Scalable Manufacture of Curcumin-Loaded Chitosan Nanocomplex for pH-Responsive Delivery by Coordination-Driven Flash Nanocomplexation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
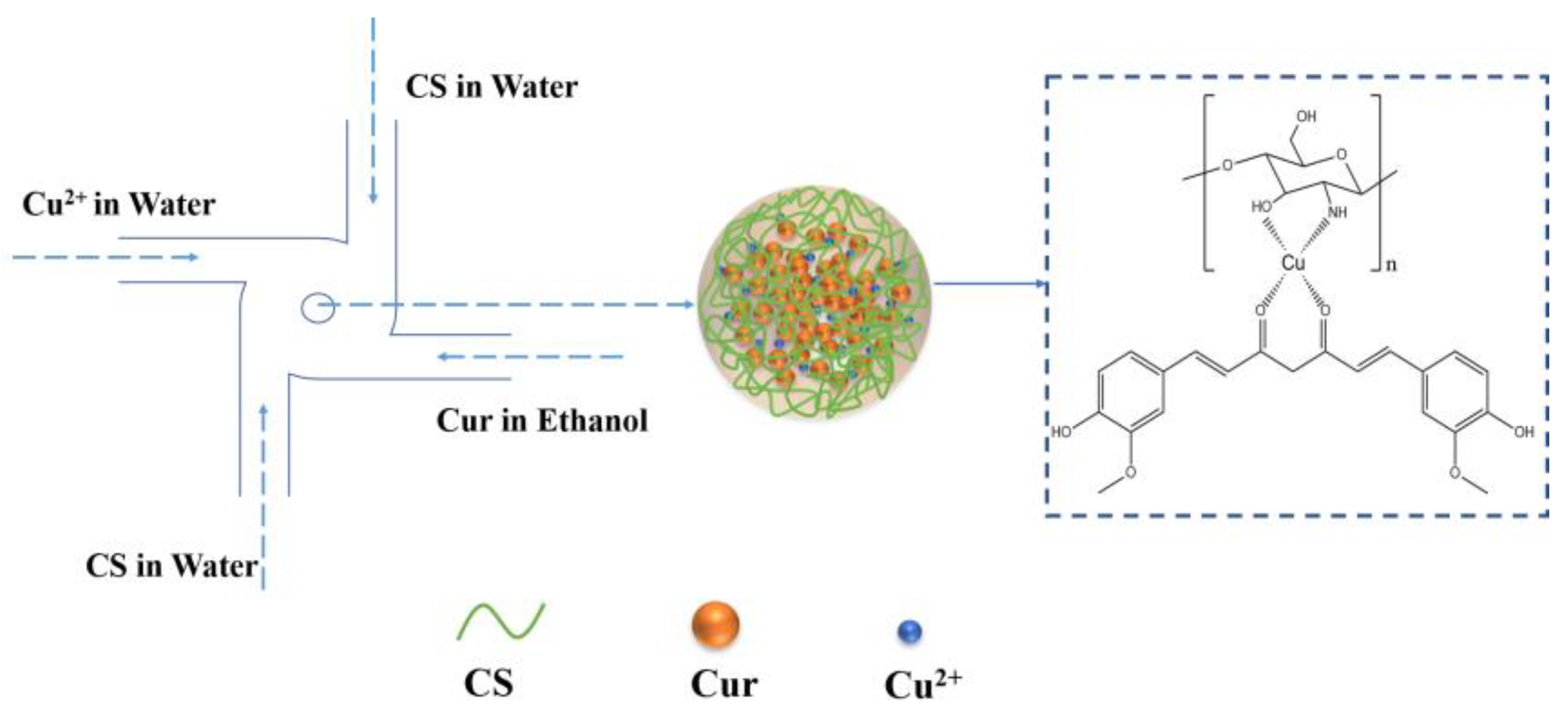
2.2. Preparation of Cur-Loaded CS Nanocomplex
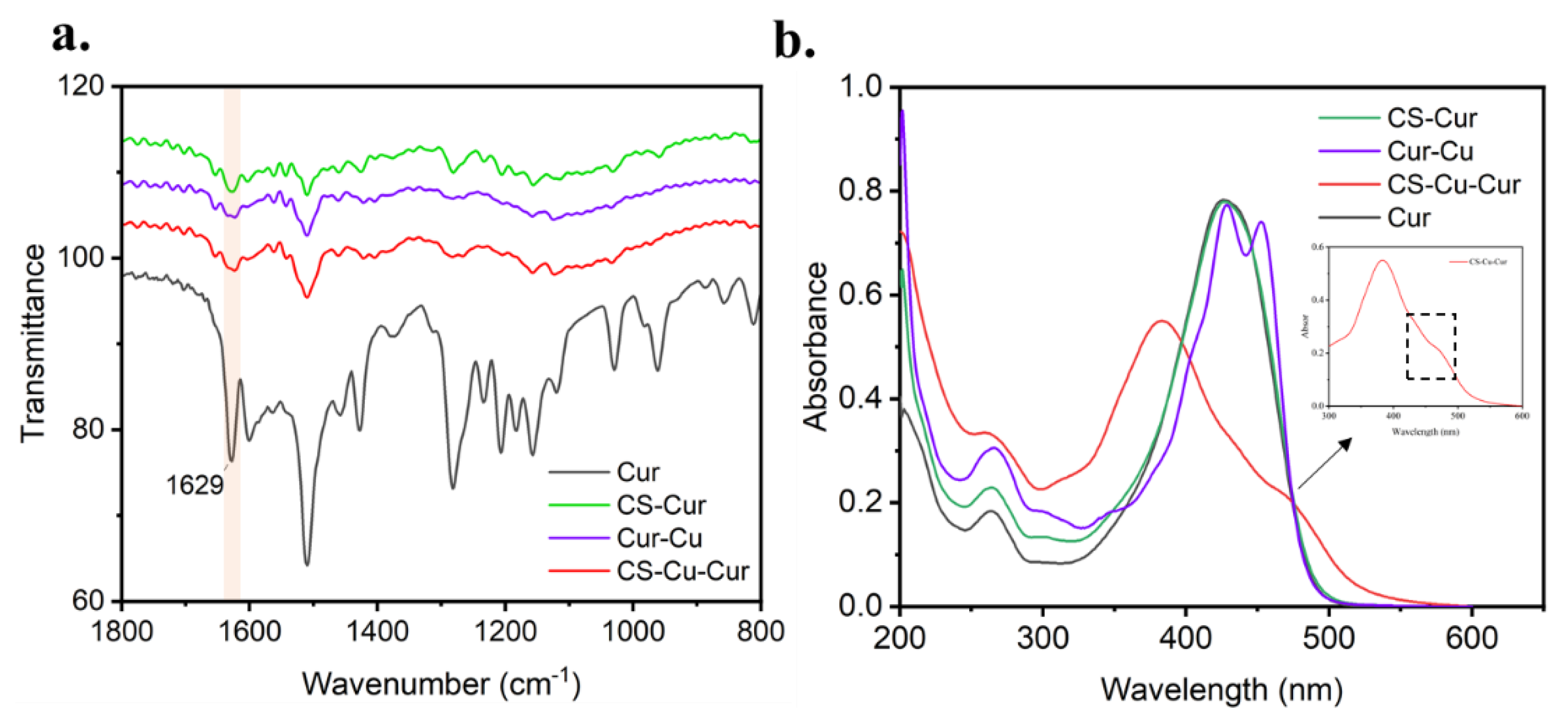
2.3. Morphology and Structure Characterization
2.4. Encapsulation Efficiency and Drug Loading Capacity
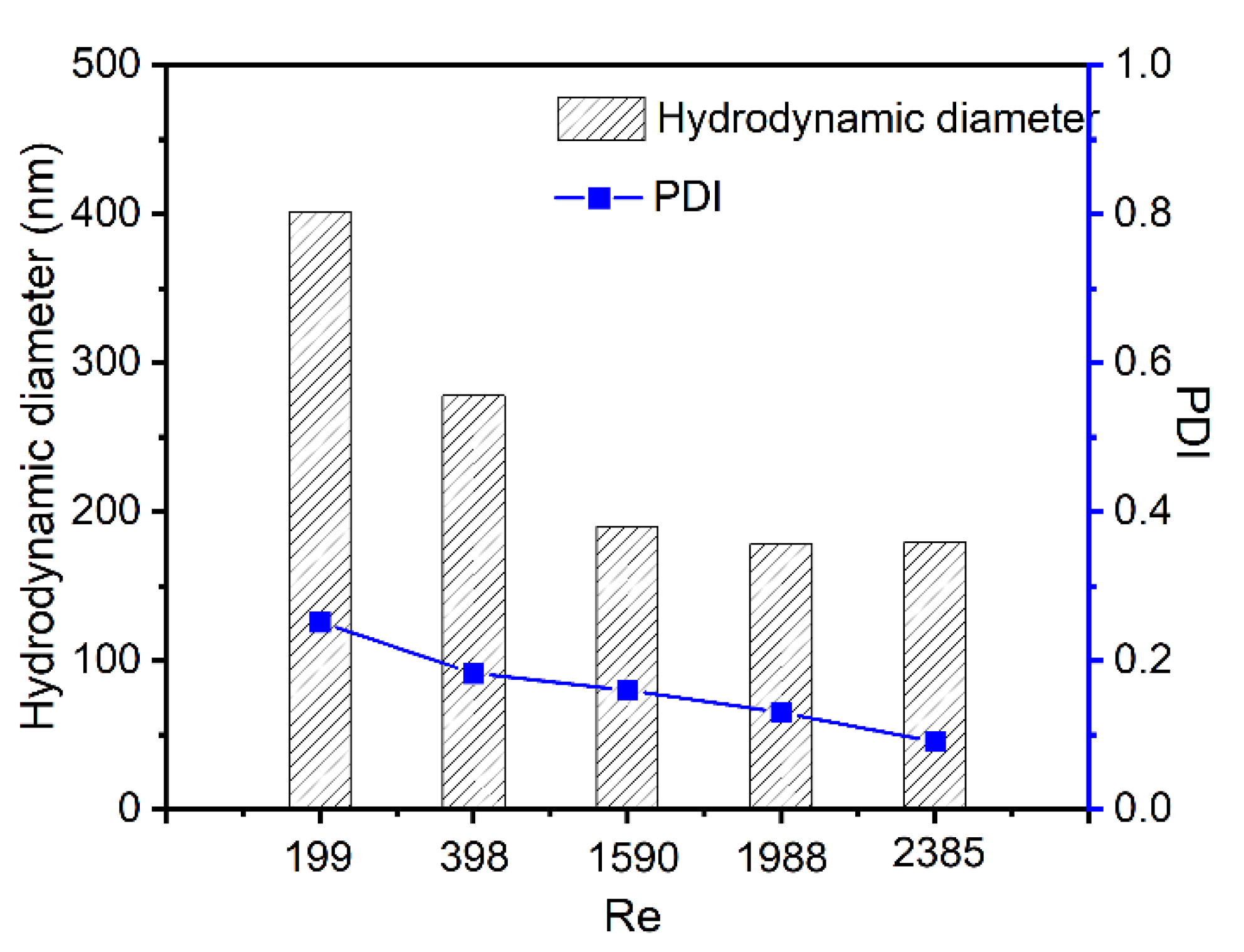
2.5. Kinetically Control of Cur-Loaded CS Nanocomplex Size
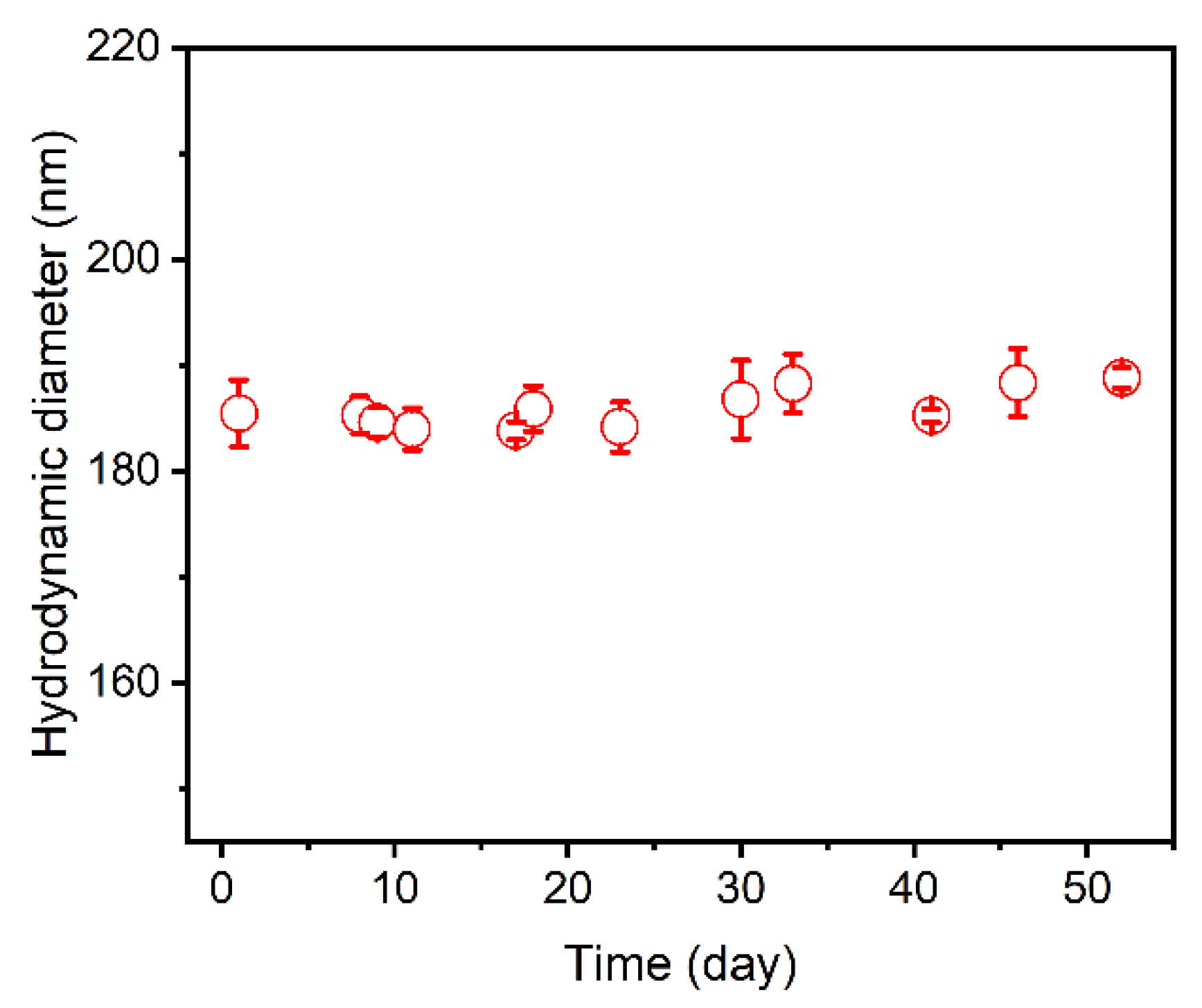
2.6. Stability Test
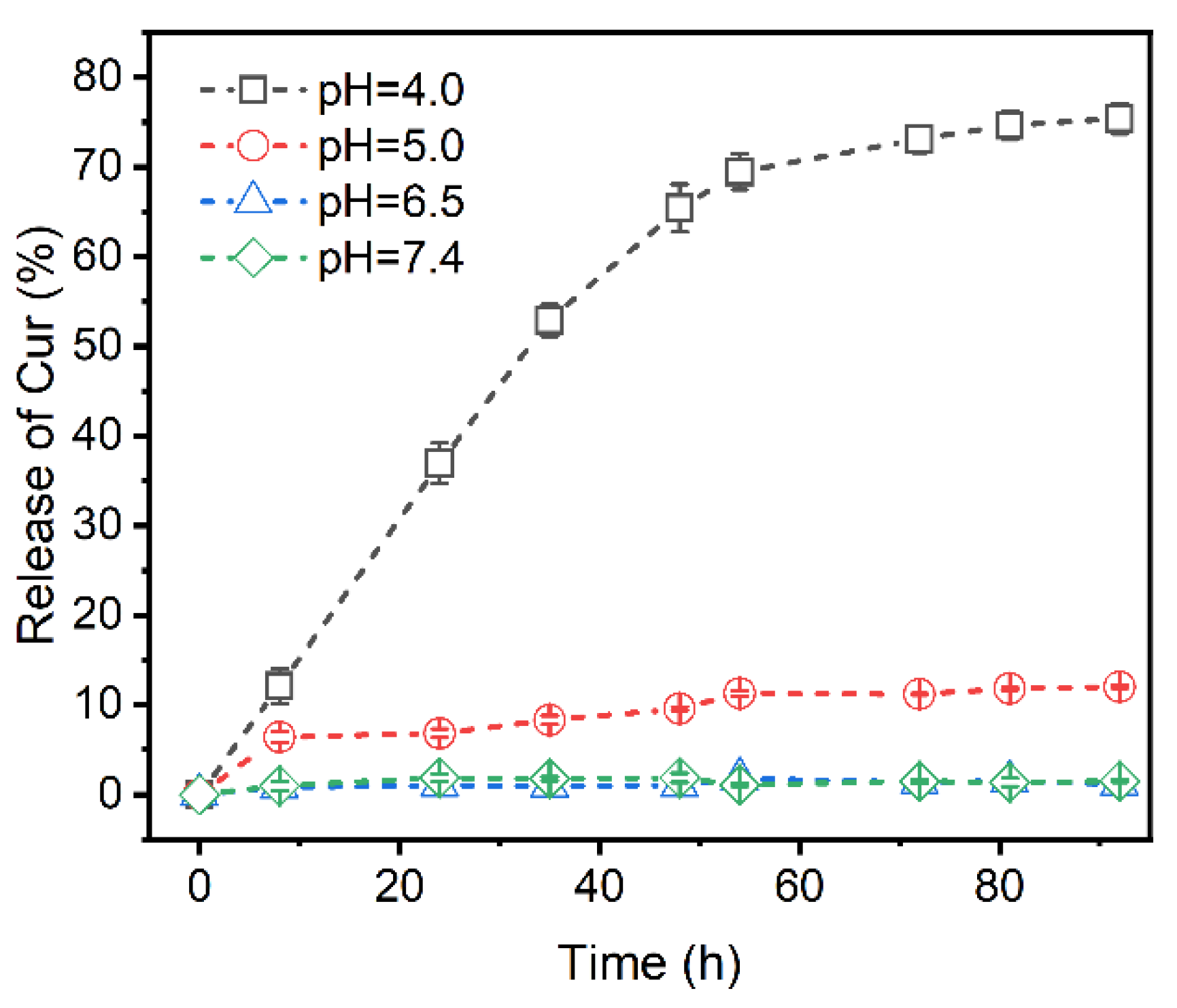
2.7. In Vitro Drug Release Profiles
2.8. In Vitro Cellular Uptake
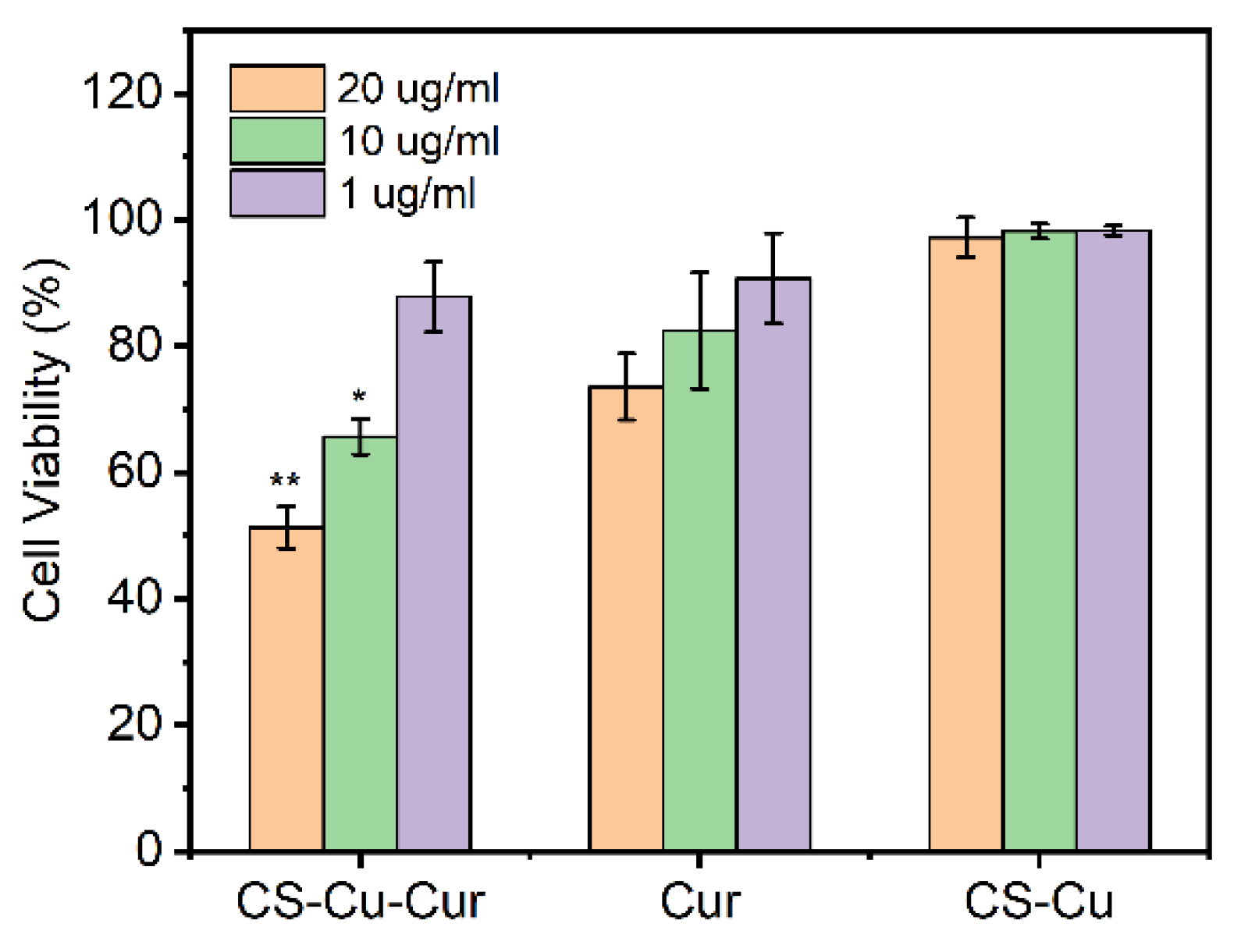
2.9. In Vitro Antitumor Activity
3. Results
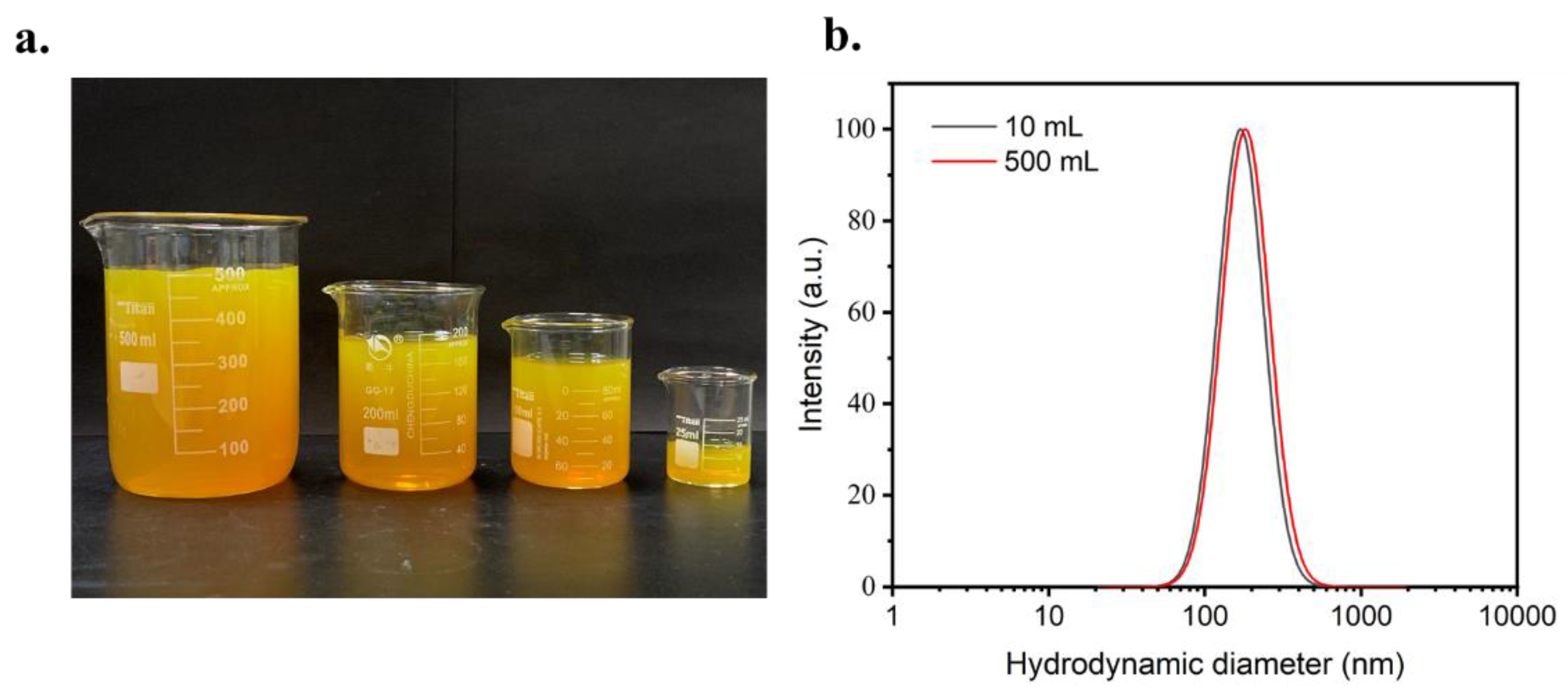
3.1. Preparation and Characteristic of Cur-Loaded CS Nanocomplex
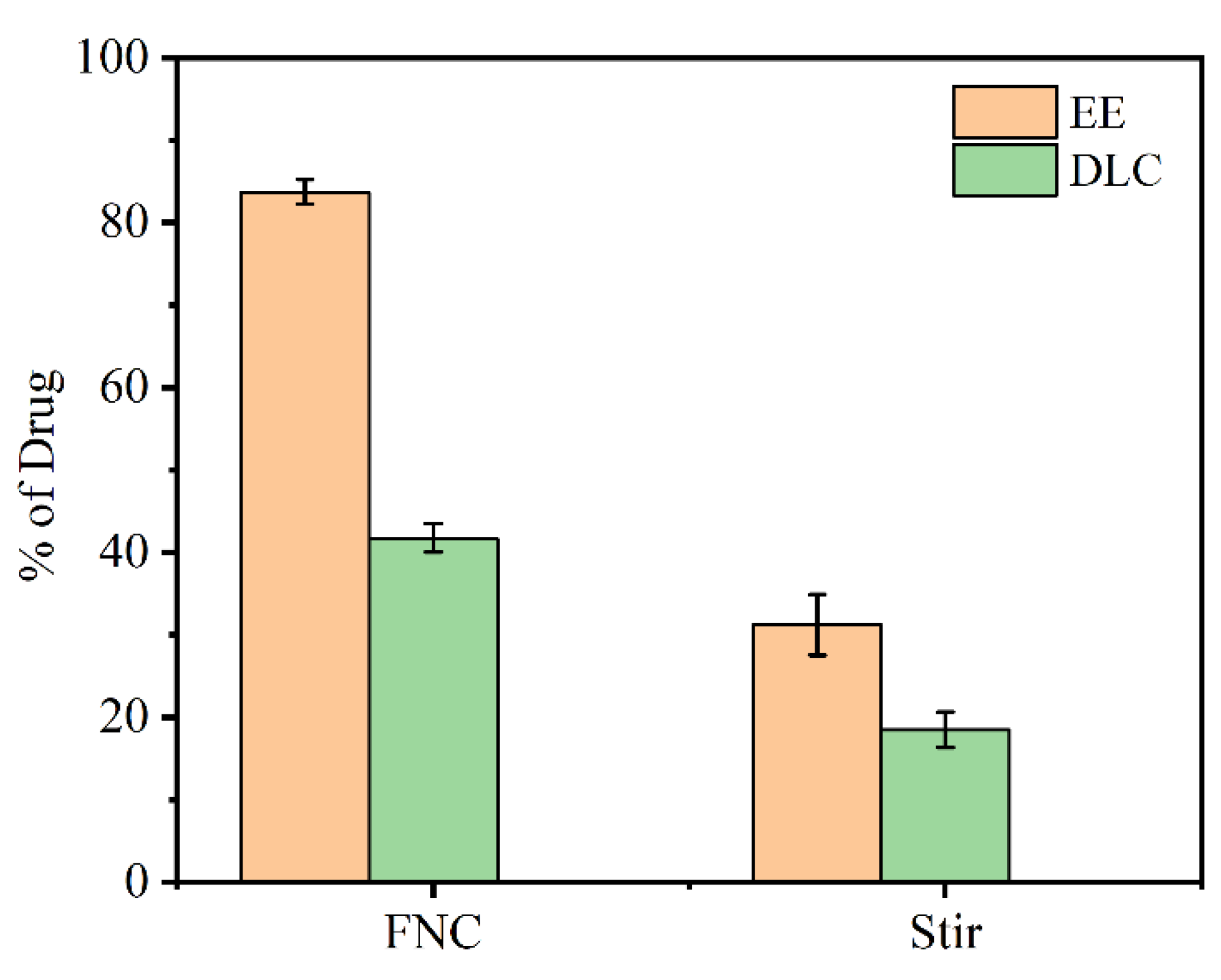
3.2. Encapsulation Efficiency (EE) and Drug Loading Capacity (DLC)
3.3. Stability of Cur-Loaded CS Nanocomplex
3.4. Size Control of Cur-Loaded Particles by Reynolds Number
3.5. Profiles of Curcumin Release at Different pH
3.6. Cellular Uptake Study
3.7. In Vitro Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Zhang, Y. Combination of tumor fragments and nanotechnology as a therapeutic approach: Treating a tumor with tumor. Nano Today 2020, 35, 100993. [Google Scholar] [CrossRef]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.-M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef] [PubMed]
