Near-Infrared Light-Remote Localized Drug Delivery Systems Based on Zwitterionic Polymer Nanofibers for Combination Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PCL-b-PMPC (P1 and P2)
2.3. Preparation of Electrospun Nanofiber Membranes
2.4. General Characterization
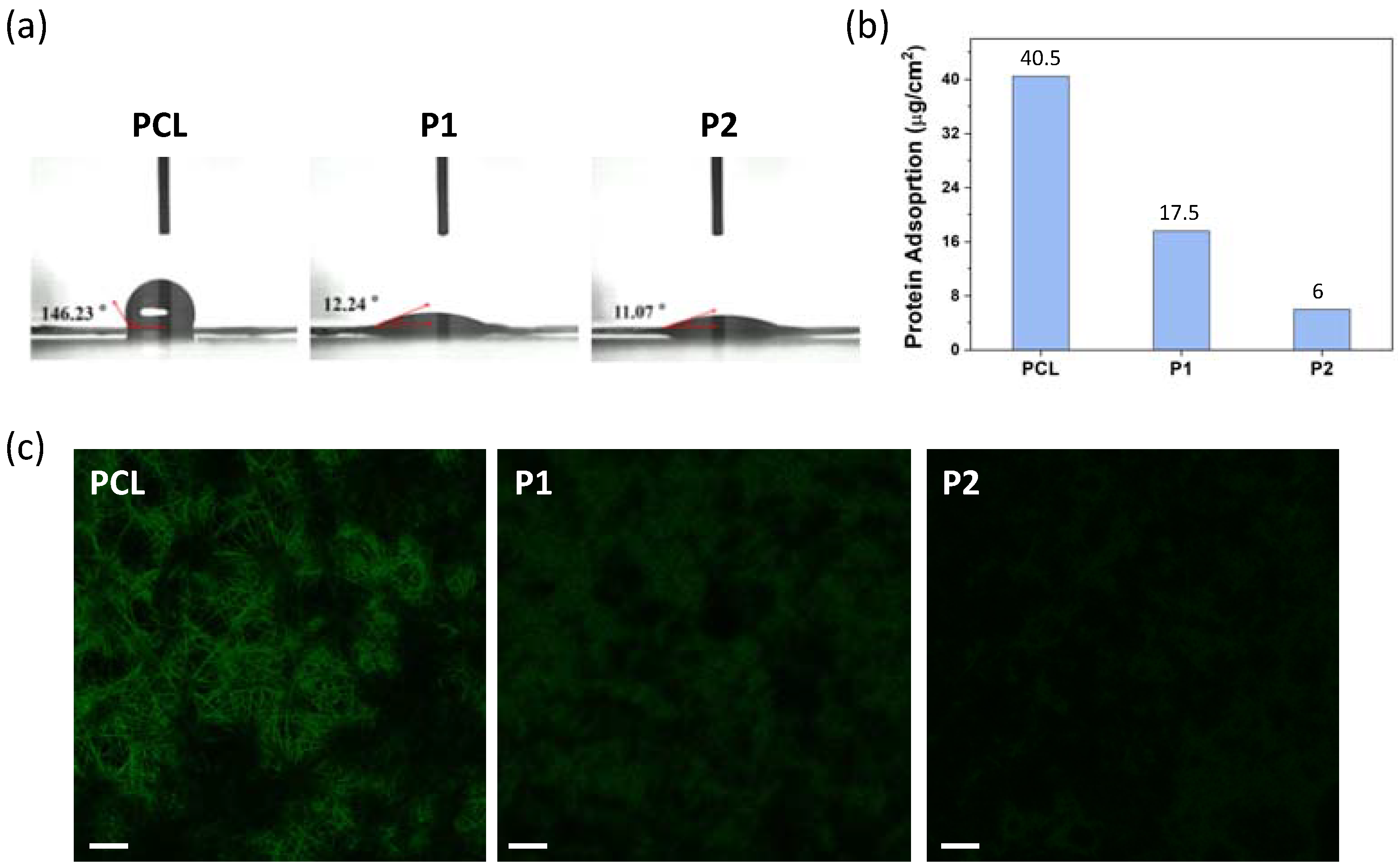
2.5. Quantitative and Qualitative Evaluation of Protein Adsorption
2.6. Photothermal Effect
2.7. Drug Release Behavior
2.8. Cell Culture and In Vitro Cytotoxicity and Phototoxicity
2.9. Subcellular Localization Study
3. Results and Discussion
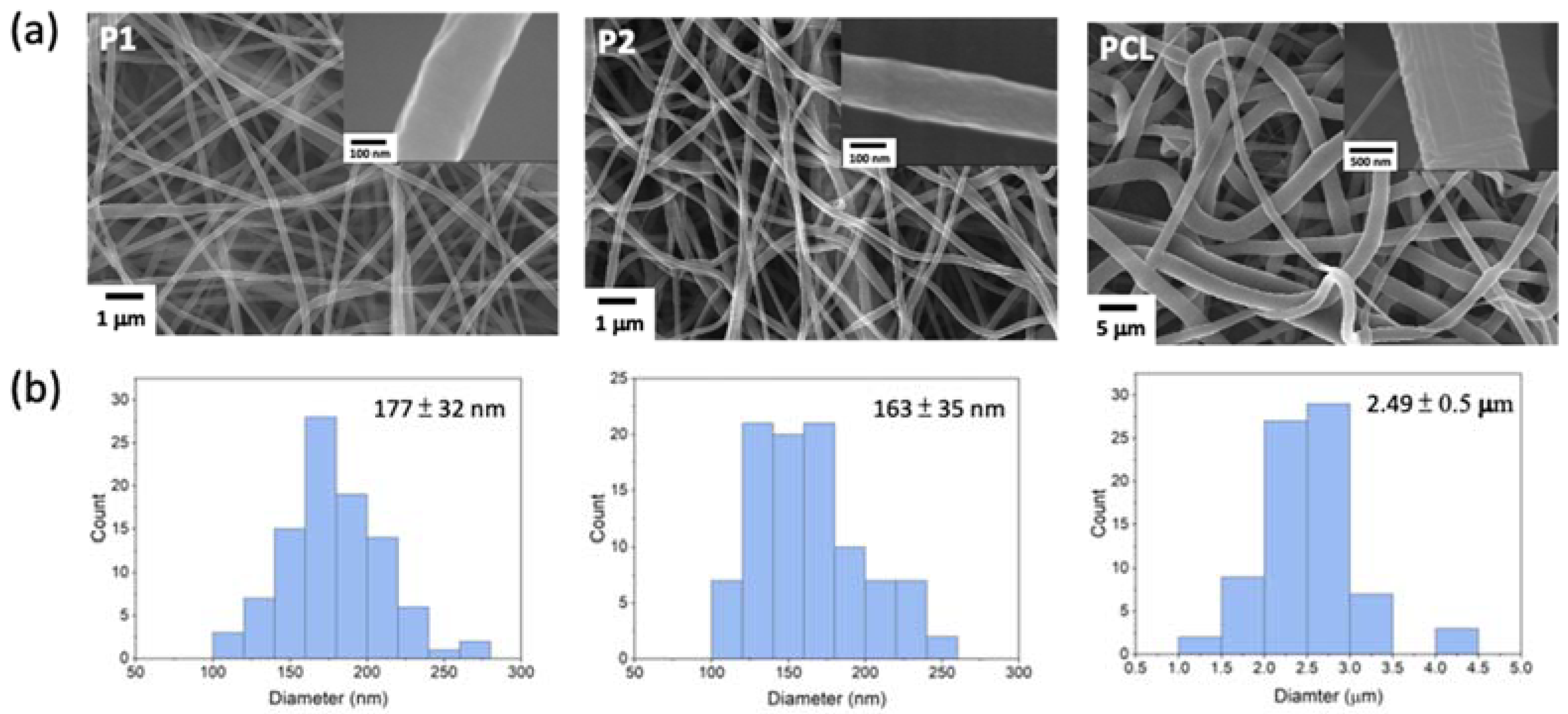
3.1. Preparation and Characterization of PCL-b-PMPC Copolymers and Electrospun Nanofibers
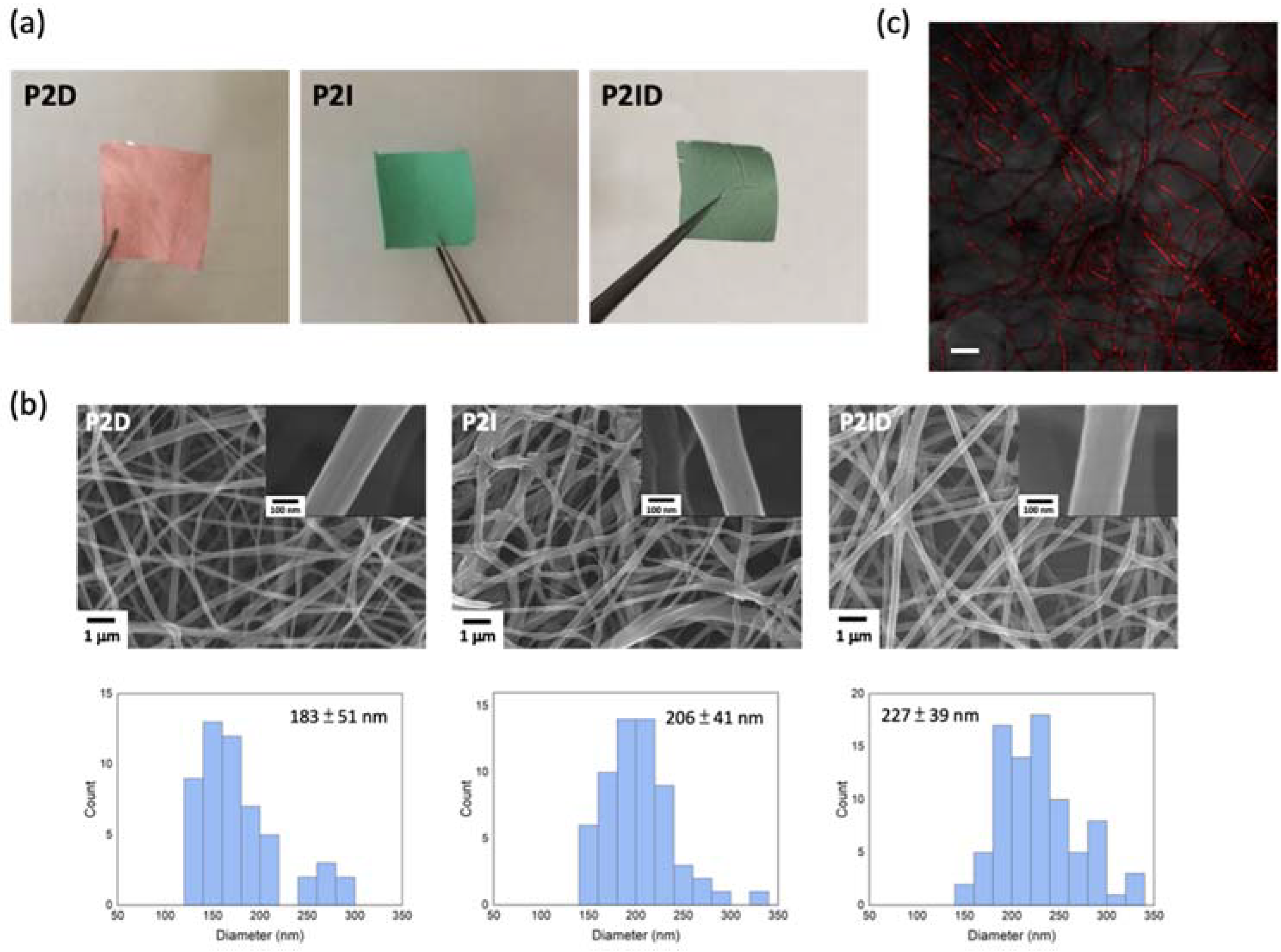
3.2. Drugs Encapsulation in Electrospun Nanofibers
3.3. Photothermal Effect under NIR Irradiation
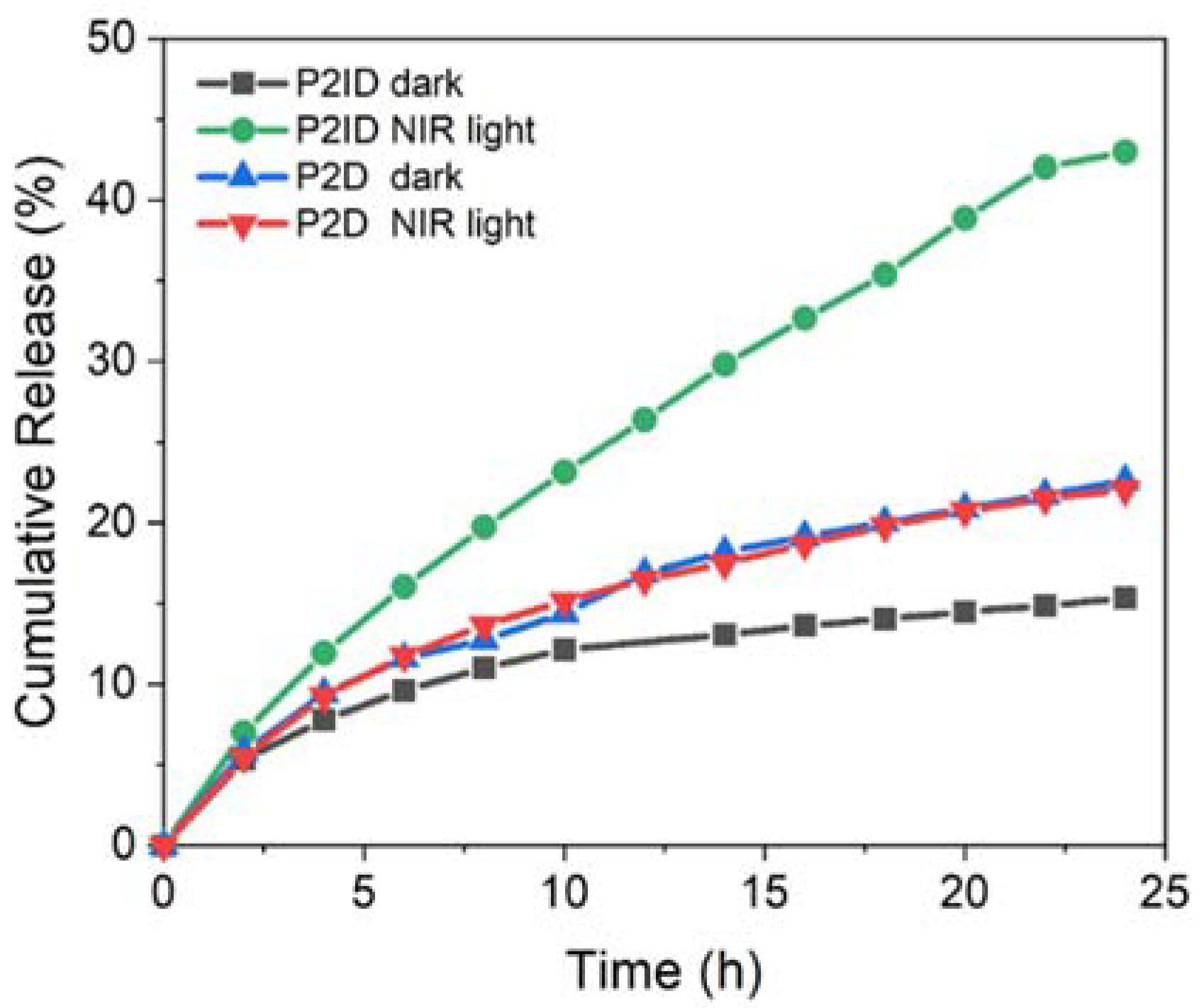
3.4. In Vitro NIR Light Remote Release Behavior
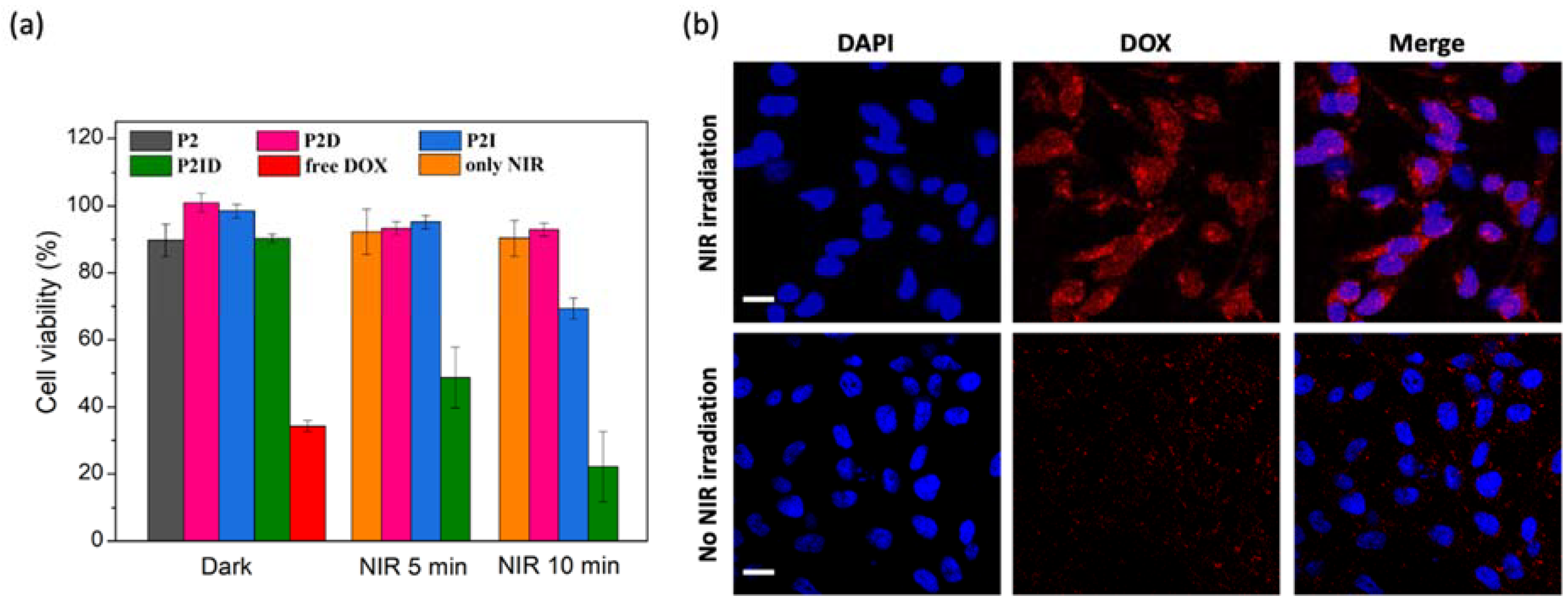
3.5. In Vitro Biocompatibility, Anticancer Efficiency, and Cellular Uptake of Released DOX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based nanofiber–nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Gheluwe, L.V.; Chourpa, I.; Gaigne, C.; Munnier, E. Polymer-based smart drug delivery systems for skin application and demonstration of stimuli-responsiveness. Polymers 2021, 13, 1285. [Google Scholar] [CrossRef]
