Abstract
Here, a dual-modification strategy using KMnO4 (potassium permanganate) and AlCl3·6H2O (aluminum chloride, hexahydrate) as co-modifiers to improve the Cr(VI) removal capacity of K2CO3 activated biochar is introduced. As a result, the dual-modified biochar with KMnO4 and AlCl3·6H2O has the calculated adsorption energy of −0.52 eV and −1.64 eV for HCrO4−, and −0.21 eV and −2.01 eV for Cr2O72−. The Al2O3 (aluminum oxide) and MnO (manganese oxide) embedded on the surface of dual-modified biochar bring more Cr(VI) absorption sites comparing to single-modified biochar, resulting in a maximum Cr(VI) saturated adsorption capacity of 152.86 mg g−1. The excellent removal performance is due to the synthetic effect of electrostatic attraction, reduction reaction, complexation reaction, and physical adsorption. The experimental results also indicated that the spontaneous adsorption process agreed well with the pseudo-second order and Langmuir models. This dual-modification strategy is not limited to the treatment of Cr(VI) with biochar, and may also be incorporated with the treatment of other heavy metals in aqueous environment.
1. Introduction
Discharge of chromium-containing wastewater has led to destruction of the aquatic ecological environment. Among all the oxidation states of chromium (Cr), Cr(VI) is the most toxic, with a toxicity 100 times higher than that of Cr(III) [1]. It has been reported that 0.1 mg L−1 of Cr(VI) is the threshold for aquatic organisms to stay alive [2]. Moreover, chromium in soil can affect crop metabolism and nutrient uptake. The Cr(VI) accumulation in the human body through the food chain harms internal organs, induces gene mutations, and causes cancer. Therefore, effective methods are needed to remove Cr(VI) in wastewater to protect the aquatic environment and human health.
Various methods, such as adsorption [3], chemical precipitation [4], ion exchange [5], and electrochemical techniques [6] have been applied to remove heavy metals. In addition, biological methods such as algae and microorganisms are also commonly used to remove hexavalent chromium from water [7,8]. Among these methods, adsorption is the most frequently adopted due to its simple operation, high efficiency, and low cost. Chen et al. prepared magnetic modified Enteromorpha prolifera biochar with a maximum adsorption capacity of 88.17 mg g−1 for Cr(VI) [2]. Sharma et al. prepared biochar with agricultural waste (Cornulaca-monacantha stem) as raw material, and immersed biochar in the mixture of NaOH (sodium hydroxide, 6%) and NaClO (sodium hypochlorite, 5.6%) to prepare modified biochar. The results showed that the maximum adsorption capacity of biochar and modified biochar for Cr(VI) were 37.17 mg g−1 and 67.88 mg g−1, respectively [9]. Many adsorbents, such as biochar [10], metal oxide nanoparticles [11], and graphene [12] have been explored for heavy metal removal. Biochar is considered as a promising adsorbent for its eco-friendly recycling. However, biochar has a limited capacity to remove heavy metal ions due to its small specific surface area and few functional groups [3,10]. Therefore, various strategies have been applied to modify biochar to improve its adsorption performances.
Chemical modification, including acid/base treatment [13], chemical oxidation [3], and impregnation with mineral oxides [14], is one of the most effective approaches to functionalize biochar. For example, biochar functionalized with KMnO4 as a strong oxidizing agent exhibited a high adsorption capacity of organic/inorganic pollutants due to the increased complexation effect of oxygen-containing functional groups [15]. Moreover, biochar modified with aluminum chloride (AlCl3) had an improved adsorption capacity of As(V) [16]. While many methods focused on the improvement of adsorption capacity via single modification, the impact of dual-modifiers on the removal performance of biochar still needs to be explored.
The removal of Cr(VI) from aqueous solution by bimetallic oxide-modified biochar has thus far been rarely studied. Here, a dual-modification strategy to prepare a novel biochar to remove Cr(VI) in solution is reported. The prepared bimetallic oxide modified biochar may have a large specific surface area and rich functional groups (MOx), which will have better Cr(VI) removal ability. The formation mechanism of biochar and its Cr(VI) removal mechanism were also investigated. The recycling of forest waste resources has been realized, and the technology of preparing biochar with dual-modifiers has been enriched in the academic circle. Specifically, the biochar (BC), prepared from chinar (Platanus orientalis Linn.) leaves, was first activated with K2CO3. Then the dual modifiers, KMnO4 and AlCl3, were embedded on the pre-activated biochar (KBC) by one-time modification. As a result, the dual-modified biochar (AMKBC) showed a significant improvement of physico-chemical properties and Cr(VI) removal capacity compared to the single-modified biochar.
