The Study of Cyclosporin A Nanocrystals Uptake and Transport across an Intestinal Epithelial Cell Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CsA-NCs
2.3. Particle Characterization
2.4. Characterization of CsA NCs
2.5. Particle Size Stability Studies
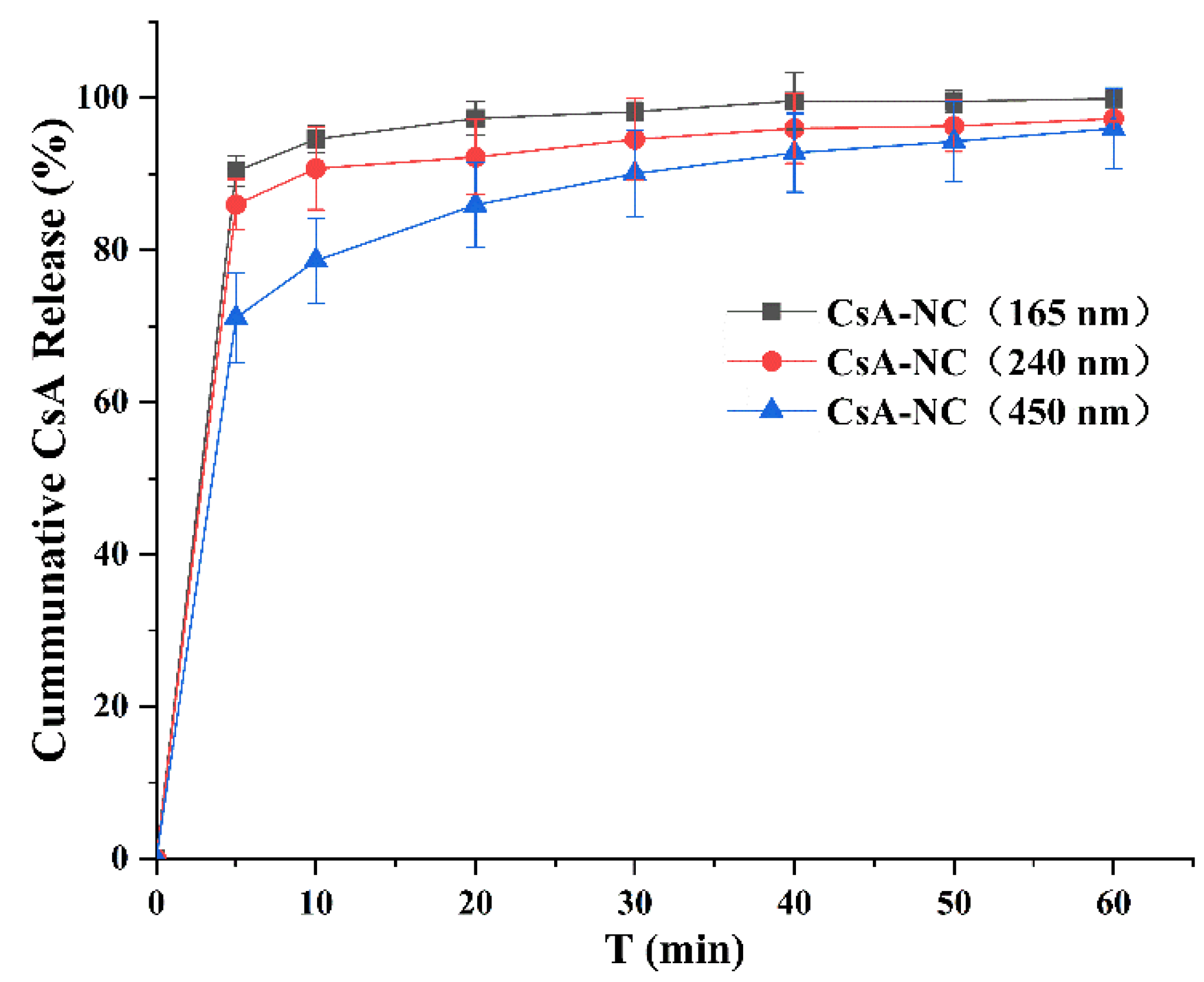
2.6. Dissolution Studies
2.7. HPLC Analysis
2.8. In Vitro Cellular Uptake and Monolayer Permeation
2.8.1. Cell Culture
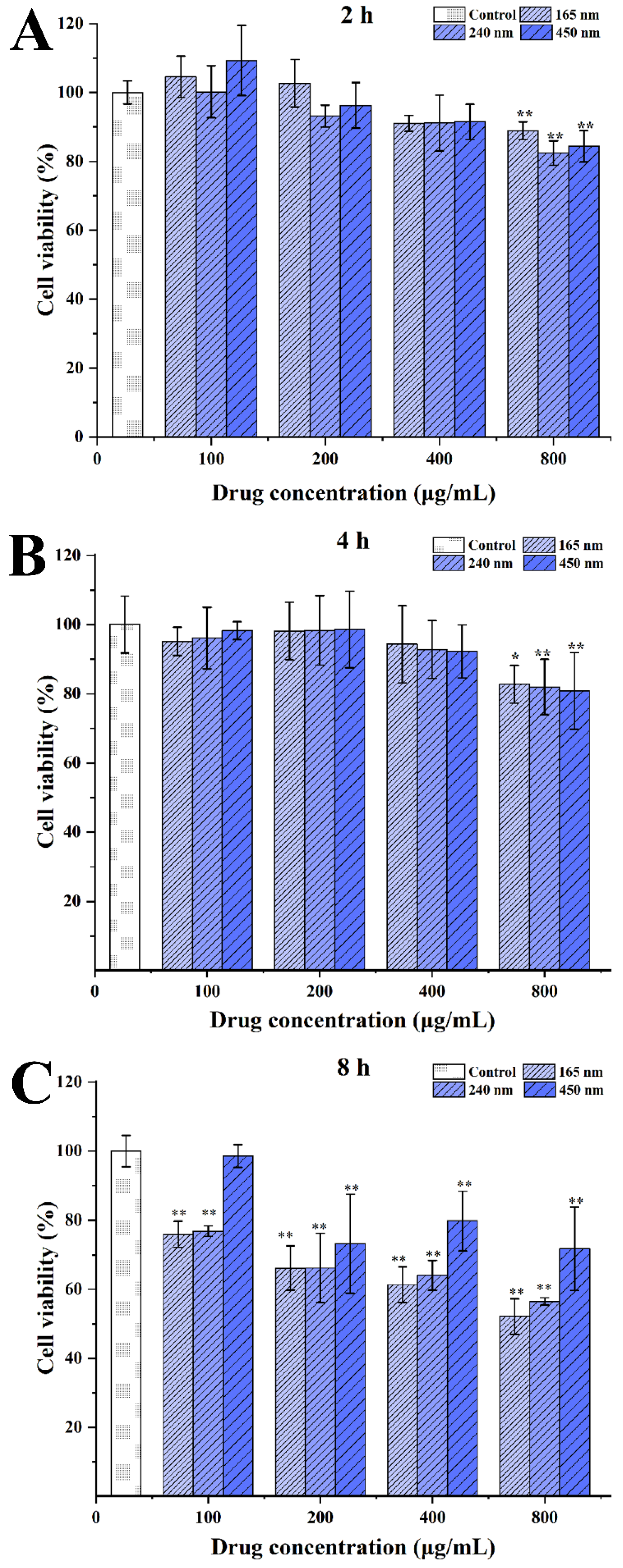
2.8.2. In Vitro Cytotoxicity
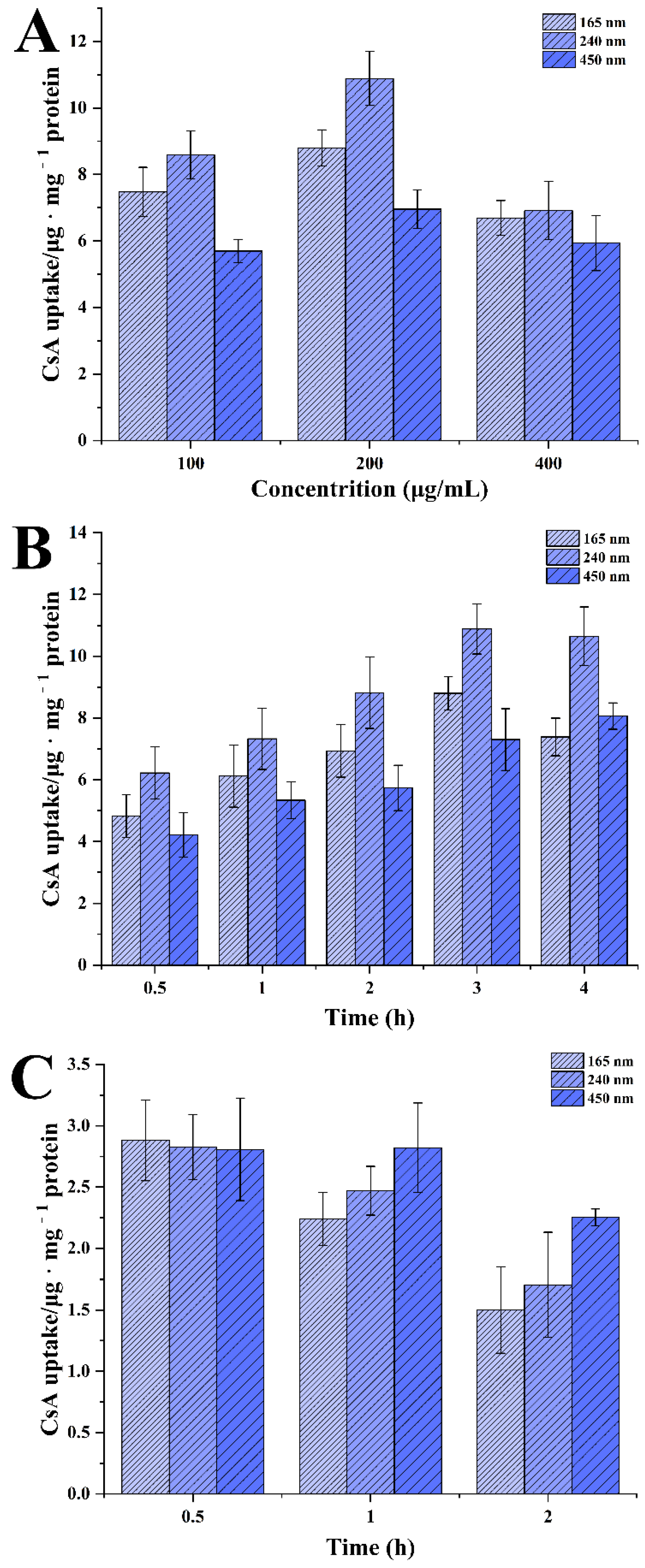
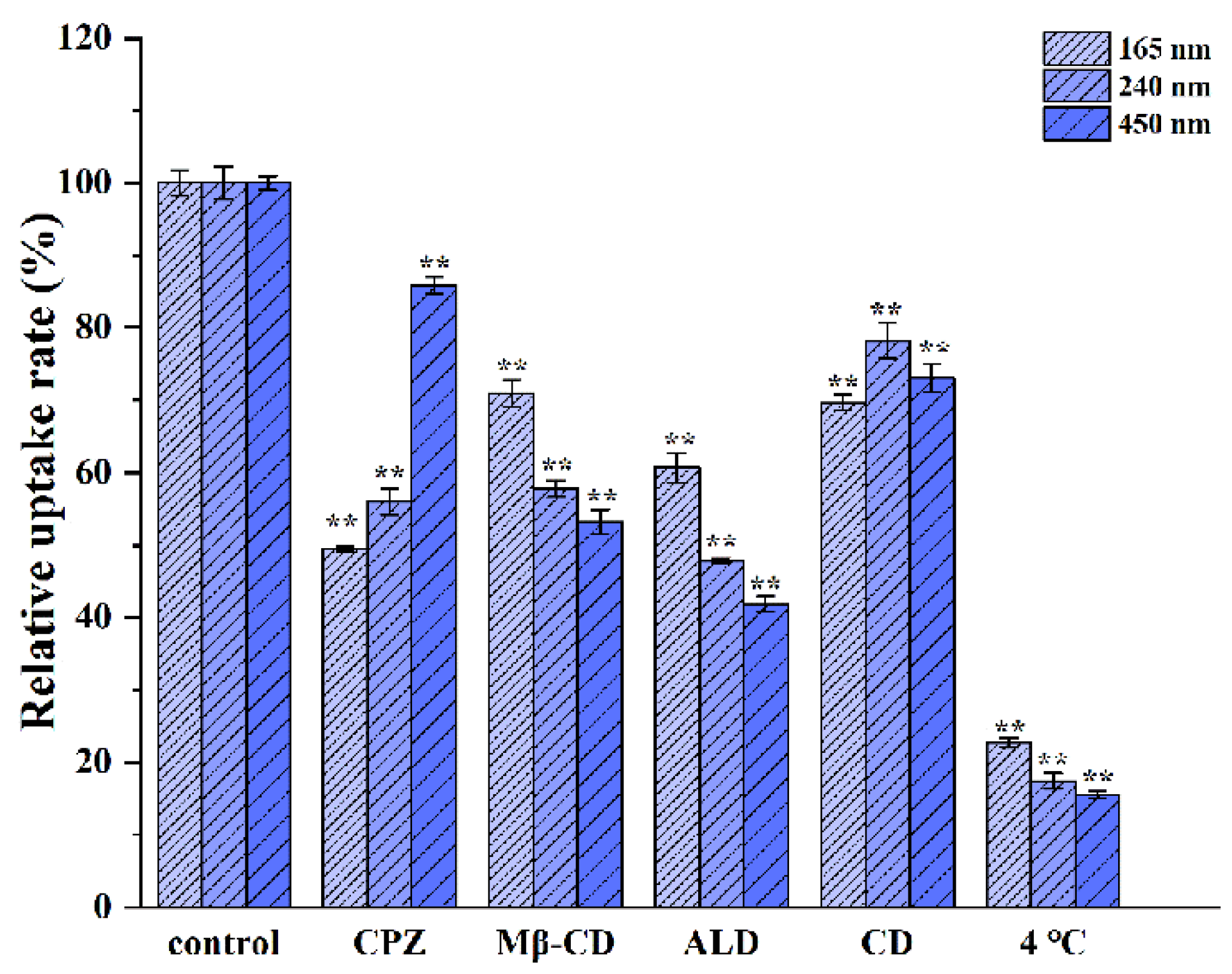
2.8.3. Cellular Uptake and Uptake Mechanism Analysis
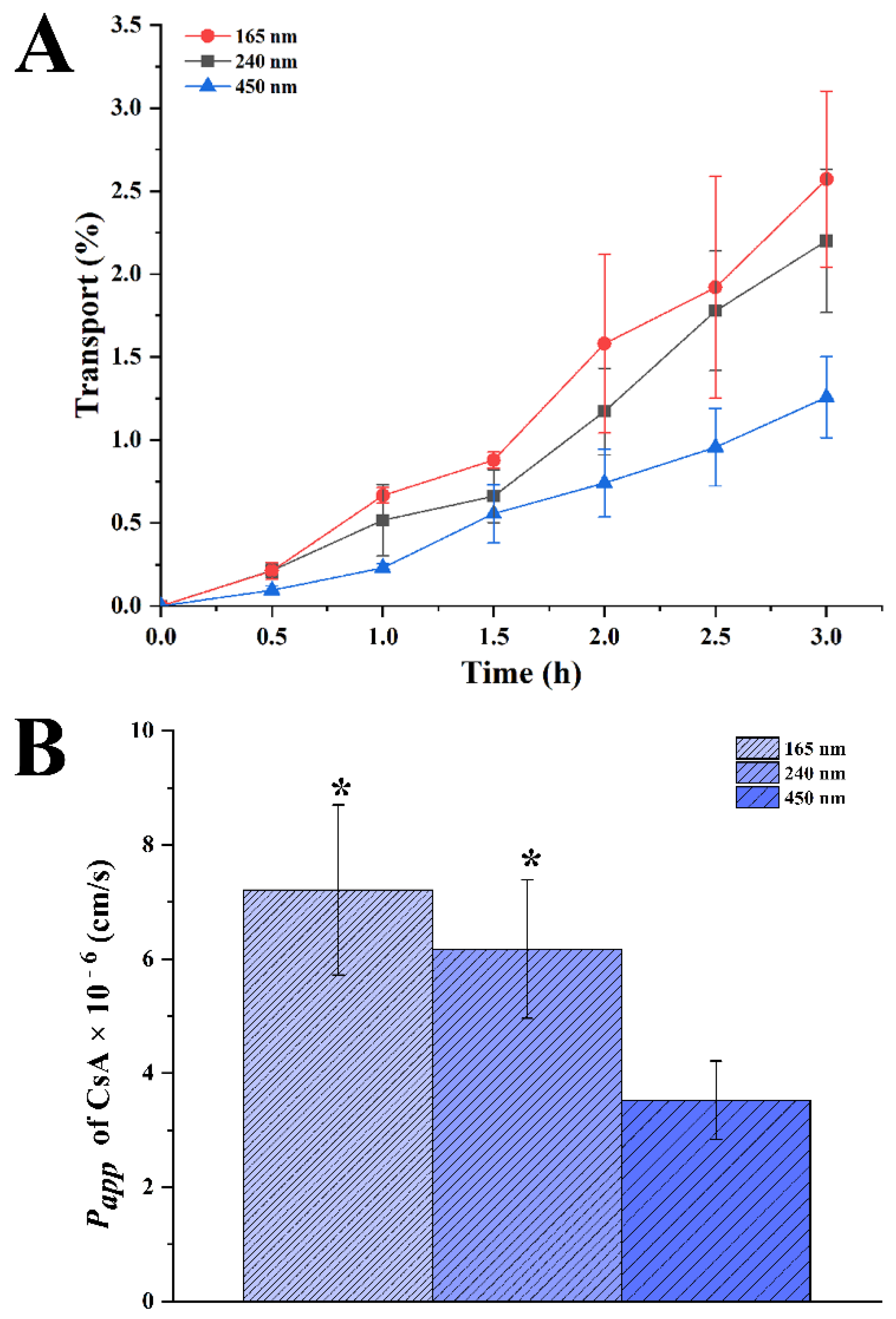
2.8.4. Transport of CsA-NCs across the Caco-2 Cell Monolayers
2.9. LC-MS/MS Measurement
3. Results and Discussion
3.1. Characterizations of the CsA-NCs
3.2. Dissolution Studies
3.3. In Vitro Cellular Uptake and Uptake Mechanism Analysis
3.3.1. Cytotoxicity
3.3.2. Effect of Particle Size, Incubation Concentration and Time on Uptake of CsA-NCs by Caco-2 Cells
3.3.3. Possible Uptake Pathways for Different-Sized CsA-NCs by Caco-2 Cells
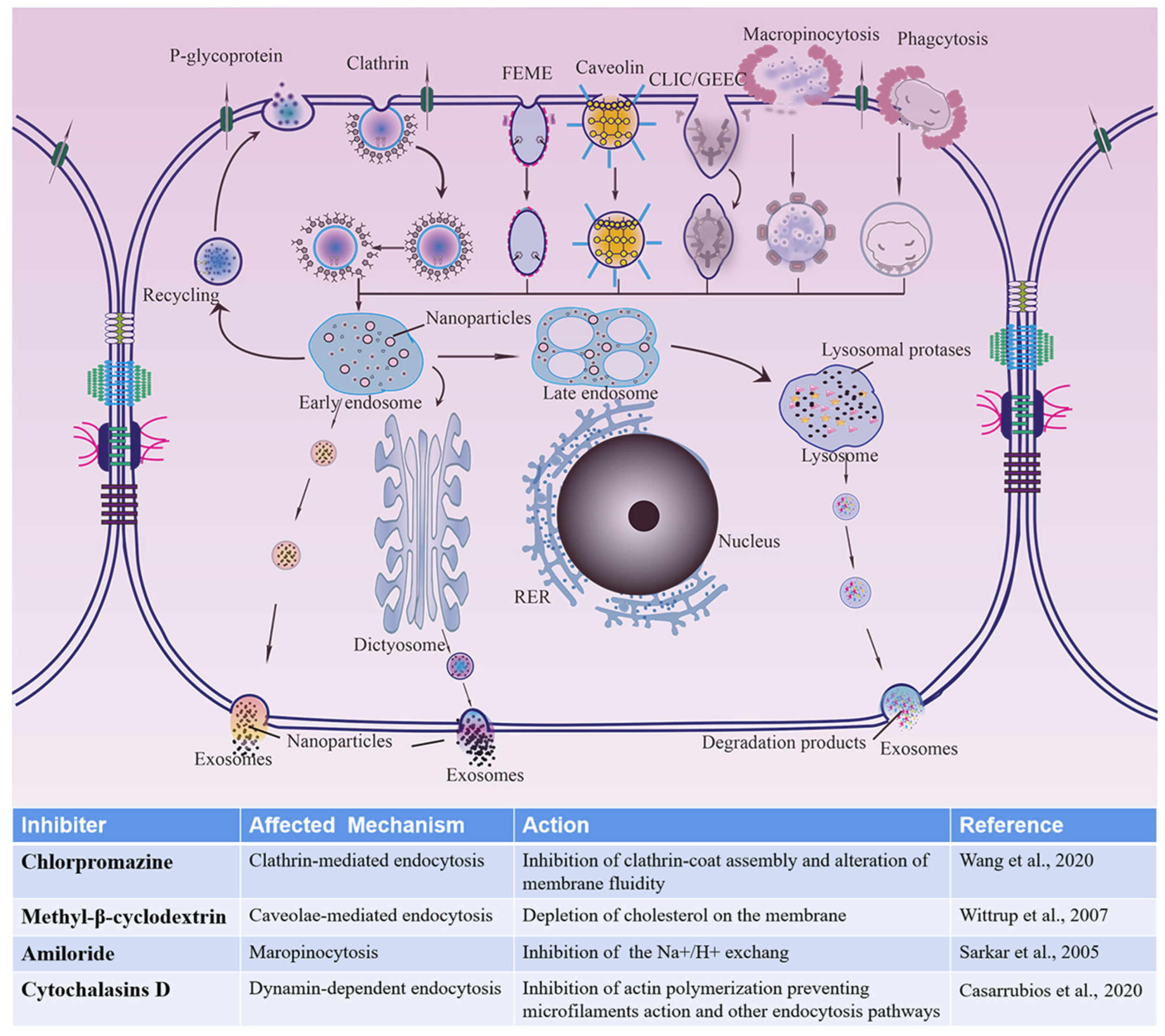
3.4. Transport of CsA-NCs across the Caco-2 Cell Monolayers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing--oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Shi, B.; Xia, F.; Qi, J.; Dong, X.; Zhao, W.; Li, T.; Wu, W.; Lu, Y. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies. J. Control. Release 2018, 270, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, Y.; Shen, B.; Xie, Y.; Qi, J.; Dong, X.; Zhao, W.; Zhu, W.; Wu, W.; Yuan, H.; et al. Self-discriminating fluorescent hybrid nanocrystals: Efficient and accurate tracking of translocationviaoral delivery. Nanoscale 2018, 10, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Hollis, C.P.; Weiss, H.L.; Evers, B.M.; Gemeinhart, R.A.; Li, T. In vivo investigation of hybrid Paclitaxel nanocrystals with dual fluorescent probes for cancer theranostics. Pharm. Res. 2014, 31, 1450–1459. [Google Scholar] [CrossRef]
- Zhang, J.; Corpstein, C.D.; Li, T. Intracellular uptake of nanocrystals: Probing with aggregation-induced emission of fluorescence and kinetic modeling. Acta Pharm. Sin. B 2021, 11, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lee, D.; Meng, Z.; Li, T. Exploring intracellular fate of drug nanocrystals with crystal-integrated and environment-sensitive fluorophores. J. Control. Release 2017, 267, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Zhang, Q.; Reipa, V.; Wang, N.S.; Stratmeyer, M.E.; Hitchins, V.M.; Goering, P.L. Comparison of cytotoxic and inflammatory responses of photoluminescent silicon nanoparticles with silicon micron-sized particles in RAW 264.7 macrophages. J. Appl. Toxicol. 2009, 29, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef]