- Penalva, R.; Esparza, I.; Larraneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Chen, X. Semiconducting Perylene Diimide Nanostructure: Multifunctional Phototheranostic Nanoplatform. Accounts Chem. Res. 2019, 52, 1245–1254. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-J.; Xing, L.; Cui, P.-F.; Zhang, J.-L.; Zhu, Y.; Qiao, J.-B.; Lyu, J.-Y.; Zhang, M.; Luo, C.-Q.; Zhou, Y.-X.; et al. Transferrin-inspired vehicles based on pH-responsive coordination bond to combat multidrug-resistant breast cancer. Biomaterials 2017, 113, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yao, H.; Guo, Y.; Yang, B.; Shi, J. Enhancing Tumor Catalytic Therapy by Co-Catalysis. Angew. Chem. Int. Ed. Engl. 2022, 134, e202200480. [Google Scholar] [CrossRef]
- Yang, B.; Yao, H.; Tian, H.; Yu, Z.; Guo, Y.; Wang, Y.; Yang, J.; Chen, C.; Shi, J. Intratumoral synthesis of nano-metalchelate for tumor catalytic therapy by ligand field-enhanced coordination. Nat. Commun. 2021, 12, 3393. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Feng, W.; Ding, L.; Zhang, H.; Dong, C.; Chen, Y.; Shi, J. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioact. Mater. 2022, 10, 131–144. [Google Scholar] [CrossRef]
- Sun, S.-K.; Wu, J.-C.; Wang, H.; Zhou, L.; Zhang, C.; Cheng, R.; Kan, D.; Zhang, X.; Yu, C. Turning solid into gel for high-efficient persistent luminescence-sensitized photodynamic therapy. Biomaterials 2019, 218, 119328. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild Magnetic Hyperthermia-Activated Innate Immunity for Liver Cancer Therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guan, X.; Lin, H.; Pu, Y.; Fang, Y.; Yue, W.; Zhou, B.; Wang, Q.; Chen, Y.; Xu, H. Nanomedicine-Enabled Photonic Thermogaseous Cancer Therapy. Adv. Sci. 2020, 7, 1901954. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Xue, H.; Xing, R.; Yan, X. Tumor microenvironment-oriented adaptive nanodrugs based on peptide self-assembly. Chem. Sci. 2020, 11, 8644–8656. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.; Dadkhah Tehrani, A.; Adeli, M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Che, S. Molecular design of coordination bonding architecture in mesoporous nanoparticles for rational pH-responsive delivery. Microporous Mesoporous Mater. 2013, 168, 73–80. [Google Scholar] [CrossRef]
- Liang, H.; Pei, Y.; Li, J.; Xiong, W.; He, Y.; Liu, S.; Li, Y.; Li, B. pH-Degradable antioxidant nanoparticles based on hydrogen-bonded tannic acid assembly. RSC Adv. 2016, 6, 31374–31385. [Google Scholar] [CrossRef]
- Peng, H.; Gan, Z.; Xiong, H.; Luo, M.; Yu, N.; Wen, T.; Wang, R.; Li, Y. Self-Assembly of Protein Nanoparticles from Rice Bran Waste and Their Use as Delivery System for Curcumin. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Q.; Yuan, C.; Li, S.; Xing, R.; Yan, X. Amino Acid Coordination Driven Self-Assembly for Enhancing both the Biological Stability and Tumor Accumulation of Curcumin. Angew. Chem. Int. Ed. Engl. 2018, 57, 17084–17088. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Shen, G.; Chang, R.; Zhang, Y.; Yan, X. Coordination self-assembly of natural flavonoids into robust nanoparticles for enhanced in vitro chemo and photothermal cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124805. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Li, M.; Shao, D.; Mao, H.-Q.; Leong, K.W. Flash technology-based self-assembly in nanoformulation: Fabrication to biomedical applications. Mater. Today 2021, 42, 99–116. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, W.; Guo, Z.; Wu, Y.; Zhu, W. Engineering Nanoparticulate Organic Photocatalysts via a Scalable Flash Nanoprecipitation Process for Efficient Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2021, 60, 15590–15597. [Google Scholar] [CrossRef]
- Santos, J.L.; Ren, Y.; Vandermark, J.; Archang, M.M.; Williford, J.-M.; Liu, H.-W.; Lee, J.; Wang, T.-H.; Mao, H.-Q. Continuous Production of Discrete Plasmid DNA-Polycation Nanoparticles Using Flash Nanocomplexation. Small 2016, 12, 6214–6222. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Hao, Y.; Gong, L.; Pang, M.; Howard, G.P.; Ahn, H.-H.; Brummet, M.; Chen, K.; Liu, H.-W.; et al. Kinetic Control in Assembly of Plasmid DNA/Polycation Complex Nanoparticles. ACS Nano 2019, 13, 10161–10178. [Google Scholar] [CrossRef]
- Ke, X.; Tang, H.; Mao, H.-Q. Effective encapsulation of curcumin in nanoparticles enabled by hydrogen bonding using flash nanocomplexation. Int. J. Pharm. 2019, 564, 273–280. [Google Scholar] [CrossRef]