2. Materials and Methods
2.1. Synthesis of Biochar
As a kind of forestry waste, the carbon content of chinar leaves is high, which has great advantages in preparing biochar. The high-value utilization of chinar leaves can also reduce the pollution caused by incineration. Leaves of chinar were collected on the campus of Qilu University of Technology (Jinan, China). A certain amount of crushed leaves was pyrolyzed in a tube furnace (Jinan Kester Experimental Equipment Co., Ltd., Jinan, China) at 800 °C for 2 h (BC). Crushed leaves mixed with K2CO3 (Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd., Tianjin, China) at a mass ratio of 50% was similarly pyrolyzed to make KBC. For the preparation of KMnO4 (Yantai Yuandong Fine Chemicals Co., Ltd., Yantai, China)/AlCl3·6H2O (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) modified biochar composites, KBC (0.25, 0.5, 1, and 1.5 g) was added into a 4 mL mixed solution of AlCl3·6H2O (0.6953 g) and KMnO4 (0.0759 g). The mixtures were dried and then pyrolyzed at 600 °C for 1 h to obtain AMKBC. Based on the mass ratio of KMnO4/AlCl3·6H2O to the added KBC, the KMnO4/AlCl3·6H2O modified KBC composites were named AMKBC3/1, AMKBC3/2, AMKBC3/4, and AMKBC1/2.
2.2. Materials Characterization
A scanning electron microscope (SEM) (JSM-7610F, JEOL, Akishima, Japan) combined with energy dispersive spectrometer (EDS) (X-max, Oxford, UK) was used to analyze the surface morphology and elemental composition of various biochars. The specific surface area (SSA) and pore-size distribution were assayed by N2 adsorption–desorption tests using a Brunner–Emmet–Teller (BET) surface area and pore size analyzer (BK300C, Gao Bo Science and Technology Co., Ltd., Beijing, China). The crystalline phases were obtained by X-ray diffraction analysis (XRD) (D8-ADVANCE, Bruker AXS, Karlsruhe, Germany). The chemical states and elemental composition of the tested metal oxides were assessed using X-ray photoelectron spectroscopy (XPS) (ESCALAB Xi+, ThermoFisher, Brno, Czech Republic).
2.3. Adsorption Measurements
To six identical 30 mL Cr(VI) solutions (100 mg L−1), 0.01 g of BC, KBC, AMKBC3/1, AMKBC3/2, AMKBC3/4, or AMKBC1/2 were added. The Cr(VI) concentration after 5 h shaking was measured according to our previously published method [5]. Three replicates were set for the adsorption experiments, and statistical analyses and least significant difference test for mean comparisons were conducted in SPSS (17.0, IBM company, Stanford, CA, USA). Differences at p ≤ 0.05 were considered significant. The adsorption capacity (q) of these materials was calculated (Equation (1)) [5].
where C0 (mg/L) and C (mg/L) represent the concentration of Cr(VI) before and after adsorption, respectively, V (L) is the volume of experimental solution, and m (g) is the mass of the adsorbent.
2.3.1. Effect of pH on the Removal of Cr(VI) in Solution
The optimal biochar (0.01 g) was added into Cr(VI) solution (100 mg L−1), and the pH value of the mixture was adjusted to 3, 5, 7, or 9. The Cr(VI) removal capacity from the solution was measured according to our previously published method after shaking for 13 h [5].
2.3.2. Adsorption Kinetic and Isothermal Adsorption Experiments
The adsorption kinetic (Equations (2)–(4)) and isothermal adsorption (Equations (5) and (6)) experiments were performed in this study according to our previous method [5]. The experimental parameters of adsorption kinetic were as follows: AMKBC3/4 dosage was 0.67 g L−1, the effect of contact time was 0–13 h, and solution concentration was 100 mg L−1 (pH = 3). The concentration of solution was changed (50–200 mg L−1) for the isothermal adsorption experiment, with the other parameters having been chosen because they were the best parameters obtained in the above experiment.
where qt (mg g−1) and qe (mg g−1) represent the adsorption amounts at time t (h) and equilibrium state during the adsorption process, respectively. K1 (h−1) and K2 (g mg−1 h−1) are the rate constants for the pseudo-first order and pseudo-second order adsorption kinetics. Kid (mg g−1 min−1/2) is the intra-particle diffusion rate constant, and C is a constant related to the thickness of the boundary layer. Coefficient qm (mg g−1) is the maximum adsorption capacity, Ce (mg L−1) is the equilibrium concentration of solution, and n is empirical index. Coefficients KL (L mg−1) and KF are indicators of adsorption capacity for Langmuir and Freundlich models.
2.4. Computational Method
The MnO (001)-terminated and Al2O3 (010)-terminated surfaces were chosen as computational models based on 2 × 2 supercell and 2 × 3 supercell, respectively. The constructed supercells were isolated with a 15 Å vacuum space in the c direction. The computational calculations were performed with the QUANTUM-ESPRESSO code [17], which is based on the density functional theory (DFT) [18], with the Hubbard model (DFT + U) [19]. The effective U value of 3.9 eV was applied for Mn (3d). The electronic wave function was expressed by the combination of plane wave basis, and the generalized gradient approximation (GGA) method with the Perdew–Burke–Ernzerhof (PBE) functional was employed for exchange and correlation interactions [20]. The Brillouin zones were performed by the Monkhorst-Pack scheme sampled into 3 × 3 × 1 for both MnO and Al2O3.
