Preparation and Characterization of Electrosprayed Aerogel/Polytetrafluoroethylene Microporous Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
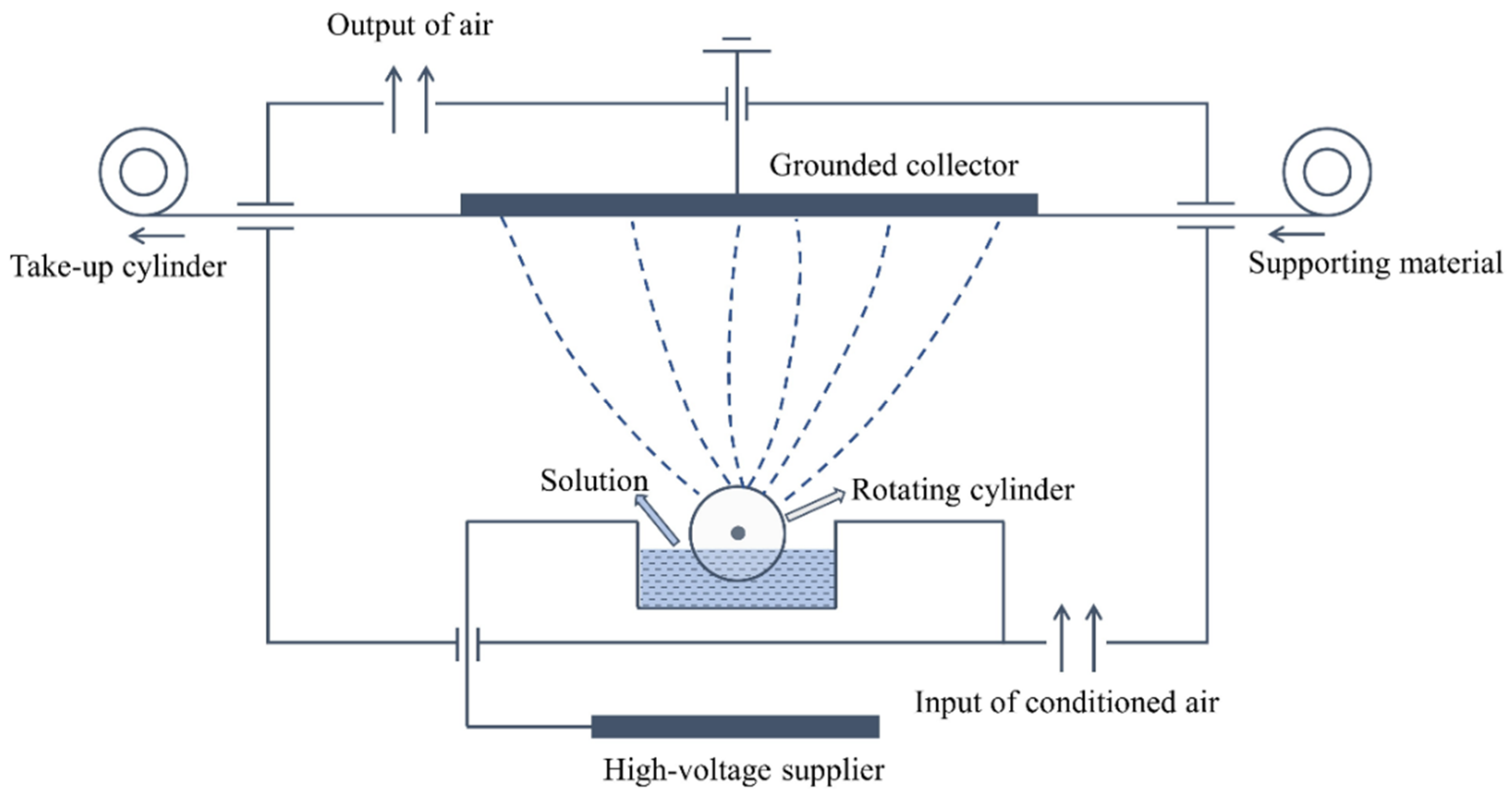
2.2. Preparation of Electro-Sprayed PTFE/Aerogel
2.3. Characterization
2.3.1. Morphologies
2.3.2. Particle Size Distribution
2.3.3. Thickness and Areal Density of the Materials
2.3.4. Surface Roughness
2.3.5. Water Contact Angle
2.3.6. Thermal Conductivity
3. Results and Discussion
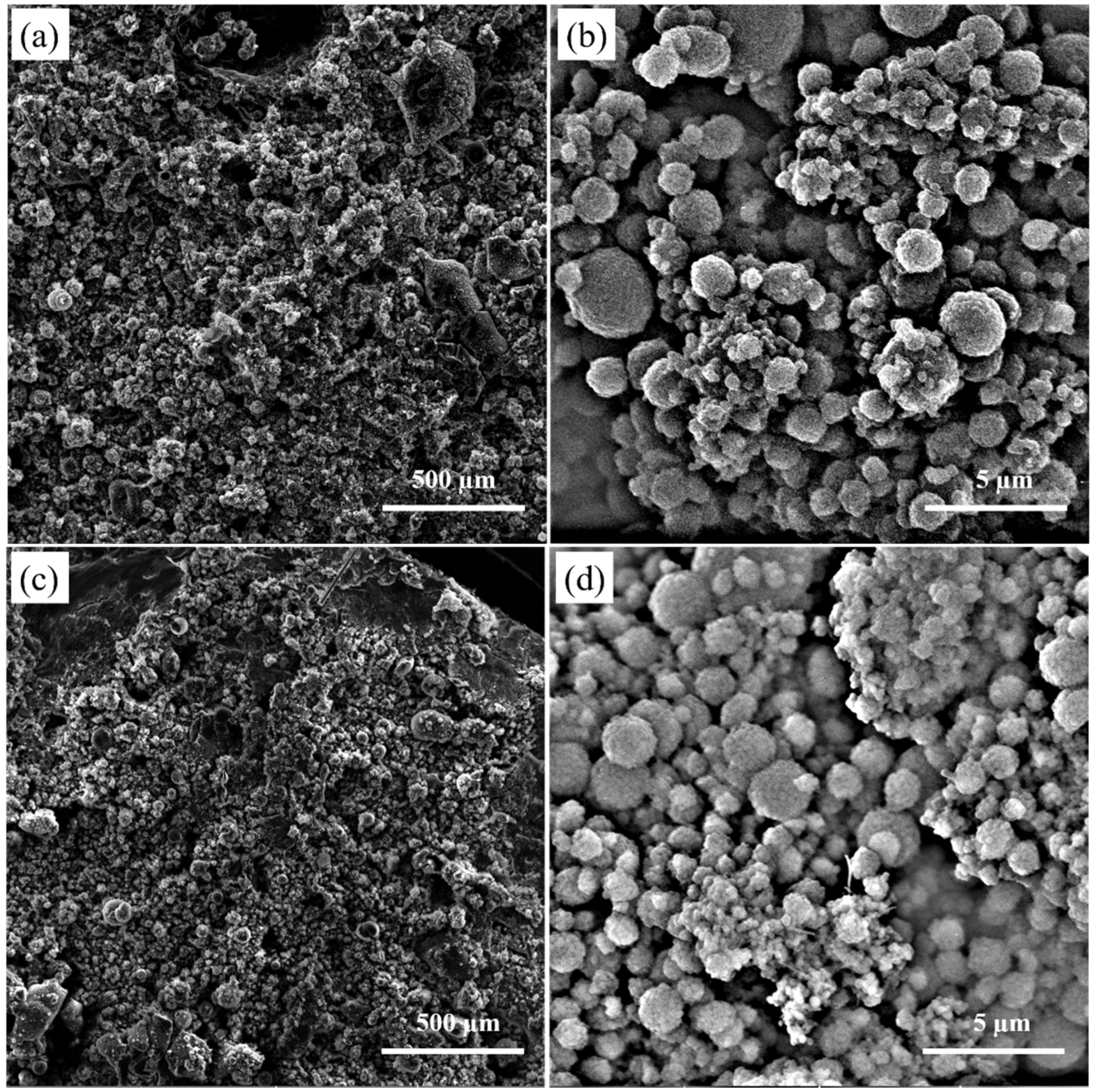
3.1. Cross-Section and Surface Morphology of the Electro-Sprayed Materials
3.2. Particle Size Distribution of the Electrosprayed Particles
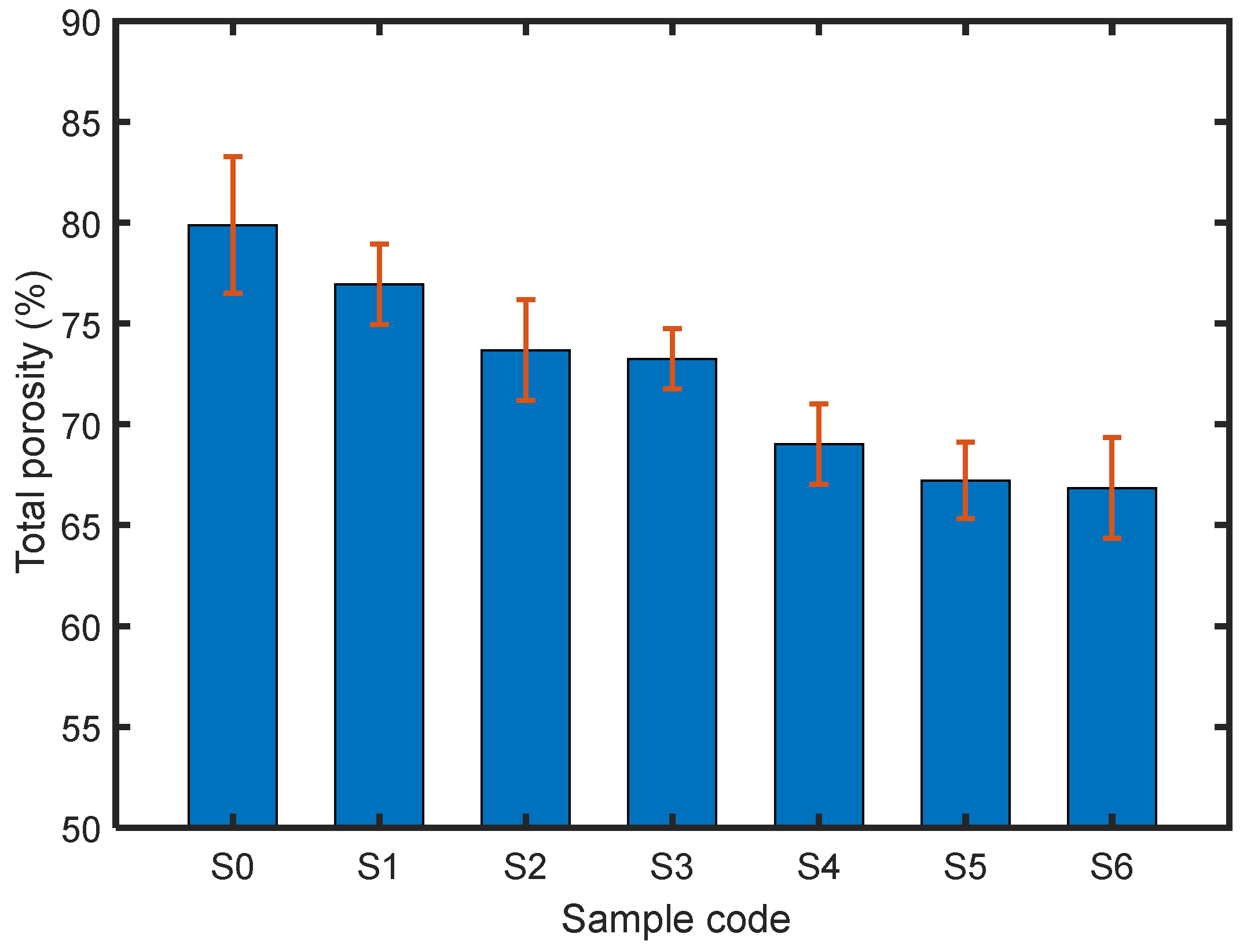
3.3. Structural Characteristics of the Materials
3.4. Analysis of Surface Roughness
3.5. Effect of Aerogels on Surface Hydrophobicity
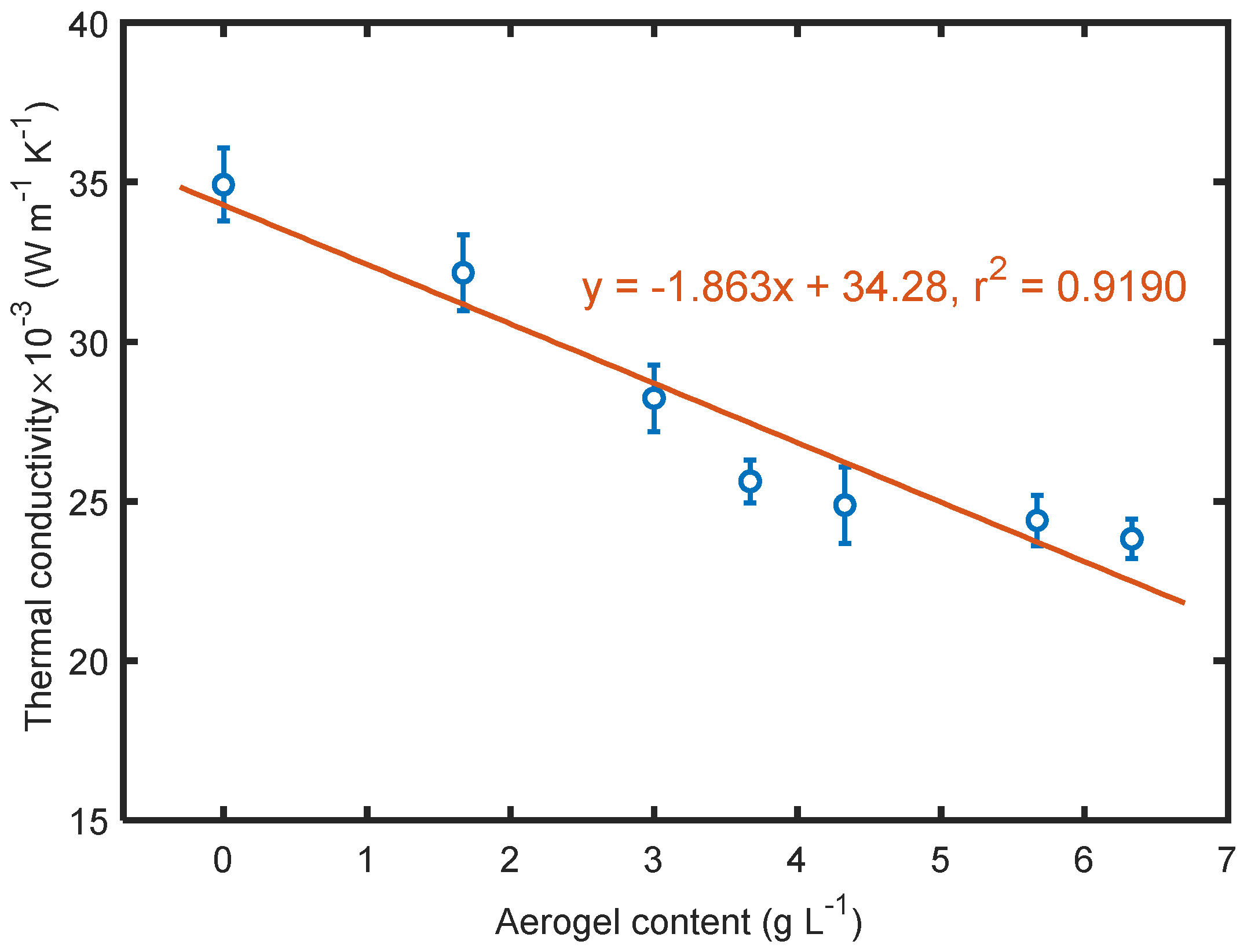
3.6. Effect of Aerogels on Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebnesajjad, S. Expanded PTFE Applications Handbook: Technology, Manufacturing and Applications; William Andrew Publishing: Oxford, UK, 2016. [Google Scholar]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Gardiner, J. Fluoropolymers: Origin, production, and industrial and commercial applications. Aust. J. Chem. 2015, 68, 13–22. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid. Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Thomas, P. The use of fluoropolymers for non-stick cooking utensils. Surf. Coat. Int. 1998, 81, 604–609. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, X. A study on friction and wear properties of PTFE coatings under vacuum conditions. Wear 2010, 269, 291–297. [Google Scholar] [CrossRef]
