Utility of Thermal Cross-Linking in Stabilizing Hydrogels with Beta-Tricalcium Phosphate and/or Epigallocatechin Gallate for Use in Bone Regeneration Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sponges (Precursors of Hydrogels)
2.2. Leakage Test of β-TCP Granules from Hydrogels
2.3. Analysis of Biocompatibility of the Hydrogels
2.4. Animal Experiment
2.5. Statistical Analysis
3. Results
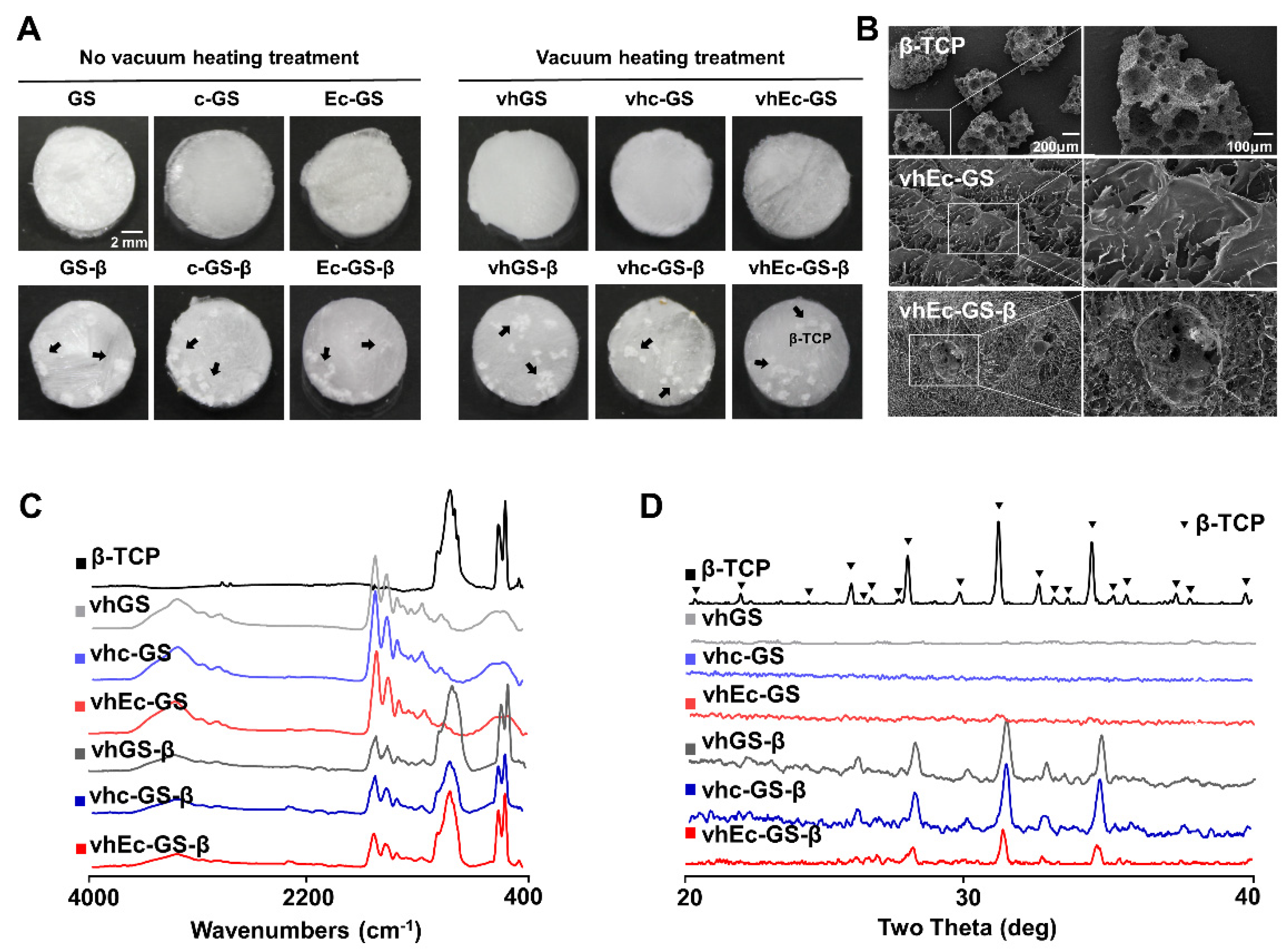
3.1. Characterization of Sponges (Precursors of Hydrogels)
3.2. Leakage of β-TCP Granules from Hydrogels
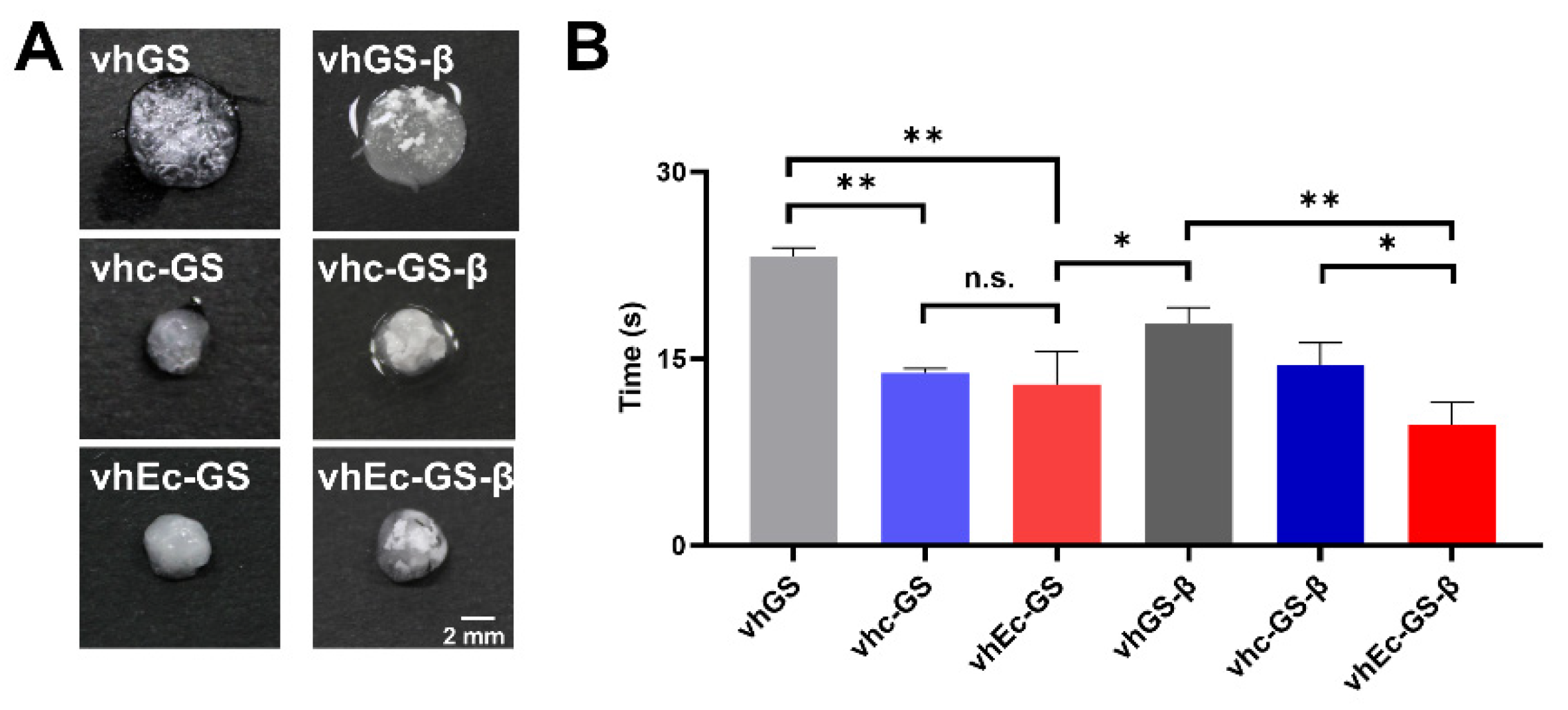
3.3. Hydrogel Form and Water Absorption Rate
3.4. In Vitro Biocompatibility Test
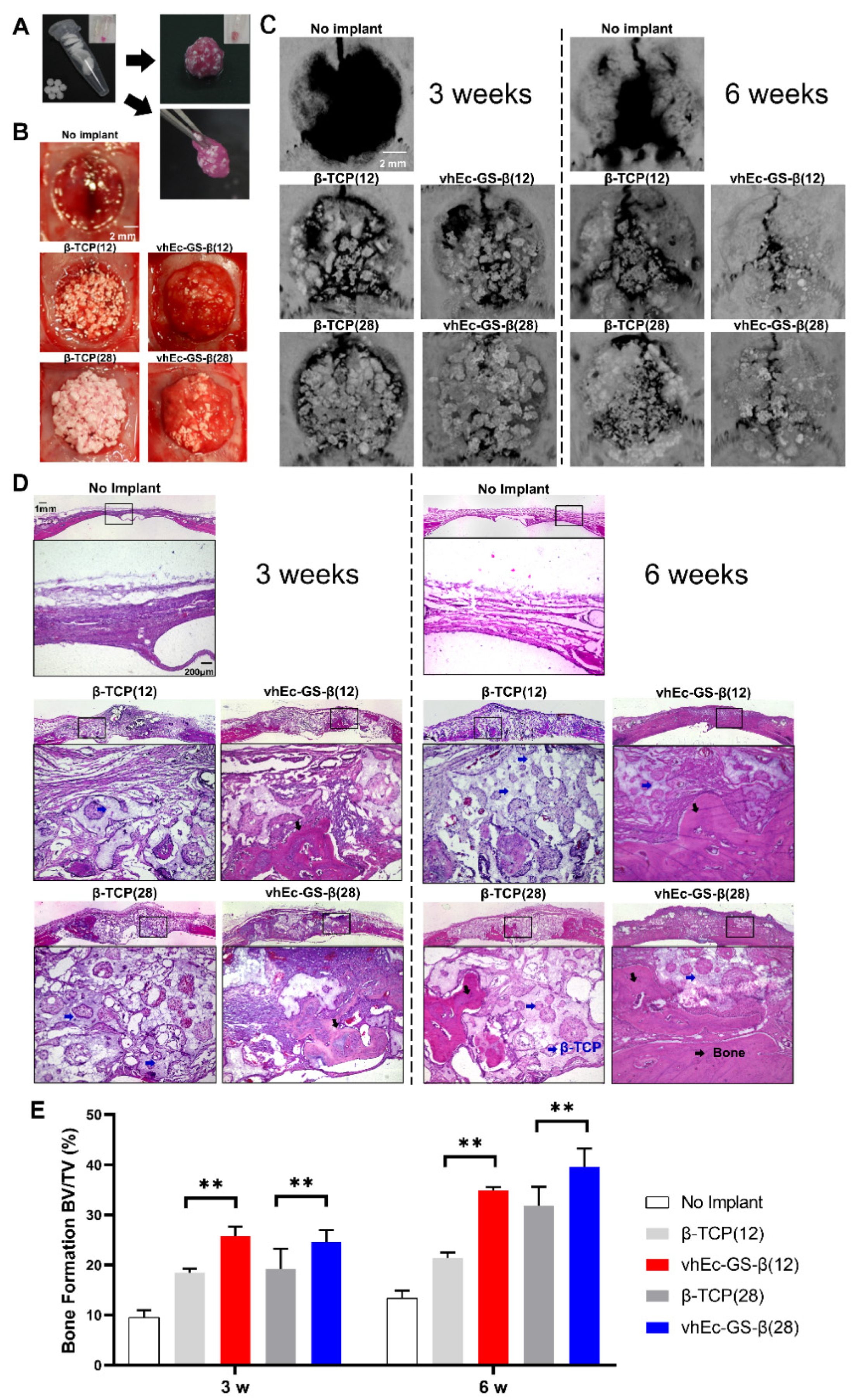
3.5. Bone Regeneration and β-TCP Resorption
3.6. β-TCP Resorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, T.M.; Moreno, E.C.; Patel, J.M.; Brown, W.E. Solubility of β-Ca3(PO4)2 in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37 °C. J. Res. Natl. Bur. Stand A Phys. Chem. 1974, 78, 667. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. A detailed history of calcium orthophosphates from 1770s till 1950. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3085–3110. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Honeycomb blocks composed of carbonate apatite, beta-tricalcium phosphate, and hydroxyapatite for bone regeneration: Effects of composition on biological responses. Mater. Today Bio 2019, 4, 100031. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, A.; Tanaka, T.; Chazono, M.; Komaki, H.; Kitasato, S.; Inagaki, N.; Akiyama, S.; Marumo, K. Effects of micro-porosity and local BMP-2 administration on bioresorption of beta-TCP and new bone formation. Biomater. Res. 2019, 23, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yuan, H.; Chandrakar, A.; Moroni, L.; Habibovic, P. 3D porous Ti6Al4V-beta-tricalcium phosphate scaffolds directly fabricated by additive manufacturing. Acta Biomater. 2021, 126, 496–510. [Google Scholar] [CrossRef]