The adsorption energy (Eads (eV)) for HCrO4− and Cr2O72− on Al2O3 and MnO was estimated based on the following equation (Equation (7)) [17,18,19,20]:
where EMOx+Cr (eV) and EMOx (eV), respectively, represent the total energy of the biochar with and without adsorbed Cr(VI), ECr (eV) denotes the energy of an isolated Cr(VI). MOx stands for Al2O3 or MnO. By definition, Eads < 0 corresponds to favorable or exothermic adsorption of Cr(VI) on MOx.
Eads = EMOx+Cr − (EMOx + ECr)
3. Results
3.1. AMKBC3/4 Greatly Decreased Cr(VI) Content in Solution
The Cr(VI) adsorption capacity of BC and KBC were 10.76 mg g−1 and 39.82 mg g−1 (Figure 1), respectively. Notably, the adsorption capacity of AMKBC had a bell curve as the mass ratio of KMnO4/AlCl3·6H2O to KBC increased (Figure 1). Among all the samples, AMKBC3/4 exhibited the highest adsorption capacity of 56.87 mg g−1. Compared with the maximum adsorption capacity (38 mg g−1) of Mg/Al modified biochar for Cr(VI) in the literature [1], the biochar prepared in this study has better adsorption performance. The further decrease of Cr(VI) adsorption capacity was attributed to the blocking effect of Al/Mn metal oxides on the pores of the biochar.
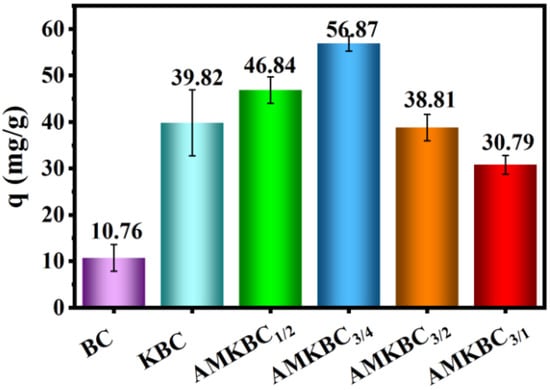
Figure 1.
Cr(VI) adsorption capacity of various biochars: initial pH; T = 5 h; m = 0.01 g; V = 30 mL; C0 = 100 mg L−1.
3.2. Characterization of BC, KBC, and AMKBC3/4
In the SEM images, BC had a small number of pores (Figure 2a), while KBC had a superior pore structure (Figure 2b). The gases (CO and CO2) produced from the chemical reaction between K2CO3 and carbon advantageously formed additional pores on the biochar surface except for the oxidation of carbon, which resulted in an increased interlayer distance [5].
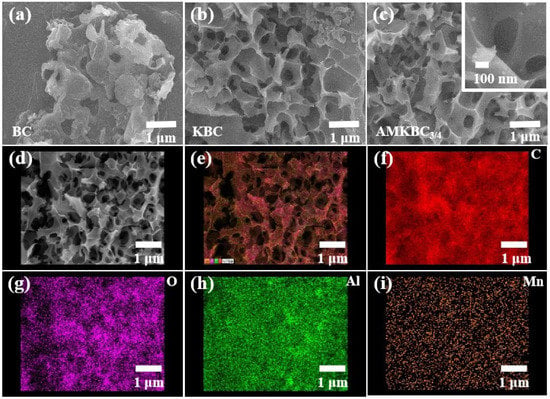
Figure 2.
SEM images of BC (a), KBC (b) and AMKBC3/4 (c,d); EDS merged image of total elements (e); the distribution maps of C (f), O (g), Al (h), and Mn (i) for AMKBC3/4.
AMKBC3/4 with porous structure was loaded with spherical particles, which might be Al/Mn oxides (Figure 2c, [21]). Similarly, KBC with a large surface area could be the carrier for nano Al/Mn oxides to reduce agglomeration, which facilitated a high adsorption capacity of the pollutants [22]. The EDS results indicated that Al and Mn oxides had loaded on the surface of AMKBC3/4 (Table S1), which matched well with the results from elemental distribution diagrams (Figure 2d–i). The presence of C and O indicated the existence of rich oxygen-containing functional groups on the surface of AMKBC3/4 (Figure 2f,g). As shown in Figure 2e, the uniform display of each element on the surface of AMKBC3/4 indicates that Al/Mn oxides did not agglomerate, which would have provided additional adsorption sites for Cr(VI) removal.