- Liu, Z.; Le, Z.; Lu, L.; Zhu, Y.; Yang, C.; Zhao, P.; Wang, Z.; Shen, J.; Liu, L.; Chen, Y. Scalable fabrication of metal–phenolic nanoparticles by coordination-driven flash nanocomplexation for cancer theranostics. Nanoscale 2019, 11, 9410–9421. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Nie, T.; Tang, H.; Zhu, J.; Chen, K.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.-Q. Size-controlled lipid nanoparticle production using turbulent mixing to enhance oral DNA delivery. Acta Biomater. 2018, 81, 195–207. [Google Scholar] [CrossRef]
- He, Z.; Liu, Z.; Tian, H.; Hu, Y.; Liu, L.; Leong, K.W.; Mao, H.-Q.; Chen, Y. Scalable production of core–shell nanoparticles by flash nanocomplexation to enhance mucosal transport for oral delivery of insulin. Nanoscale 2018, 10, 3307–3319. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Zhang, F.; Li, M.; Tu, Z.; Mu, L.; Dawulieti, J.; Lao, Y.H.; Xiao, Z.; Yan, H.; et al. A Versatile and Robust Platform for the Scalable Manufacture of Biomimetic Nanovaccines. Adv. Sci. 2021, 8, 2002020. [Google Scholar] [CrossRef]
- Lian, Z.; Pan, R.; Wang, J. Microencapsulation of norfloxacin in chitosan/chitosan oligosaccharides and its application in shrimp culture. Int. J. Biol. Macromol. 2016, 92, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Ganglo, C.; Rui, J.; Zhu, Q.; Shan, J.; Wang, Z.; Su, F.; Liu, D.; Xu, J.; Guo, M.; Qian, J. Chromium (III) coordination capacity of chitosan. Int. J. Biol. Macromol. 2020, 148, 785–792. [Google Scholar] [CrossRef]