- Yusof, M.R.; Shamsudin, R.; Zakaria, S.; Abdul Hamid, M.A.; Yalcinkaya, F.; Abdullah, Y.; Yacob, N. Fabrication and characterization of carboxymethyl Starch/Poly(l-Lactide) Acid/beta-Tricalcium Phosphate composite nanofibers via electrospinning. Polymers 2019, 11, 1468. [Google Scholar] [CrossRef] [Green Version]
- Omata, K.; Matsuno, T.; Asano, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Enhanced bone regeneration by gelatin-beta-tricalcium phosphate composites enabling controlled release of bFGF. J. Tissue Eng. Regen. Med. 2014, 8, 604–611. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lin, K.C.; Chen, Y.S.; Yao, C.H. A novel porous gelatin composite containing naringin for bone repair. Evid. Based Complement. Alternat. Med. 2013, 2013, 283941. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Prasertsung, I.; Mongkolnavin, R.; Damrongsakkul, S.; Wong, C.S. Surface modification of dehydrothermal crosslinked gelatin film using a 50 Hz oxygen glow discharge. Surf. Coat. Technol. 2010, 205, S133–S138. [Google Scholar] [CrossRef]
- Ho, C.H.; Chu, P.Y.; Peng, S.L.; Huang, S.C.; Lin, Y.H. The development of Hyaluronan/Fucoidan-based nanoparticles as macrophages targeting an Epigallocatechin-3-Gallate delivery system. Int. J. Mol. Sci. 2020, 21, 6327. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tomiyama, D.; Yui, K.; Katsuki, M.; Ikeda, K.; Nonaka, A.; Miyamoto, T. Mechanism for the antibacterial action of epigallocatechin gallate (EGCG) on Bacillus subtilis. Biosci. Biotechnol. Biochem. 2015, 79, 845–854. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Mah, Y.J.; Song, J.S.; Kim, S.O.; Lee, J.H.; Jeon, M.; Jung, U.W.; Moon, S.J.; Kim, J.H.; Choi, H.J. The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch. Oral Biol. 2014, 59, 539–549. [Google Scholar] [CrossRef]