In Figure 3a, the graphite crystal plane diffraction peaks ((002) and (100)) were measured to be 23° and 43°, respectively, for both BC and KBC [23]. Notably, the remarkable diffraction peaks of Al2O3 appeared at 18.71° (JCPDS: 88–1609) and 28.42° (JCPDS: 88–0107), and the peak situated at 40.49° is assigned to MnO [24]. Previous studies have found that the existence of metal oxides on the surface of biochar could enhance the removal capacity of anions via coordination reaction [25]. The loaded Al/Mn oxides on the biochar surface can react with HCrO4− and Cr2O72−, resulting in effective Cr(VI) removal [26].
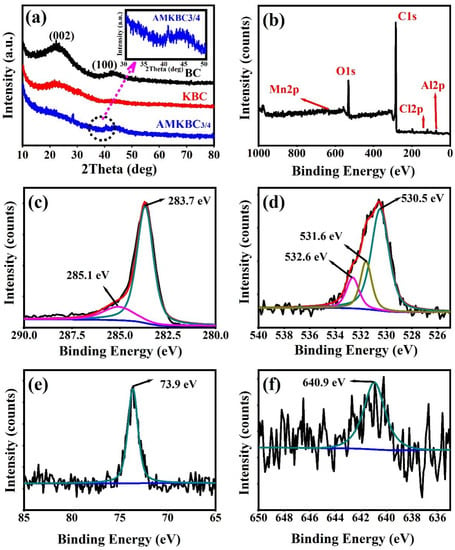
Figure 3.
X-ray diffraction analysis of BC, KBC, and AMKBC3/4 (a). For AMKBC3/4: XPS spectra (b); high resolution XPS spectra of C1s (c); O1s (d); Al2p (e); Mn2p3/2 (f).
The visible peaks of C1s, O1s, Al2p, and Mn2p for AMKBC3/4 (Figure 3b–f) confirmed the loading effect of Al/Mn oxides, which was consistent with the EDS analysis. The peaks located at 283.7 eV and 285.1 eV represented the functional groups of C–N and C=C of the AMKBC3/4 in the high-resolution XPS spectrum (Figure 3c) [27]. The O1s spectrum had three peaks (Figure 3d): the peak of 531.6 eV can be ascribed to the lattice oxygen (CO, C=O) [28]; the peaks of 532.6 eV and 530.5 eV are attributed to the oxygen from organic matter (–OH or C–O–C) [29] and inorganic matter (MO and M–OH, M was metal), respectively [28]. The spectrum of Al2p and Mn2p3/2 represented elemental aluminum and manganese from Al2O3 and MnO (Figure 3e,f), respectively [30]. The loaded Al/Mn oxides with different chemical states on the surface of AMKBC3/4 could react with Cr2O72− and HCrO4− through a complex reaction and electrostatic attraction, which enhanced Cr(VI) removal (Equations (8) and (9)). A similar result was obtained by Wu, who found that Al/Mn oxides could form complexes with As(III) and As(V) [4].
AMKBC3/4 had a higher percentage of micropores than BC when the relative pressure (P/P0) < 0.1 (Figure 4a). The hysteresis loop indicated that both BC and AMKBC3/4 had mesoporous structure (Figure 4a, [31]). Moreover, the upward curve indicated the existence of macropores for these biochars when the relative pressure (P/P0) was close to 1.0 (Figure 4a, [32]). A similar result was obtained from the pore volume distribution (Figure 4b). Accordingly, the specific surface area and the total pore volume of AMKBC3/4 were 1173.36 m2 g−1 and 0.54 cm3 g−1, respectively (Table 1), which was 15.25 and 8.87 times higher than that of biochar. The surface area and volume of micropore reached 93.97% and 83.92%, which was significantly higher than those of BC (85.37% and 47.54%). The increased surface area and pore volume of AMKBC3/4 might be attributed to the large surface area of loaded Al/Mn oxides and gasification of K2CO3 [5], which enhanced Cr(VI) removal.
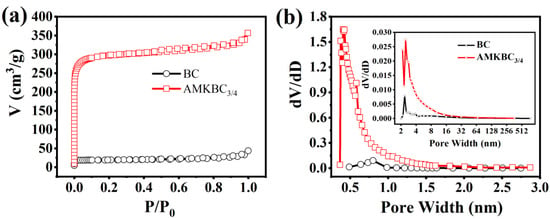
Figure 4.
N2 adsorption-desorption isotherms (a), and pore size distribution (b) of BC and AMKBC3/4.

Table 1.
The textural parameters of BC and AMKBC3/4.