- Qi, Y.; Shi, E.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Jergens, A.; Barrett, T.; Wu, Y.; Wang, Q. Ex Vivo Study of Telluride Nanowires in Minigut. J. Biomed. Nanotechnol. 2018, 14, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nat. 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Jergens, A.; Wannemuehler, M.; Wang, Q. Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery. Mar. Drugs 2021, 19, 282. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Qi, Y.; Bussiere, L.D.; Wannemuehler, M.; Miller, C.L.; Wang, Q.; Yu, C. Transport of artificial virus-like nanocarriers through intestinal monolayers via microfold cells. Nanoscale 2020, 12, 16339–16347. [Google Scholar] [CrossRef]
- Reding, B.; Carter, P.; Qi, Y.; Li, Z.; Wu, Y.; Wannemuehler, M.; Bratlie, K.M.; Wang, Q. Manipulate intestinal organoids with niobium carbide nanosheets. J. Biomed. Mater. Res. A 2021, 109, 479–487. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Cummins, C.L.; Salphati, L.; Reid, M.J.; Benet, L.Z. In Vivo Modulation of Intestinal CYP3A Metabolism by P-Glycoprotein: Studies Using the Rat Single-Pass Intestinal Perfusion Model. J. Pharmacol. Exp. Ther. 2003, 305, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Togami, K.; Tosaki, Y.; Chono, S.; Morimoto, K.; Hayasaka, M.; Tada, H. Enantioselective uptake of fexofenadine by Caco-2 cells as model intestinal epithelial cells. J. Pharm. Pharmacol. 2013, 65, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; Garcia-Rodriguez, A.; Cortes, C.; Velazquez, A.; Xamena, N.; Sampayo-Reyes, A.; Marcos, R.; Hernandez, A. Effects of cerium oxide nanoparticles on differentiated/undifferentiated human intestinal Caco-2cells. Chem. Biol. Interact. 2018, 283, 38–46. [Google Scholar] [CrossRef]