- Shin, Y.S.; Seo, J.Y.; Oh, S.H.; Kim, J.H.; Kim, S.T.; Park, Y.B.; Moon, H.S. The effects of ErhBMP-2-/EGCG-coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Dis. 2014, 20, 281–287. [Google Scholar] [CrossRef]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a photosensitive material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef] [Green Version]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campiglio, C.E.; Contessi Negrini, N.; Fare, S.; Draghi, L. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [Green Version]
- Kujawa, J.; Glodek, M.; Li, G.; Al-Gharabli, S.; Knozowska, K.; Kujawski, W. Highly effective enzymes immobilization on ceramics: Requirements for supports and enzymes. Sci. Total Environ. 2021, 801, 149647. [Google Scholar] [CrossRef]
- Lai, J.Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local controlled release of polyphenol conjugated with gelatin facilitates bone formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrmann, A. Non-toxic crosslinking of electrospun gelatin nanofibers for tissue engineering and biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Wan Ishak, W.; Ahmad, I.; Ramli, S.; Mohd Amin, M. Gamma irradiation-assisted synthesis of cellulose nanocrystal-reinforced gelatin hydrogels. Nanomaterials 2018, 8, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledari, S.A.; Milani, J.M.; Lanbar, F.S. Improving gelatin-based emulsion films with cold plasma using different gases. Food Sci. Nutr. 2020, 8, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Giner, S.; Gimeno-Alcaniz, J.V.; Ocio, M.J.; Lagaron, J.M. Comparative performance of electrospun collagen nanofibers cross-linked by means of different methods. ACS Appl. Mater. Interfaces 2009, 1, 218–223. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Basu, P.; Saha, N.; Alexandrova, R.; Andonova-Lilova, B.; Georgieva, M.; Miloshev, G.; Saha, P. Biocompatibility and biological efficiency of inorganic calcium filled bacterial cellulose based hydrogel scaffolds for bone bioengineering. Int. J. Mol. Sci. 2018, 19, 3980. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Huang, A.; Zhao, J.; Han, X.; Kurushima, Y.; Gong, Y.; Kanzaki, H.; Katsumata, Y.; Yamada, Y.; Hashimoto, Y.; et al. Sustained release of catechin from gelatin and its effect on bone formation in critical sized defects in rat calvaria. J. Hard Tissue Biol. 2020, 29, 77–84. [Google Scholar] [CrossRef]
- Rebers, L.; Granse, T.; Tovar, G.E.M.; Southan, A.; Borchers, K. Physical interactions strengthen chemical gelatin methacryloyl gels. Gels 2019, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Wang, J.; Qian, K.J.; Yu, J.; Zhu, H.Y. Effects of nanofibers on mesenchymal stem cells: Environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J. Zhejiang Univ. Sci. B 2020, 21, 871–884. [Google Scholar] [CrossRef]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of multipotent progenitor cells using the epigallocatechin gallate-modified gelatin sponge scaffold in the rat congenital cleft-jaw model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef] [Green Version]
- Hara, E.; Honda, Y.; Suzuki, O.; Tanaka, T.; Matsumoto, N. Epigallocatechin gallate-modified gelatins with different compositions alter the quality of regenerated bones. Int. J. Mol. Sci. 2018, 19, 3232. [Google Scholar] [CrossRef] [Green Version]
- Kaida, K.; Honda, Y.; Hashimoto, Y.; Tanaka, M.; Baba, S. Application of green tea catechin for inducing the osteogenic differentiation of human dedifferentiated fat cells in vitro. Int. J. Mol. Sci. 2015, 16, 27988–28000. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Dai, H.; Wu, Y.; Li, B.; Yi, J.; Xu, C.; Wu, X. In vitro and in vivo mechanism of hepatocellular carcinoma inhibition by beta-TCP nanoparticles. Int. J. Nanomed. 