3.3. Effect of pH on the Removal of Cr(VI) in Solution
Chromium mainly exists in the form of HCrO4− and Cr2O72− under acidic conditions; removal efficiency reached 83.86% when the pH value of solution was 3 (Figure 5), which could be ascribed to the following three favorable factors: (i) low adsorption energy for HCrO4− and Cr2O72− according to DFT calculations, as shown in Figure 6 and Figure 7 and Table S2 [14]; (ii) high redox potential of Cr(VI) promoting the reduction of Cr(VI) to Cr(III) at low pH conditions [29]; (iii) enhanced electrostatic attraction due to the increased zeta potential of AMKBC3/4 while in the form of CrO42− under alkaline conditions [5]. The removal rate of Cr(VI) is due to the Al/Mn oxides dopant [1]. Conversely, the decreased Cr(VI) removal rate with the increased pH was because OH– and CrO42− ions competed for unfilled adsorption sites of AMKBC3/4 [14]. Based on the above analysis, the optimum pH of the solution and the corresponding optimal dosage of AMKBC3/4 were set to 3 and 0.67 g L−1, respectively, in the subsequent experiments to obtain excellent adsorption performance.
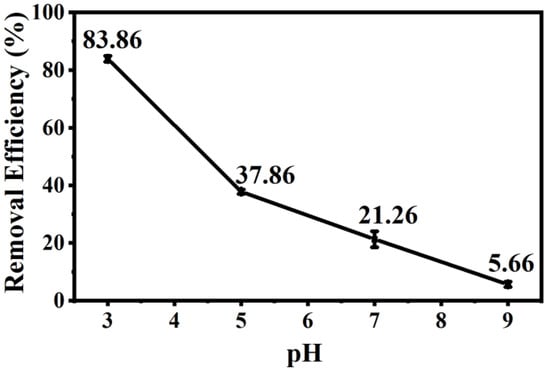
Figure 5.
Effect of pH on the removal of Cr(VI) in solution: pH = 3–9; T = 13 h; m = 0.01 g; V = 15 mL; C0 = 100 mg L−1.
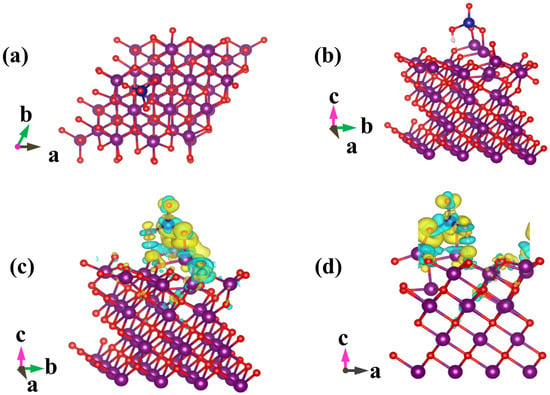
Figure 6.
Optimized geometric configurations for HCrO4− adsorbed on MnO(001) (a,b), and isosurface representations of charge difference (c,d). The accumulation and depletion of electrons are represented by the yellow and cyan regions, respectively. Isosurface value = 0.002 e/bohr3. Manganese atoms are purple, oxygen atoms are red, chromium atoms are blue, and hydrogen atoms are white.
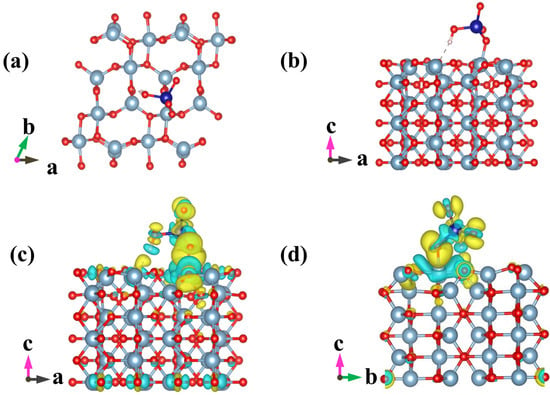
Figure 7.
Optimized geometric configurations for HCrO4− adsorbed on Al2O3(010) (a,b), and isosurface representations of charge difference (c,d). The accumulation and depletion of electrons are represented by the yellow and cyan regions, respectively. Isosurface value = 0.002 e/bohr3. Aluminum atoms are gray, oxygen atoms are red, chromium atoms are blue, and hydrogen atoms are white.
3.4. Adsorption Kinetics
The influence of contact time on the Cr(VI) adsorption is presented in Figure S1a. Specifically, the Cr(VI) adsorption capacity of AMKBC3/4 increased quickly in the first hour, and then it slowly increased until the adsorption achieved equilibrium of 125.66 mg g−1 at the 8th hour. The rapid Cr(VI) adsorption was due to the large amount of effective adsorption sites and functional groups on the surface of AMKBC3/4. Likewise, the reduced Cr(VI) adsorption rate was due to the reduced adsorption sites and the increased diffusion resistance with the extension of the adsorption process.