- Jain, S.; Kambam, S.; Thanki, K.; Jain, A.K. Cyclosporine A loaded self-nanoemulsifying drug delivery system (SNEDDS): Implication of a functional excipient based co-encapsulation strategy on oral bioavailability and nephrotoxicity. RSC Advances 2015, 5, 49633–49642. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Shayeganpour, A.; Alshamsan, A.; Montazeri Aliabadi, H.; Lavasanifar, A. The immunosuppressive activity of polymeric micellar formulation of cyclosporine A: In vitro and in vivo studies. AAPS J. 2011, 13, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Italia, J.L.; Bhardwaj, V.; Kumar, M.N. Disease, destination, dose and delivery aspects of ciclosporin: The state of the art. Drug Discov. Today 2006, 11, 846–854. [Google Scholar] [CrossRef]
- Romero, G.B.; Arntjen, A.; Muller, R.H. Amorphous cyclosporin A nanoparticles for enhanced dermal bioavailability. Int. J. Pharm. 2016, 498, 217–224. [Google Scholar] [CrossRef]
- He, Y.; Xia, D.; Li, Q.; Tao, J.; Gan, Y.; Wang, C. Enhancement of cellular uptake, transport and oral absorption of protease inhibitor saquinavir by nanocrystal formulation. Acta Pharmacol. Sin. 2015, 36, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Wang, X.; Jia, X.; Wang, H.; Li, J. Impacts of particle size on the cytotoxicity, cellular internalization, pharmacokinetics and biodistribution of betulinic acid nanosuspensions in combined chemotherapy. Int. J. Pharm. 