2019, 14, 3491–3502. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Jeon, H.J.; Park, J.; Chang, M.S. Epigallocatechin-3-gallate prevents oxidative stress-induced cellular senescence in human mesenchymal stem cells via Nrf2. Int. J. Mol. Med. 2016, 38, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.; Rasheed, S.; Yougen, C. Silk fibroin/hydroxyapatite scaffold: A highly compatible material for bone regeneration. Sci. Technol. Adv. Mater. 2020, 21, 242–266. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Brum, I.; Frigo, L.; Goncalo Pinto Dos Santos, P.; Nelson Elias, C.; Da Fonseca, G.; Jose de Carvalho, J. Performance of nano-hydroxyapatite/beta-tricalcium phosphate and xenogenic hydroxyapatite on bone regeneration in rat calvarial defects: Histomorphometric, immunohistochemical and ultrastructural analysis. Int. J. Nanomed. 2021, 16, 3473–3485. [Google Scholar] [CrossRef]
- Da Silva Brum, I.; Frigo, L.; Lana Devita, R.; Da Silva Pires, J.L.; Hugo Vieira de Oliveira, V.; Rosa Nascimento, A.L.; De Carvalho, J.J. Histomorphometric, immunohistochemical, ultrastructural characterization of a Nano-hydroxyapatite/Beta-tricalcium phosphate composite and a bone xenograft in sub-critical size bone defect in rat calvaria. Materials 2020, 13, 4598. [Google Scholar] [CrossRef]
- Masaki, S.; Hirokazu, I.; Yutaka, S.; Takashi, M.; Yukihiko, H.; Mamoru, I. Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci. Biotechnol. Biochem. 1997, 61, 1504–1506. [Google Scholar]
- Huang, A.; Honda, Y.; Li, P.; Tanaka, T.; Baba, S. Integration of epigallocatechin gallate in gelatin sponges attenuates matrix metalloproteinase-dependent degradation and increases bone formation. Int. J. Mol. Sci. 2019, 20, 6042. [Google Scholar] [CrossRef] [Green Version]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhurakkat Perikamana, S.K.; Lee, S.M.; Lee, J.; Ahmad, T.; Lee, M.S.; Yang, H.S.; Shin, H. Oxidative epigallocatechin gallate coating on polymeric substrates for bone tissue regeneration. Macromol. Biosci. 2019, 19, e1800392. [Google Scholar] [CrossRef]