- Price, D.M.; Jarratt, M. Thermal conductivity of PTFE and PTFE composites. Thermochim. Acta 2002, 392, 231–236. [Google Scholar] [CrossRef]
- Cai, W.Z.; Tu, S.T.; Tao, G.L. Thermal conductivity of PTFE composites with three-dimensional randomly distributed fillers. J. Thermoplast. Compos. Mater. 2005, 18, 241–253. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, Z.; Gao, T.; Yue, K.; Zhang, X. Thermal property improvement of polytetrafluoroethylene nanocomposites with graphene nanoplatelets. RSC Adv. 2018, 8, 11367–11374. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Chen, T.; Chen, H. Study on the composites of two sized silica filled in PTFE. J. Reinf. Plast. Comp. 2006, 25, 1053–1058. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Lee, Y. The effects of filler content and size on the properties of PTFE/SiO2 composites. J. Poly Res. 2003, 10, 247–258. [Google Scholar] [CrossRef]
- Valdés-Solís, T.; Fuertes, A.B. High-surface area inorganic compounds prepared by nano-casting techniques. Mater. Res. Bull. 2006, 41, 2187–2197. [Google Scholar] [CrossRef]
- Thapliyal, P.; Singh, K. Aerogels as promising thermal insulating materials: An overview. J. Mater. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fricke, J.; Tillotson, T. Aerogels: Production, characterization, and applications. Thin Solid Films 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Venkataraman, M.; Jašíková, D.; Yang, T.; Mishra, R.; Militký, J.; Petrů, M. An experimental evaluation of convective heat transfer in multi-layered fibrous materials composed by different middle layer structures. J. Ind. Text. 2021, 51, 362–379. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, T.; Mishra, R.; Kanai, H.; Militky, J. Thermal and compression characteristics of aerogel-encapsulated textiles. J. Ind. Text. 2017, 7, 1998–2013. [Google Scholar] [CrossRef]
- Venkataraman, M.; Mishra, R.; Militky, J.; Xiong, X.; Marek, J.; Yao, J.; Zhu, G. Electrospun nanofibrous membranes embedded with aerogel for advanced thermal and transport properties. Polym. Adv. Technol. 2018, 29, 2583–2592. [Google Scholar] [CrossRef]
- Huang, D.; Shen, Y.; Yuan, Q.; Wang, C.; Shi, L. Preparation and characterization of silica aerogel/polytetrafluoroethylene composites. Mater. Res. Express 2019, 6, 115021. [Google Scholar] [CrossRef]
- Murali, K.P.; Rajesh, S.; Prakash, O.; Kulkarni, A.R.; Ratheesh, R. Preparation and properties of silica filled PTFE flexible laminates for microwave circuit applications. Compos. Part. A Appl. Sci. Manuf. 2009, 40, 1179–1185. [Google Scholar] [CrossRef]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Chen, B.; Simmons, K.; Wang, W. Nanoporous polytetrafluoroethylene/silica composite separator as a high-performance all-vanadium redox flow battery membrane. Adv. Energy Mater. 2013, 3, 1215–1220. [Google Scholar] [CrossRef]