2020, 588, 119799. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Zode, S.S.; Bansal, A.K. Formulation aspects of intravenous nanosuspensions. Int. J. Pharm. 2020, 586, 119555. [Google Scholar] [CrossRef]
- Toziopoulou, F.; Malamatari, M.; Nikolakakis, I.; Kachrimanis, K. Production of aprepitant nanocrystals by wet media milling and subsequent solidification. Int. J. Pharm. 2017, 533, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Guada, M.; Lasa-Saracibar, B.; Lana, H.; Dios-Vieitez Mdel, C.; Blanco-Prieto, M.J. Lipid nanoparticles enhance the absorption of cyclosporine A through the gastrointestinal barrier: In vitro and in vivo studies. Int. J. Pharm. 2016, 500, 154–161. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol). J. Control. Release 2002, 80, 129–144. [Google Scholar] [CrossRef]
- Galli, C. Experimental determination of the diffusion boundary layer width of micron and submicron particles. Int. J. Pharm. 2006, 313, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Da Violante, G.; Zerrouk, N.; Richard, I.; Provot, G.; Chaumeil, J.C.; Arnaud, P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol. Pharm. Bull. 2002, 25, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Fang, X. Transport characteristics of 9-nitrocamptothecin in the human intestinal cell line Caco-2 and everted gut sacs. Int. J. Pharm. 