- Chen, S.T.; Kang, L.; Wang, C.Z.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Chou, S.H.; Lu, C.C.; Shen, P.C.; Lin, Y.S.; et al. (-)-Epigallocatechin-3-Gallate decreases osteoclastogenesis via modulation of RANKL and osteoprotegrin. Molecules 2019, 24, 156. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos. Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Liu, J.; Jin, C.; Meng, Y.; Pei, D. Influence of epigallocatechin-3-gallate in promoting proliferation and osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.M.; Asiyanbi, H.T.; Idris, M.; Fadzillah, N.; Mirghani, M.E.S. A Review of gelatin source authentication Methods. Trop. Life Sci. Res. 2018, 29, 213–227. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and biological properties of gelatin extracted from marine snail rapana venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef] [Green Version]
- Hannink, G.; Arts, J.J. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Saito, K.; Anada, T.; Shiwaku, Y.; Chiba, S.; Miyatake, N.; Suzuki, K.; Tsuchiya, K.; Suzuki, O. Dose-dependent enhancement of octacalcium phosphate biodegradation with a gelatin matrix during bone regeneration in a rabbit tibial defect model. RSC Adv. 2016, 6, 64165–64174. [Google Scholar] [CrossRef]
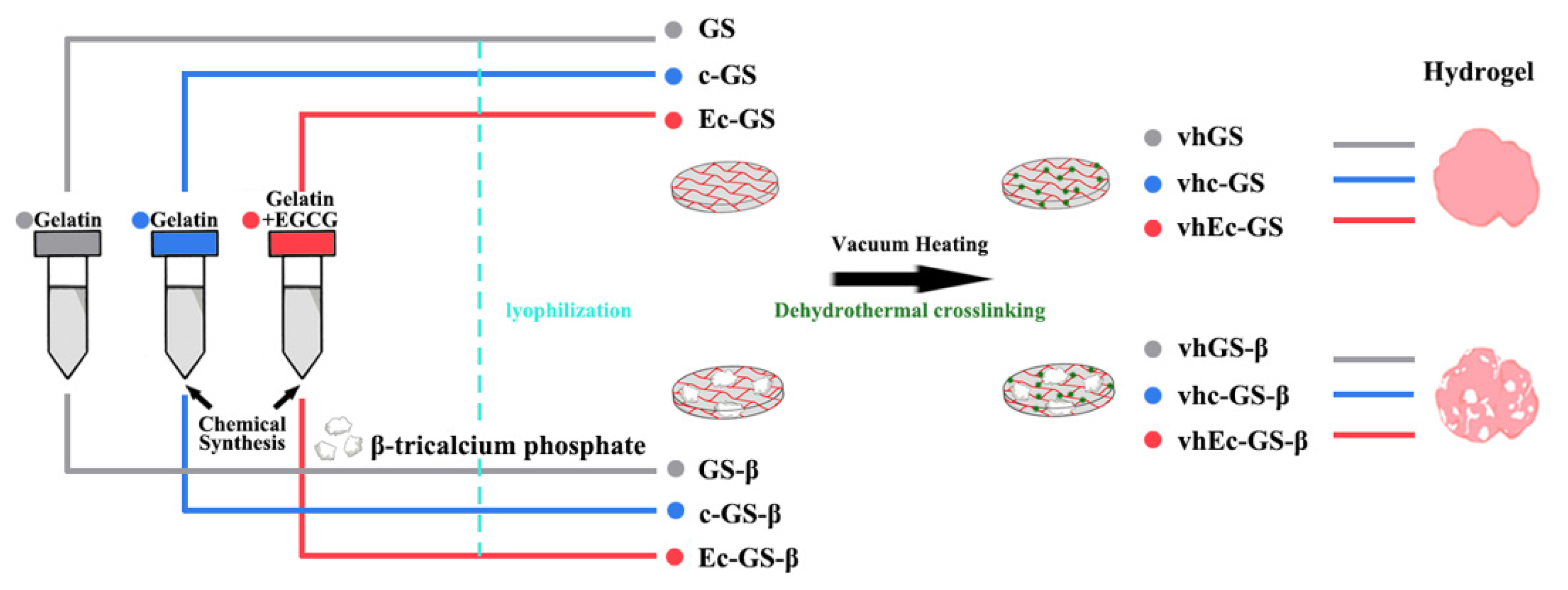



| Sample Name | Abbreviation | Gelatin (mg) | EGCG (mg) | β-TCP (mg) | Chemical Synthesis | Vacuum Heating |
|---|---|---|---|---|---|---|
| Gelatin sponge | GS | 1 | 0 | 0 | No | No |
| Chemically synthesized gelatin sponge | c-GS | 1 | 0 | 0 | Yes | No |
| Chemically synthesized gelatin sponge modified with EGCG | Ec-GS | 1 | 0.0028 | 0 | Yes | No |