- Guo, X.; Bai, C.; Shan, J.; Lei, W.; Ding, R.; Zhang, Y.; Yang, H. Fabrication and characterization of MSQ aerogel coating on ePTFE thin films for cable sheaths. Molecules 2019, 24, 1246. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Liu, F.; Cui, Z.; Wang, H.; Song, X.; Geng, L.; Wang, H.; Peng, X. Novel PTFE/CNT composite nanofiber membranes with enhanced mechanical, crystalline, conductive, and dielectric properties fabricated by emulsion electrospinning and sintering. Compos. Sci. Technol. 2021, 214, 108980. [Google Scholar] [CrossRef]
- Jirsak, O.; Sanetrnik, F.; Lukas, D.; Kotek, V.; Martinova, L.; Chaloupek, J. A Method of Nanofibres Production from a Polymer Solution using Electrostatic Spinning and a Device for Carrying Out the Method. European Patent EP 1,673,493, 8 September 2004. [Google Scholar]
- Brown, N.A.; Zhu, Y.; German, G.K.; Yong, X.; Chiarot, P.R. Electrospray deposit structure of nanoparticle suspensions. J. Electrostat. 2017, 90, 67–73. [Google Scholar] [CrossRef]
- Costa, L.M.M.; Bretas, R.E.S.; Gregorio, R. Effect of solution concentration on the electrospray/electrospinning transition and on the crystalline phase of PVDF. Mater. Sci. Appl. 2010, 1, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, M.; Yang, K.; Xiong, X.; Militky, J.; Kremenakova, D.; Zhu, G.; Yao, J.; Wang, Y.; Zhang, G. Preparation of electrosprayed, microporous particle filled Layers. Polymers 2020, 12, 1352. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, I.; Hes, L.; Bal, K. A non-destructive single plate method for measurement of thermal resistance of polymer sheets and fabrics. Int. J. Occup. Saf. Ergon. 2019, 25, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lenggoro, I.W.; Xia, B.; Okuyama, K. Sizing of colloidal nanoparticles by electrospray and differential mobility analyzer methods. Langmuir 2002, 18, 4584–4591. [Google Scholar] [CrossRef]
- Gomez, A.; Tang, K. Charge and fission of droplets in electrostatic sprays. Phys. Fluids 1994, 6, 404–414. [Google Scholar] [CrossRef]
- Castillo, J.L.; Martin, S.; Rodriguez-Perez, D.; Higuera, F.J.; Garcia-Ybarra, P.L. Nanostructured porous coatings via electrospray atomization and deposition of nanoparticle suspensions. J. Aerosol. Sci. 2018, 125, 148–163. [Google Scholar] [CrossRef]
- Bernett, M.K.; Zisman, W.A. Relation of wettability by aqueous solutions to the surface constitution of low-energy solids. J. Phys. Chem. 1959, 63, 1241. [Google Scholar] [CrossRef]
- Wloch, J.; Terzyk, A.P.; Wiśniewski, M.; Kowalczyk, P. Nanoscale water contact angle on PTFE surfaces characterized by the MD-AFM imaging. Langmuir 2018, 34, 4526–4534. [Google Scholar] [CrossRef]
- Shi, H.; Cui, J.; Shen, H.; Wu, H. Preparation of silica aerogel and its adsorption performance to organic molecule. Adv. Mater. Sci. Eng. 2014, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Altay, P.; Atakan, R.; Özcan, G. Silica aerogel application to polyester fabric for outdoor clothing. Fibers Polym. 