- Liu, J.; Wu, R.; Gu, R.; Qiu, F.; Pan, J. Simple formation of chitosan tablet with self-supporting blocks: Fe3+-mediated supramolecular coordination. Chem. Eng. J. 2018, 341, 648–657. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, K.; Li, Q.; Huang, C. pH responsive release of paclitaxel by self-assembling Chitosan-ethyl vanillin@GNRs nanocomposites. Int. J. Pharm. 2021, 607, 121047. [Google Scholar] [CrossRef]
- Krishnankutty, K.; John, V.D. Synthesis, Characterization, and Antitumour Studies of Metal Chelates of Some Synthetic Curcuminoids. Synth. React. Inorganic, Met. Nano-Metal Chem. 2011, 33, 343–358. [Google Scholar] [CrossRef]
- Shehata, T.M.; Ibrahim, M.M.; Elsewedy, H.S. Curcumin Niosomes Prepared from Proniosomal Gels: In Vitro Skin Permeability, Kinetic and In Vivo Studies. Polymers 2021, 13, 791. [Google Scholar] [CrossRef]
- Chuah, L.H.; Roberts, C.J.; Billa, N.; Abdullah, S.; Rosli, R. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 116, 228–236. [Google Scholar] [CrossRef]
- Chen, K.; Fu, Z.; Wang, M.; Lv, Y.; Wang, C.; Shen, Y.; Wang, Y.; Cui, H.; Guo, X. Preparation and Characterization of Size-Controlled Nanoparticles for High-Loading λ-Cyhalothrin Delivery through Flash Nanoprecipitation. J. Agric. Food Chem. 2018, 66, 8246–8252. [Google Scholar] [CrossRef]
- Zhu, Z. Flash Nanoprecipitation: Prediction and Enhancement of Particle Stability via Drug Structure. Mol. Pharm. 2014, 11, 776–786. [Google Scholar] [CrossRef]
- Fu, Z.; Li, L.; Li, F.; Bhutto, R.A.; Niu, X.; Liu, D.; Guo, X. Facile Morphology Control during Rapid Fabrication of Nanosized Organosilica Particles. Ind. Eng. Chem. Res. 2020, 59, 14797–14805. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of Novel Biodegradable Methoxy Poly(ethylene glycol)–Zein Micelles for Effective Delivery of Curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef]