| GS incorporating β-TCP | GS-β | 1 | 0 | 4 | No | No |
| c-GS incorporating β-TCP | c-GS-β | 1 | 0 | 4 | Yes | No |
| Ec-GS incorporating β-TCP | Ec-GS-β | 1 | 0.0028 | 4 | Yes | No |
| Vacuum-heated GS | vhGS | 1 | 0 | 0 | No | Yes |
| Vacuum-heated c-GS | vhc-GS | 1 | 0 | 0 | Yes | Yes |
| Vacuum-heated Ec-GS | vhEc-GS | 1 | 0.0028 | 0 | Yes | Yes |
| Vacuum-heated GS-β | vhGS-β | 1 | 0 | 4 | No | Yes |
| Vacuum-heated c-GS-β | vhc-GS-β | 1 | 0 | 4 | Yes | Yes |
| Vacuum-heated Ec-GS-β | vhEc-GS-β | 1 | 0.0028 | 4 | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, B.; Honda, Y.; Yamada, Y.; Tanaka, T.; Takeda, Y.; Nambu, T.; Baba, S. Utility of Thermal Cross-Linking in Stabilizing Hydrogels with Beta-Tricalcium Phosphate and/or Epigallocatechin Gallate for Use in Bone Regeneration Therapy. Polymers 2022, 14, 40. https://doi.org/10.3390/polym14010040
Gao B, Honda Y, Yamada Y, Tanaka T, Takeda Y, Nambu T, Baba S. Utility of Thermal Cross-Linking in Stabilizing Hydrogels with Beta-Tricalcium Phosphate and/or Epigallocatechin Gallate for Use in Bone Regeneration Therapy. Polymers. 2022; 14(1):40. https://doi.org/10.3390/polym14010040
Chicago/Turabian StyleGao, Beiyuan, Yoshitomo Honda, Yoichi Yamada, Tomonari Tanaka, Yoshihiro Takeda, Takayuki Nambu, and Shunsuke Baba. 2022. "Utility of Thermal Cross-Linking in Stabilizing Hydrogels with Beta-Tricalcium Phosphate and/or Epigallocatechin Gallate for Use in Bone Regeneration Therapy" Polymers 14, no. 1: 40. https://doi.org/10.3390/polym14010040
APA StyleGao, B., Honda, Y., Yamada, Y., Tanaka, T., Takeda, Y., Nambu, T., & Baba, S. (2022). Utility of Thermal Cross-Linking in Stabilizing Hydrogels with Beta-Tricalcium Phosphate and/or Epigallocatechin Gallate for Use in Bone Regeneration Therapy. Polymers, 14(1), 40. https://doi.org/10.3390/polym14010040