2004, 272, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Yazdanian, M.; Audus, K.L. Microparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epithelium. J. Pharm. Pharm. 2001, 53, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, M.; Kersting, M.; Rosenkranz, N.; Loza, K.; Breisch, M.; Rosteck, A.; Prymak, O.; SChurmeyer, L.; Westphal, G.; Koller, M.; et al. Cell-biological effects of zinc oxide spheres and rods from the nano- to the microscale at sub-toxic levels. Cell Biol. Toxicol. 2020, 37, 573–593. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Dizaj, S.M.; Khosroushahi, A.Y. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39. [Google Scholar] [CrossRef] [PubMed]
- Fricker, G.; Drewe, J.; Huwyler, J.; Gutmann, H.; Beglinger, C. Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: In vitro-in vivo correlation. Br. J. Pharmacol. 2012, 118, 1841–1847. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre. Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuhorn, I.S.; Kalicharan, R.; Hoekstra, D. Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J. Biol. Chem. 2002, 277, 18021–18028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitha, J.; Irie, T.; Sklar, P.B.; Nye, J.S. Drug solubilizers to aid pharmacologists: Amorphous cyclodextrin derivatives. Life Sci. 1988, 43, 493–502. [Google Scholar] [CrossRef]
- Means, N.; Elechalawar, C.K.; Chen, W.R.; Bhattacharya, R.; Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Aspects Med. 2022, 83, 100993. [Google Scholar] [CrossRef]