- Liang, H.; Sun, X.; Gao, J.; Zhou, B. Chitosan coordination driven self-assembly for effective delivery of curcumin. Int. J. Biol. Macromol. 2020, 165, 2267–2274. [Google Scholar] [CrossRef]
- Sun, L.; Le, Z.; He, S.; Liu, J.; Liu, L.; Leong, K.W.; Mao, H.-Q.; Liu, Z.; Chen, Y. Flash Fabrication of Orally Targeted Nanocomplexes for Improved Transport of Salmon Calcitonin across the Intestine. Mol. Pharm. 2020, 17, 757–768. [Google Scholar] [CrossRef]
- He, Z.; Santos, J.L.; Tian, H.; Huang, H.; Hu, Y.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.-Q. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials 2017, 130, 28–41. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Tian, H.; Le, Z.; Liu, L.; Leong, K.W.; Mao, H.-Q.; Chen, Y. Scalable Manufacturing of Enteric Encapsulation Systems for Site-Specific Oral Insulin Delivery. Biomacromolecules 2018, 20, 528–538. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Z.; Li, L.; Ma, E.; Guo, X. A Cost-Effective Nano-Sized Curcumin Delivery System with High Drug Loading Capacity Prepared via Flash Nanoprecipitation. Nanomaterials 2021, 11, 734. [Google Scholar] [CrossRef]
- Fu, Z.; Li, L.; Wang, Y.; Chen, Q.; Zhao, F.; Dai, L.; Chen, Z.; Liu, D.; Guo, X. Direct preparation of drug-loaded mesoporous silica nanoparticles by sequential flash nanoprecipitation. Chem. Eng. J. 2020, 382, 122905. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, C.; Peng, B.; Shu, M.; Che, S. pH-Responsive Drug Delivery System Based on Coordination Bonding in a Mesostructured Surfactant/Silica Hybrid. J. Phys. Chem. C 2011, 115, 7230–7237. [Google Scholar] [CrossRef]
- He, H.; Chen, S.; Zhou, J.; Dou, Y.; Song, L.; Che, L.; Zhou, X.; Chen, X.; Jia, Y.; Zhang, J.; et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials 2013, 34, 5344–5358. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Fu, Z.; Li, L.; Ma, E.; Sun, L.; Ma, Q.; Guo, X. Scalable Manufacture of Curcumin-Loaded Chitosan Nanocomplex for pH-Responsive Delivery by Coordination-Driven Flash Nanocomplexation. Polymers 2022, 14, 2133. https://doi.org/10.3390/polym14112133
Xia Z, Fu Z, Li L, Ma E, Sun L, Ma Q, Guo X. Scalable Manufacture of Curcumin-Loaded Chitosan Nanocomplex for pH-Responsive Delivery by Coordination-Driven Flash Nanocomplexation. Polymers. 2022; 14(11):2133. https://doi.org/10.3390/polym14112133
Chicago/Turabian StyleXia, Ziwei, Zhinan Fu, Li Li, Enguang Ma, Liang Sun, Qinyu Ma, and Xuhong Guo. 2022. "Scalable Manufacture of Curcumin-Loaded Chitosan Nanocomplex for pH-Responsive Delivery by Coordination-Driven Flash Nanocomplexation" Polymers 14, no. 11: 2133. https://doi.org/10.3390/polym14112133
APA StyleXia, Z., Fu, Z., Li, L., Ma, E., Sun, L., Ma, Q., & Guo, X. (2022). Scalable Manufacture of Curcumin-Loaded Chitosan Nanocomplex for pH-Responsive Delivery by Coordination-Driven Flash Nanocomplexation. Polymers, 14(11), 2133. https://doi.org/10.3390/polym14112133






