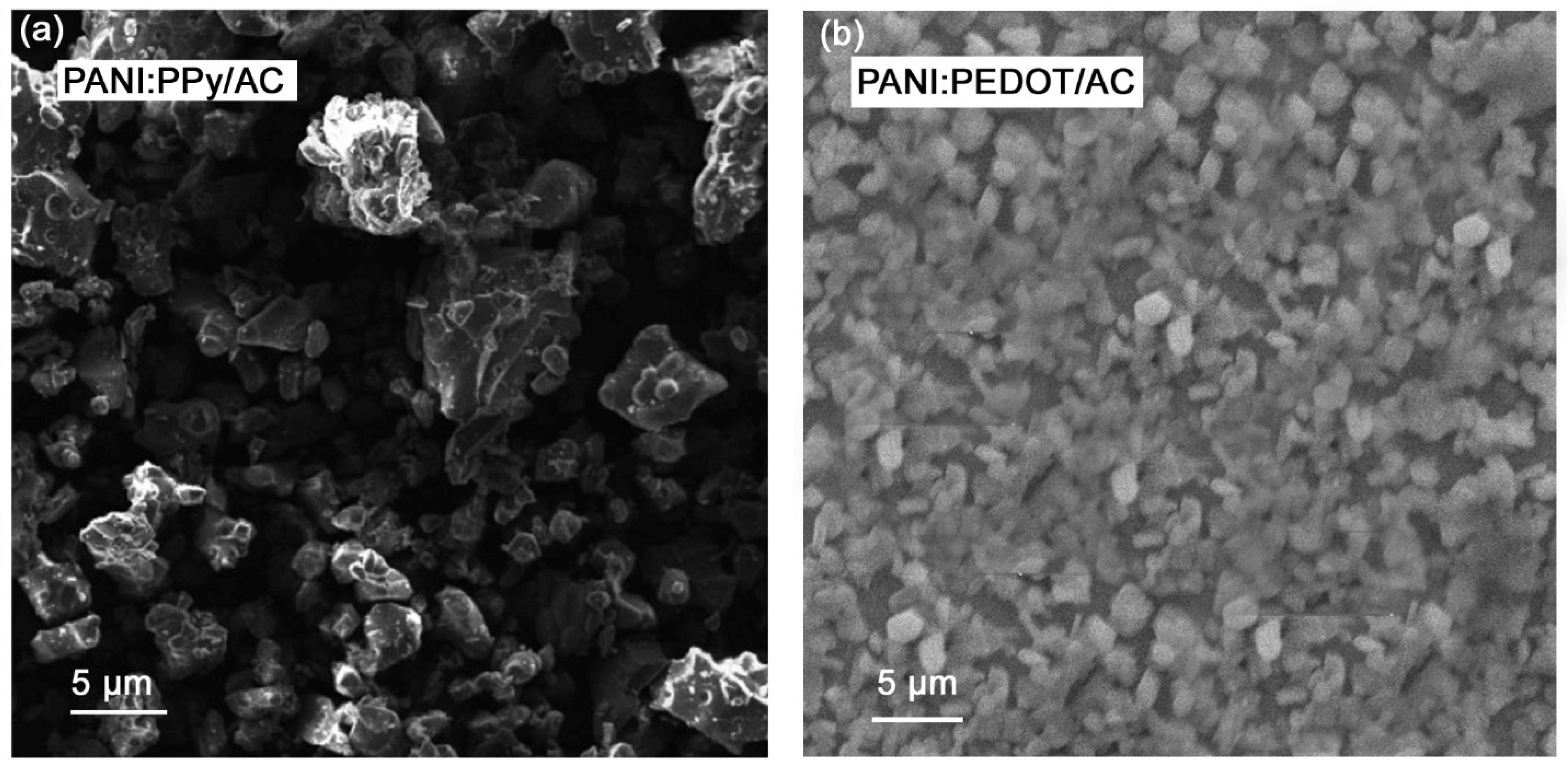
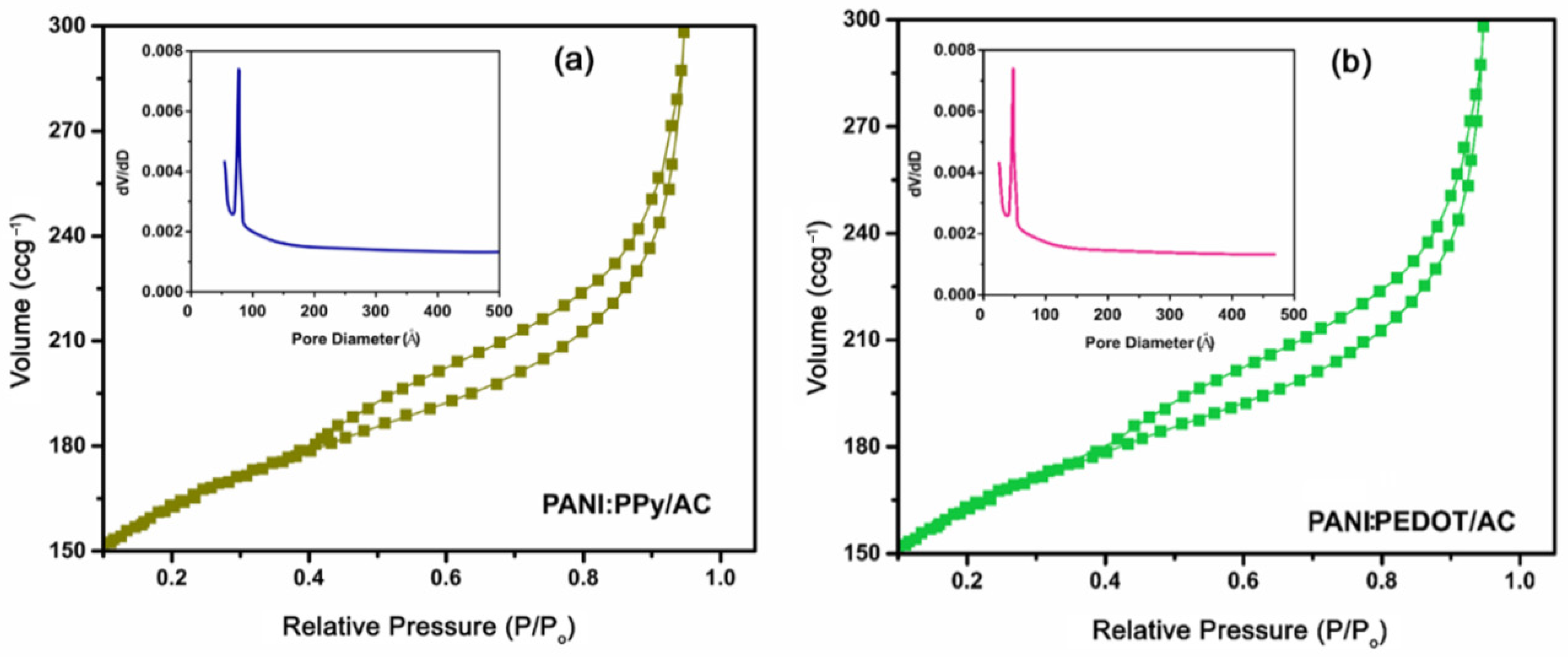
| Current Density (Ag−1) | Specific Capacitance (Fg−1) | Energy Density (Whkg−1) | Power Density (Wkg−1) |
|---|---|---|---|
| 1 | 586 | 40 | 349 |
| 2 | 571 | 39 | 698 |
| 4 | 514 | 35 | 1396 |
| 6 | 429 | 29 | 2094 |
| Current Density (Ag−1) | Specific Capacitance (Fg−1) | Energy Density (Whkg−1) | Power Density (Wkg−1) |
|---|---|---|---|
| 1 | 611 | 44 | 360 |
| 2 | 583 | 42 | 720 |
| 4 | 556 | 40 | 1440 |
| 6 | 500 | 36 | 2160 |
| Composite | Synthesis Technique | Specific Capacitance | Cyclic Stability | References |
|---|---|---|---|---|
| PANI/Porous Carbon | electrochemical polymerization | 180 Fg−1 | 91% after 1000 cycles | [86] |
| PANI/MWCNTS | chemical oxidative polymerization | 320 Fg−1 | 8% after 50 cycles | [49] |
| GRAPHENE/PANI NANOFIBER | in situ polymerization | 480 Fg−1 | 70% after 1000 cycles | [87] |
| GO/PANI | a soft chemical route | 531 Fg−1 | N/A | [88] |
| PANI/CUO | in situ polymerization | 286.35 Fg−1 | N/A | [89] |
| RUO2/PANI | in situ oxidative polymerization | 425 Fg−1 | N/A | [90] |
| PANI/Fe3O4 | in situ polymerization | 572 Fg−1 | 82% over 5000 cycles | [91] |
| SNO2/PANI | in situ oxidative polymerization | 335.5 Fg−1 | 99% after 1000 cycles | [92] |