The Cr(VI) diffusion to the AMKBC3/4 surface according to Weber–Morris intraparticle diffusion modeling is presented in Figure S1b, and the external surface adsorption and the intraparticle diffusion along the pores of the AMKBC3/4 occurred before the Cr(VI) adsorption achieved equilibrium. A similar result was reported by our previous study as well [5]. The pseudo-first order model and the pseudo-second order model reflected the Cr(VI) adsorption process of AMKBC3/4 (Figure S1c,d): the correlation coefficient of pseudo-second order model was higher than that of pseudo-first order model, indicating that the Cr(VI) adsorption on AMKBC3/4 was mainly chemical adsorption. Moreover, the theoretical value of the adsorbed Cr(VI) according to the pseudo-second order model was 126.58 mg g−1, which was close to the experimental value of 125.66 mg g−1.
3.5. Adsorption Isotherms
The isothermal Cr(VI) adsorption models on AMKBC3/4 is shown in Figure S2. The correlation coefficient of the Langmuir model was higher than that of the Freundlich model, indicating that the monolayer Cr(VI) adsorption occurred on AMKBC3/4. Meanwhile, the calculated Cr(VI) adsorption capacity on AMKBC3/4 from the Langmuir model was 158.73 mg g−1, which was close to the maximum adsorption capacity of 152.86 mg g−1. Compared with the modified biochar (67.88 mg g−1) prepared by soaking biochar in the mixture of NaOH and NaClO by Sharma et al. [9], the biochar prepared in this study has a larger adsorption capacity for Cr(VI), and the doping of metal oxides plays a significant role in this process. The Cr(VI) adsorption capacity by various modifiers-produced biochars is compared in Table 2, which indicates that the AMKBC3/4 has a superior Cr(VI) adsorption capacity. Compared with single modification, the biochar prepared in this study has a higher adsorption capacity, which proves that the dual modification can indeed enhance the adsorption capacity of biochar compared to the single modification. The following reasons could explain the Cr(VI) removal in the solution: chromate ions implanted the pores of the AMKBC3/4 due to its surface structure destruction from the release of Al3+/Mn4+ [33], and electrostatic attraction occurred between chromate ions and reattached Al3+/Mn4+ by AMKBC3/4 (Equation (10)). The existence of metal oxides on the surface of AMKBC3/4 could enhance the removal capacity of HCrO4− and Cr2O72− via coordination reaction [25].

Table 2.
Adsorption capacities of Cr(VI) by modified biochars derived from various feedstocks.
3.6. Removal Mechanism of Cr(VI) in Solution
While the KBC served as the carrier for Al/Mn oxides with reduced agglomeration, the loaded Al/Mn oxides could also enhance the adsorbent surface area. Therefore, the Cr(VI) removal by AMKBC3/4 in solution was dependent on its pore structure and on the loaded Al/Mn oxides. Overall, the possible approaches for Cr(VI) removal by AMKBC3/4 were summarized (Figure 8): (i) the functional groups and loaded metal cations from protonated adsorbent could adsorb HCrO4−, Cr2O72−, and CrO42− via electrostatic attraction [38]; (ii) Cr(VI) could be reduced to Cr(III) by hydroxyl group at a lower pH (Equation (11)) [39], followed by the Cr(III) chelation with the Al/Mn oxides on the surface of AMKBC3/4 (Equations (12)–(14)); (iii) Cr(VI) could implant the pores of AMKBC3/4.
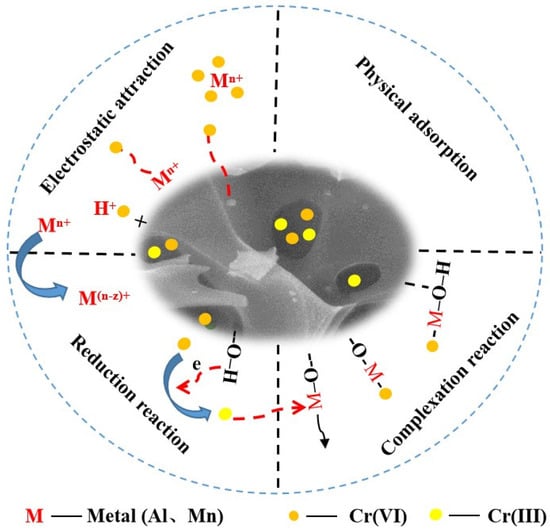
Figure 8.
Cr(VI) removal mechanism of AMKBC3/4.
4. Techno Economic Challenges and Future Research Directions
The technological challenge to preparing dual-modified biochar is to find an appropriate ratio of KBC and Al/Mn oxides in order to make metal oxides that can be evenly distributed on the biochar surface and do not block the biochar channels. In the future, dual-modified biochar can be applied to treat organic wastewater, and the removal mechanism can also be explored. Moreover, we can try to apply dual-modified biochar to treat natural water bodies.