- Jingcheng, L.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent polymers, fibers and applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Chen, Y.; Wang, Y.; Li, H.; Han, H.; Chen, T.; Jin, Q.; Ji, J. pH- and NIR light-responsive polymeric prodrug micelles for hyperthermia-assisted site-specific chemotherapy to reverse drug resistance in cancer treatment. Small 2016, 12, 2731–2740. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Hwang, T.I.; Oh, J.-M.; Maharjan, B.; Chun, S.; Kim, B.S.; Joshi, M.K.; Park, C.H.; Kim, C.S. pH/NIR-responsive polypyrrole-functionalized fibrous localized drug-delivery platform for synergistic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 20256–20270. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional platform based on electrospun nanofibers and plasmonic hydrogel: A smart nanostructured pillow for near-infrared light-driven biomedical applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Huang, H.Y.; Skripka, A.; Zaroubi, L.; Findlay, B.L.; Vetrone, F.; Skinner, C.; Oh, J.K.; Cuccia, L.A. Electrospun upconverting nanofibrous hybrids with smart NIR-light-controlled drug release for wound dressing. ACS Appl. Bio. Mater. 2020, 3, 7219–7227. [Google Scholar] [CrossRef]
- Singh, B.; Kim, K.; Park, M.-H. On-demand drug delivery systems using nanofibers. Nanomaterials 2021, 11, 3411. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Chan, H.-M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Grainger, D.W. All charged up about implanted biomaterials. Nat. Biotechnol. 2013, 31, 507–509. [Google Scholar] [CrossRef]
- Zwaal, R.F.A.; Comfurius, P.; Van Deenen, L.L.M. Membrane asymmetry and blood coagulation. Nature 1977, 268, 358–360. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Hirase, T.; Madsen, J.; Armes, S.P.; Nagasaki, Y. Non-fouling character of poly[2-(methacryloyloxy)ethyl phosphorylcholine]-modified gold surfaces fabricated by the ‘grafting to’ method: Comparison of its protein resistance with poly(ethylene glycol)-modified gold surfaces. Macromol. Rapid Commun. 2009, 30, 2136–2140. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, C.; Li, B.; Yang, H.; Cheng, Y.; Cui, W. A highly flexible paclitaxel-loaded poly(ε-caprolactone) electrospun fibrous-membrane-covered stent for benign cardia stricture. Acta Biomater. 2013, 9, 8328–8336. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, C.-Y. Folate-targeted curcumin-encapsulated micellar nanosystem for chemotherapy and curcumin-mediated photodynamic therapy. Polymers 2020, 12, 2280. [Google Scholar] [CrossRef]
- Du, J.; Armes, S.P. Preparation of biocompatible zwitterionic block copolymer vesicles by direct dissolution in water and subsequent silicification within their membranes. Langmuir 2009, 25, 9564–9570. [Google Scholar] [CrossRef]
- Rodriguez, V.B.; Henry, S.M.; Hoffman, A.S.; Stayton, P.S.; Li, X.; Pun, S.H. Encapsulation and stabilization of indocyanine green within poly(styrene-alt-maleic anhydride) block-poly(styrene) micelles for near-infrared imaging. J. Biomed. Opt. 2008, 13, 014025. [Google Scholar] [CrossRef]
- Niu, Z.; Zhao, Y.; Sun, W.; Shi, S.; Gong, Y. Biomimetic surface modification of polypropylene by surface chain transfer reaction based on mussel-inspired adhesion technology and thiol chemistry. Appl. Surf. Sci. 2016, 386, 41–50. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kim, T.H.; Wu, W.-C.; Huang, C.-M.; Wei, H.; Mount, C.W.; Tian, Y.; Jang, S.-H.; Pun, S.H.; Jen, A.K.-Y. pH-dependent, thermosensitive polymeric nanocarriers for drug delivery to solid tumors. Biomaterials 2013, 34, 4501–4509. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.H.; Hunley, M.T.; Allen, M.H., Jr.; Long, T.E. Electrospinning zwitterion-containing nanoscale acrylic fibers. Polymer 2009, 50, 4781–4787. [Google Scholar] [CrossRef]
- Sin, M.-C.; Chen, S.-H.; Chang, Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Anand, G.; Sharma, S.; Dutta, A.K.; Kumar, S.K.; Belfort, G. Conformational transitions of adsorbed proteins on surfaces of varying polarity. Langmuir 2010, 26, 10803–10811. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, J.; Gao, G.; Sheng, Z.; Cui, H.; Cai, L. Indocyanine green-loaded polydopamine-reduced graphene oxide nanocomposites with amplifying photoacoustic and photothermal effects for cancer theranostics. Theranostics 2016, 6, 1043–1052. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, P.; Luo, Z.; Gong, P.; Zheng, C.; Zhang, P.; Yue, C.; Gao, D.; Ma, Y.; Cai, L. Robust ICG theranostic nanoparticles for folate targeted cancer imaging and highly effective photothermal therapy. ACS Appl. Mater. Interfaces 2014, 6, 6709–6716. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Gao, M.; Hu, A.; Liu, Z. Remotely controlled red blood cell carriers for cancer targeting and near-Infrared light-triggered drug release in combined photothermal–chemotherapy. Adv. Funct. Mater. 