| PANI/AC | electrochemical polymerization | 200 Fg−1 | N/A | [67] |
| PANI:PEDOT | electrochemical polymerization | 0.62 mFcm−2 | N/A | [57] |
| PANI:PPy | electrochemical polymerization | 227 Fg−1 | N/A | [69] |
| PANI:PPy/AC | electrochemical polymerization | 586 Fg−1 | 92% after 10,000 cycles | * |
| PANI:PEDOT/AC | electrochemical polymerization | 611 Fg−1 | 90% after 10,000 cycles | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Alkhedher, M.; Raza, R.; Ahmad, M.A.; Majid, A.; Din, E.M.T.E. Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors. Polymers 2022, 14, 1976. https://doi.org/10.3390/polym14101976
Khan S, Alkhedher M, Raza R, Ahmad MA, Majid A, Din EMTE. Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors. Polymers. 2022; 14(10):1976. https://doi.org/10.3390/polym14101976
Chicago/Turabian StyleKhan, Shahbaz, Mohammad Alkhedher, Rizwan Raza, Muhammad Ashfaq Ahmad, Abdul Majid, and ElSayed M. Tag El Din. 2022. "Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors" Polymers 14, no. 10: 1976. https://doi.org/10.3390/polym14101976
APA StyleKhan, S., Alkhedher, M., Raza, R., Ahmad, M. A., Majid, A., & Din, E. M. T. E. (2022). Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors. Polymers, 14(10), 1976. https://doi.org/10.3390/polym14101976