5. Conclusions
In summary, a novel biochar has been developed as an adsorbent for removal of Cr(VI) in solution with a dual-step strategy. When the dosage of KBC was 1 g, AlCl3·6H2O is 0.6953 g, and KMnO4 was 0.0759 g, the modified biochar had the best adsorption performance, and had a maximum Cr(VI) saturated adsorption capacity of 152.86 mg g−1. The feasibility of dual-modified biochar was verified, which will provide reference for the preparation process and formation mechanism of other dual-modified biochar. The modified biochar of AMKBC3/4 demonstrated its potential for removing Cr(VI) due to the synthetic effect of electrostatic attraction, reduction reaction, complexation reaction and physical adsorption in solution. The spontaneous adsorption process agreed well with the pseudo-second order and Langmuir models. Overall, this present study provided not only a novel modification route for biochar, but also possibilities to remediate water polluted with other heavy metals.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/polym14010039/s1, Figure S1: Effect of contact time on the removal of Cr(VI) (a), Weber-Morris intra-particle diffusion model (b), pseudo-first order model (c) and pseudo-second order model (d) for Cr(VI) adsorption of AMKBC3/4. (pH = 3, T = 0–13 h, m = 0.01 g, V = 15 mL, C0 = 100 mg L−1), Figure S2: Langmuir isotherm (a) and Freundlich isotherm (b) for Cr(VI) adsorption of AMKBC3/4. (pH = 3, T = 13 h, m = 0.01 g, V = 15 mL, C0 = 50–200 mg L−1), Table S1: Elemental content of adsorbents, Table S2: DFT calculated adsorption energy (Eads, eV) of HCrO4− and Cr2O72− for the favored adsorption configurations on MnO (001) and Al2O3 (010).
Author Contributions
Conceptualization, Y.S., J.Y. and H.M.; methodology, Y.S., Q.C. and H.M.; software, Q.C. and Y.S.; validation, Y.S.; formal analysis, Y.S.; investigation, J.Y.; resources, H.C.; data curation, Y.S.; writing—original draft preparation, Y.Y.; writing—review and editing, W.L.; visualization, Y.Y.; supervision, H.M.; project administration, H.M.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong Province (Grant No. ZR2020ME230), State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences (Grant No. ZZ20190108), Key Laboratory of Agro-Forestry Environmental Processes and Ecological Regulation of Hainan Province (Hainan University) (Grant No. AFEPER202004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luo, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour. Technol. 2019, 276, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Xin, J.; Sun, P.; Wu, D. Adsorption behavior and mechanism of Cr(VI) by modified biochar derived from Enteromorpha prolifera. Ecotox. Environ. Safe. 2018, 164, 440–447. [Google Scholar] [CrossRef]
- An, Q.; Jiang, Y.Q.; Nan, H.Y.; Yu, Y.; Jiang, J.N. Unraveling sorption of nickel from aqueous solution by KMnO4 and KOH-modified peanut shell biochar: Implicit mechanism. Chemosphere 2019, 214, 846–854. [Google Scholar] [CrossRef]
- Wu, K.; Liu, T.; Xue, W.; Wang, X. Arsenic(III) oxidation/adsorption behaviors on a new bimetal adsorbent of Mn-oxide-doped Al oxide. Chem. Eng. J. 2012, 192, 343–349. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Mólgora, C.C.; Domínguez, A.M.; Avila, E.M.; Drogui, P.; Buelna, G. Removal of arsenic from drinking water: A comparative study between electrocoagulation-microfiltration and chemical coagulation-microfiltration processes. Sep. Purif. Technol. 2013, 118, 645–651. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent chromium removal from water by microalgal-based materials: Adsorption, desorption and recovery studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chen, S.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Bharagava, R.N. Reduction of hexavalent chromium by Microbacterium paraoxydans isolated from tannery wastewater and characterization of its reduced products. J. Water Process Eng. 2020, 39. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, K.K.; Mehta, P.; Pathania, D. Efficient adsorption of chlorpheniramine and hexavalent chromium (Cr(VI)) from water system using agronomic waste material. Sustain. Chem. Pharm. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrol. 2021, 155. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef]
- Chen, M.; He, F.; Hu, D.; Bao, C.; Huang, Q. Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem. Eng. J. 2020, 381. [Google Scholar] [CrossRef]