2015, 25, 2386–2394. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.S.; Al-Jamal, W.T.; Bossche, J.V.; Bui, T.T.; Drake, A.F.; Mason, A.J.; Kostarelos, K. Lipid–peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 2012, 6, 9335–9346. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-L.; Chen, C.-Y. Near-Infrared Light-Remote Localized Drug Delivery Systems Based on Zwitterionic Polymer Nanofibers for Combination Therapy. Polymers 2022, 14, 1860. https://doi.org/10.3390/polym14091860
Li Y-L, Chen C-Y. Near-Infrared Light-Remote Localized Drug Delivery Systems Based on Zwitterionic Polymer Nanofibers for Combination Therapy. Polymers. 2022; 14(9):1860. https://doi.org/10.3390/polym14091860
Chicago/Turabian StyleLi, Yu-Lun, and Ching-Yi Chen. 2022. "Near-Infrared Light-Remote Localized Drug Delivery Systems Based on Zwitterionic Polymer Nanofibers for Combination Therapy" Polymers 14, no. 9: 1860. https://doi.org/10.3390/polym14091860
APA StyleLi, Y.-L., & Chen, C.-Y. (2022). Near-Infrared Light-Remote Localized Drug Delivery Systems Based on Zwitterionic Polymer Nanofibers for Combination Therapy. Polymers, 14(9), 1860. https://doi.org/10.3390/polym14091860