2021, 22, 1025–1032. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Motahari, S. Mechanical, thermal, and hydrophobic properties of silica aerogel–epoxy composites. J. Appl. Polym. Sci. 2018, 135, 45706. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhong, T.; Wu, Z.; Zhan, X.; Ye, J. Thermal insulation and hydrophobization of wood impregnated with silica aerogel powder. J. Wood Sci. 2020, 66, 1–11. [Google Scholar] [CrossRef]
- Caps, R.; Fricke, J. Aerogels for Thermal Insulation. In Sol-Gel Technologies for Glass Producers and Users; Aegerter, M.A., Mennig, M., Eds.; Springer: Boston, MA, USA, 2004. [Google Scholar]






| Sample Code of the Electrosprayed Materials | Composition of the Spinning Solution | |
|---|---|---|
| Basic Solution | Aerogel (g L−1) | |
| S0 | TeflonTM PTFE 30 aqueous dispersion as received | 0 |
| S1 | 1.67 | |
| S2 | 3.00 | |
| S3 | 3.67 | |
| S4 | 4.33 | |
| S5 | 5.67 | |
| S6 | 6.33 | |
| Properties | Value Range |
|---|---|
| Distance between electrodes (mm) | 125 |
| Substrate speed (mm/min) | 15 |
| Electrode length (cm) | 50 |
| Electrode rotation (rpm) | 8 |
| Substrate | Polypropylene spun-bond nonwoven |
| Voltage (kV) | −10/45 |
| Air flow (m3 h) | 90/100 |
| Humidity/temperature | 40% RH/22 °C |
| Sample Code | Ra (μm) | Rp (μm) | Rv (μm) | Rsk (μm) |
|---|---|---|---|---|
| S0 | 2.7413 ± 0.3916 | 7.3493 ± 1.6335 | 13.1058 ± 3.1226 | −0.7058 ± 0.6786 |