- Yuan, P.; Fan, M.; Yang, D.; He, H.; Liu, D.; Yuan, A.; Zhu, J.; Chen, T. Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J. Hazard. Mater. 2009, 166, 821–829. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Yuan, L.; Zhou, D. Comparison of adsorption behavior studies of Cd(2+) by vermicompost biochar and KMnO4-modified vermicompost biochar. J. Environ. Manag. 2020, 256, 109959. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, A.-Z.; Xu, R.-K. Sorption of As(V) by Aluminum-Modified Crop Straw-Derived Biochars. Water Air Soil Pollut. 2013, 224. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, V.I.; Lichtenstein, F.; Lichtenstein, A.I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA + U method. J. Phys. Condens. Matter 1997, 9, 767–808. [Google Scholar] [CrossRef] [Green Version]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Zhang, J.; Chang, B.; Liu, T.; Zhang, F.; Jin, P.; Wang, W.; Wang, X. Removal of arsenic(III,V) by a granular Mn-oxide-doped Al oxide adsorbent: Surface characterization and performance. Environ. Sci. Pollut. Res. Int. 2017, 24, 18505–18519. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, H.; Li, H.; He, X.; Shi, Y.; Kruse, A. Microwave digestion-assisted HFO/biochar adsorption to recover phosphorus from swine manure. Sci. Total Environ. 2018, 621, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, F.; Su, Y.; Shao, N.; Yu, D.; Liu, Y.; Liu, W.; Zhang, Z. Luffa sponge-derived hierarchical meso/macroporous boron nitride fibers as superior sorbents for heavy metal sequestration. J. Hazard. Mater. 2019, 378, 120669. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, H.; Ma, Y.; Yang, Y.; Li, B.; Cui, Y.; Guo, Z.; Wang, L. Core-shell-structured Co@Co4N nanoparticles encapsulated into MnO-modified porous N-doping carbon nanocubes as bifunctional catalysts for rechargeable Zn–air batteries. J. Energy Chem. 2020, 50, 52–62. [Google Scholar] [CrossRef]
- Penke, Y.K.; Anantharaman, G.; Ramkumar, J.; Kar, K.K. Redox synergistic Mn-Al-Fe and Cu-Al-Fe ternary metal oxide nano adsorbents for arsenic remediation with environmentally stable As(0) formation. J. Hazard. Mater. 2019, 364, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.W.; Chen, Q. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model. J. Clean. Prod. 2019, 237. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Huang, Z. Carbon spheres/activated carbon composite materials with high Cr(VI) adsorption capacity prepared by a hydrothermal method. J. Hazard. Mater. 2010, 173, 377–383. [Google Scholar] [CrossRef]
- Wang, L.-j.; Yu, J.-p.; Chou, K.-c.; Seetharaman, S. Effects of MgO and Al2O3 Addition on Redox State of Chromium in CaO-SiO2-CrO x Slag System by XPS Method. Metall. Mater. Trans. B 2015, 46, 1802–1808. [Google Scholar] [CrossRef]
- Yin, W.; Guo, Z.; Zhao, C.; Xu, J. Removal of Cr(VI) from aqueous media by biochar derived from mixture biomass precursors of Acorus calamus Linn. and feather waste. J. Anal. Appl. Pyrol. 2019, 140, 86–92. [Google Scholar] [CrossRef]
- Yin, G.; Song, X.; Tao, L.; Sarkar, B.; Sarmah, A.K.; Zhang, W.; Lin, Q.; Xiao, R.; Liu, Q.; Wang, H. Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd(II) from aqueous solutions. Chem. Eng. J. 2020, 389. [Google Scholar] [CrossRef]
- Selvan, R.K.; Zhu, P.; Yan, C.; Zhu, J.; Dirican, M.; Shanmugavani, A.; Lee, Y.S.; Zhang, X. Biomass-derived porous carbon modified glass fiber separator as polysulfide reservoir for Li-S batteries. J. Colloid Interface Sci. 2018, 513, 231–239. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wang, C.; Wang, H. Biochar/MnAl-LDH composites for Cu (ΙΙ) removal from aqueous solution. Colloid Surface 2018, 538, 443–450. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, C.; Guan, Q.; Ning, P.; Gu, J.; Chen, Q.; Zhang, J.; Miao, R. Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 2018, 195, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Q.; Gao, B.; Li, M.; Fan, Z.; Sang, W.; Hao, H.; Wei, X. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation. Environ. Pollut. 2020, 265, 115018. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, C.; Lv, Y.; Zhang, W.; Du, Y.; Hao, Z.; Zhang, J. Adsorption and coadsorption mechanisms of Cr(VI) and organic contaminants on H3PO4 treated biochar. Chemosphere 2017, 186, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yue, Q.; Mao, Y.; Gao, B.; Gao, Y.; Huang, L. Enhanced adsorption of chromium onto activated carbon by microwave-assisted H3PO4 mixed with Fe/Al/Mn activation. J. Hazard. Mater. 2014, 265, 191–200. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, H.; Wang, Y.; Wang, Y.; Qu, W.; An, Q.; Liu, Y. Synthesis of novel modified magnetic chitosan particles and their adsorption performance toward Cr(VI). Bioresour. Technol. 2018, 267, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liu, Q.; Chen, Z.; Gu, J.; Zhu, C.; Lu, T.; Zhang, D.; Ma, J. N-doped porous carbon with magnetic particles formed in situ for enhanced Cr(VI) removal. Water Res. 2013, 47, 4188–4197. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).