- Miyazaki, H.; Ogura, M.; Sakaguchi, Y.; Hasegawa, T.; Atobe, S.; Terai, K. Mechanism of jet injector-induced plasmid DNA uptake: Contribution of shear stress and endocytosis. Int. J. Pharm. 2021, 609, 121200. [Google Scholar] [CrossRef]
- Gold, S.; Monaghan, P.; Mertens, P.; Jackson, T. A clathrin independent macropinocytosis-like entry mechanism used by bluetongue virus-1 during infection of BHK cells. PLoS ONE 2010, 5, e11360. [Google Scholar] [CrossRef] [Green Version]
- Wittrup, A.; Sandgren, S.; Lilja, J.; Bratt, C.; Gustavsson, N.; Morgelin, M.; Belting, M. Identification of proteins released by mammalian cells that mediate DNA internalization through proteoglycan-dependent macropinocytosis. J. Biol. Chem. 2007, 282, 27897–27904. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Kruhlak, M.J.; Erlandsen, S.L.; Shaw, S. Selective inhibition by rottlerin of macropinocytosis in monocyte-derived dendritic cells. Immunology 2005, 116, 513–524. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gomez-Cerezo, N.; Feito, M.J.; Vallet-Regi, M.; Arcos, D.; Portoles, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Chen, T.; Li, C.; Li, Y.; Xiang, Y.; Lee, S.; Zheng, Y. Oral Delivery of a Nanocrystal Formulation of Schisantherin A with Improved Bioavailability and Brain Delivery for the Treatment of Parkinson’s Disease. Mol. Pharm. 2016, 13, 3864–3875. [Google Scholar] [CrossRef] [PubMed]