| S1 | 2.8363 ± 0.3982 | 9.0997 ± 3.2011 | 13.5571 ± 2.0847 | −0.6897 ± 0.6868 |
| S2 | 3.5572 ± 1.1198 | 11.6905 ± 5.5050 | 17.9229 ± 6.8362 | −0.9110 ± 0.6688 |
| S3 | 3.6401 ± 0.8821 | 11.4426 ± 3.2350 | 14.0517 ± 4.7513 | 0.0319 ± 0.6267 |
| S4 | 3.5783 ± 0.5752 | 12.4228 ± 2.3710 | 16.6314 ± 4.6911 | −0.3828 ± 0.6133 |
| S5 | 3.7257 ± 1.5401 | 13.5657 ± 7.7107 | 18.9747 ± 8.5023 | −0.7092 ± 1.0158 |
| S6 | 5.2663 ± 1.8996 | 14.4447 ± 9.6368 | 21.711 ± 7.8459 | 0.4632 ± 0.6438 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Venkataraman, M.; Yang, T.; Militký, J.; Wiener, J. Preparation and Characterization of Electrosprayed Aerogel/Polytetrafluoroethylene Microporous Materials. Polymers 2022, 14, 48. https://doi.org/10.3390/polym14010048
Xiong X, Venkataraman M, Yang T, Militký J, Wiener J. Preparation and Characterization of Electrosprayed Aerogel/Polytetrafluoroethylene Microporous Materials. Polymers. 2022; 14(1):48. https://doi.org/10.3390/polym14010048
Chicago/Turabian StyleXiong, Xiaoman, Mohanapriya Venkataraman, Tao Yang, Jiří Militký, and Jakub Wiener. 2022. "Preparation and Characterization of Electrosprayed Aerogel/Polytetrafluoroethylene Microporous Materials" Polymers 14, no. 1: 48. https://doi.org/10.3390/polym14010048
APA StyleXiong, X., Venkataraman, M., Yang, T., Militký, J., & Wiener, J. (2022). Preparation and Characterization of Electrosprayed Aerogel/Polytetrafluoroethylene Microporous Materials. Polymers, 14(1), 48. https://doi.org/10.3390/polym14010048