- Witoonsaridsilp, W.; Panyarachun, B.; Jaturanpinyo, M.; Sarisuta, N. Phospholipid vesicle–bound lysozyme to enhance permeability in human intestinal cells. Pharm. Dev. Technol. 2013, 18, 821–827. [Google Scholar] [CrossRef] [PubMed]
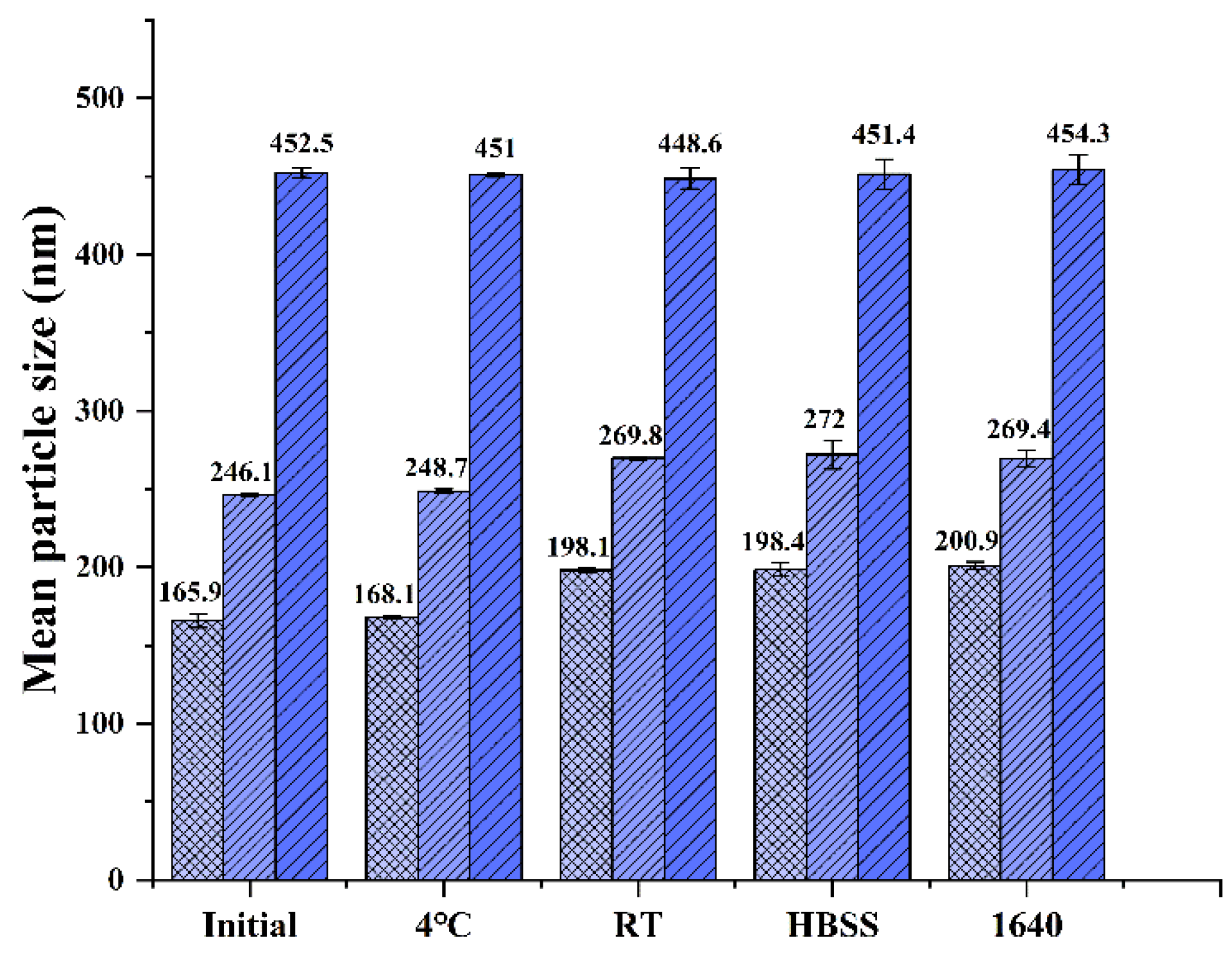
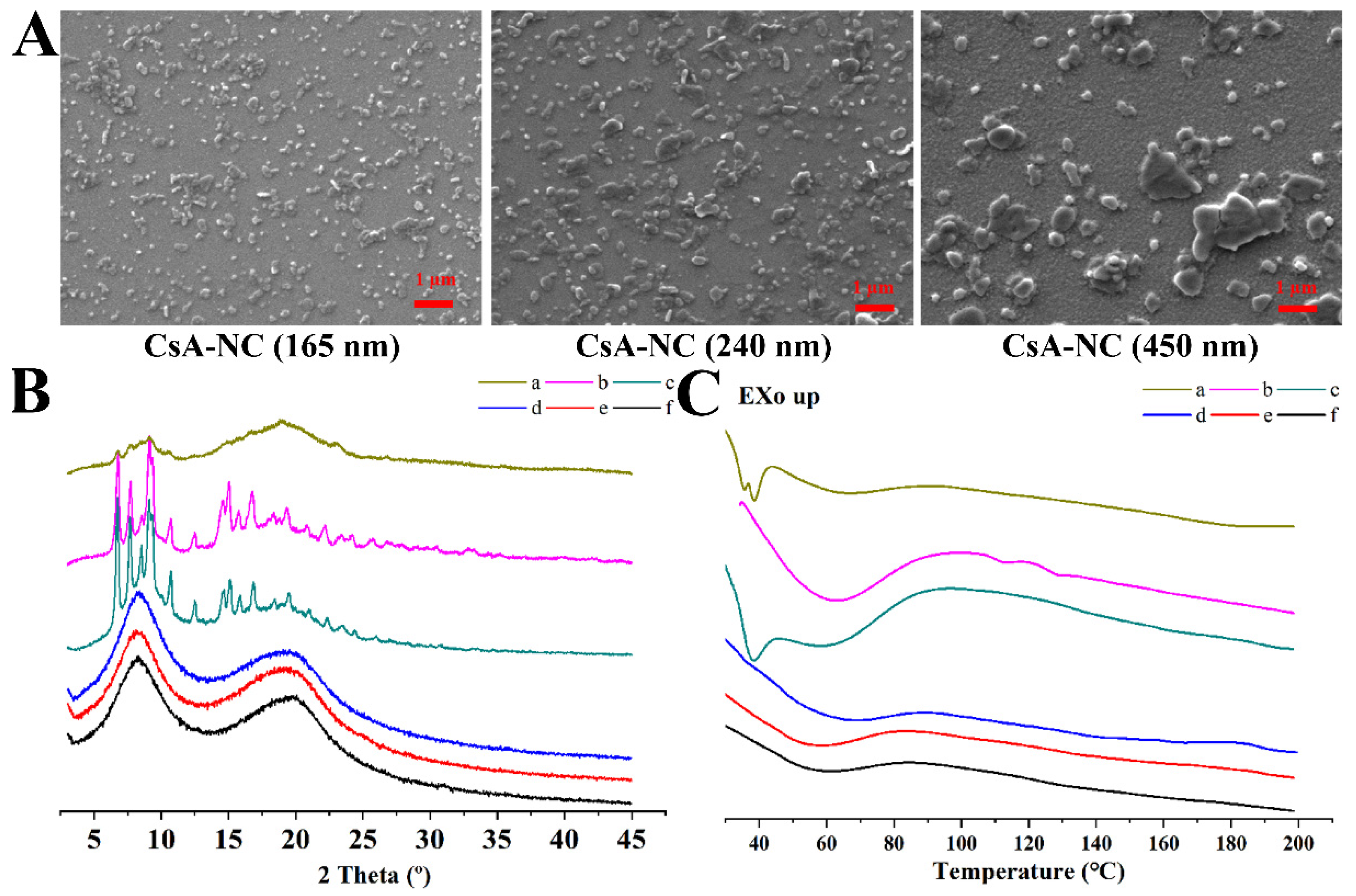
| CsA-NCs | Mean Particle Sizes (nm) | Polydispersity Index (%) | Zeta Potential (mV) |
|---|---|---|---|
| 450 nm | 452.5 ± 3.2 | 0.19 ± 0.016 | −22.3 ± 1.2 |
| 240 nm | 246.1 ± 1.2 | 0.15 ± 0.014 | −21.3 ± 0.4 |
| 165 nm | 165.9 ± 4.6 | 0.13 ± 0. 023 | −24.3 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Tian, Y.; Wang, Z.; Zhang, H.; Zheng, A. The Study of Cyclosporin A Nanocrystals Uptake and Transport across an Intestinal Epithelial Cell Model. Polymers 2022, 14, 1975. https://doi.org/10.3390/polym14101975
Sun W, Tian Y, Wang Z, Zhang H, Zheng A. The Study of Cyclosporin A Nanocrystals Uptake and Transport across an Intestinal Epithelial Cell Model. Polymers. 2022; 14(10):1975. https://doi.org/10.3390/polym14101975
Chicago/Turabian StyleSun, Wenjun, Yang Tian, Zengming Wang, Hui Zhang, and Aiping Zheng. 2022. "The Study of Cyclosporin A Nanocrystals Uptake and Transport across an Intestinal Epithelial Cell Model" Polymers 14, no. 10: 1975. https://doi.org/10.3390/polym14101975
APA StyleSun, W., Tian, Y., Wang, Z., Zhang, H., & Zheng, A. (2022). The Study of Cyclosporin A Nanocrystals Uptake and Transport across an Intestinal Epithelial Cell Model. Polymers, 14(10), 1975. https://doi.org/10.3390/polym14101975





