Sustainable Design Approach for Modeling Bioprocesses from Laboratory toward Commercialization: Optimizing Chitosan Production
Abstract
:1. Introduction
2. Materials and Methods
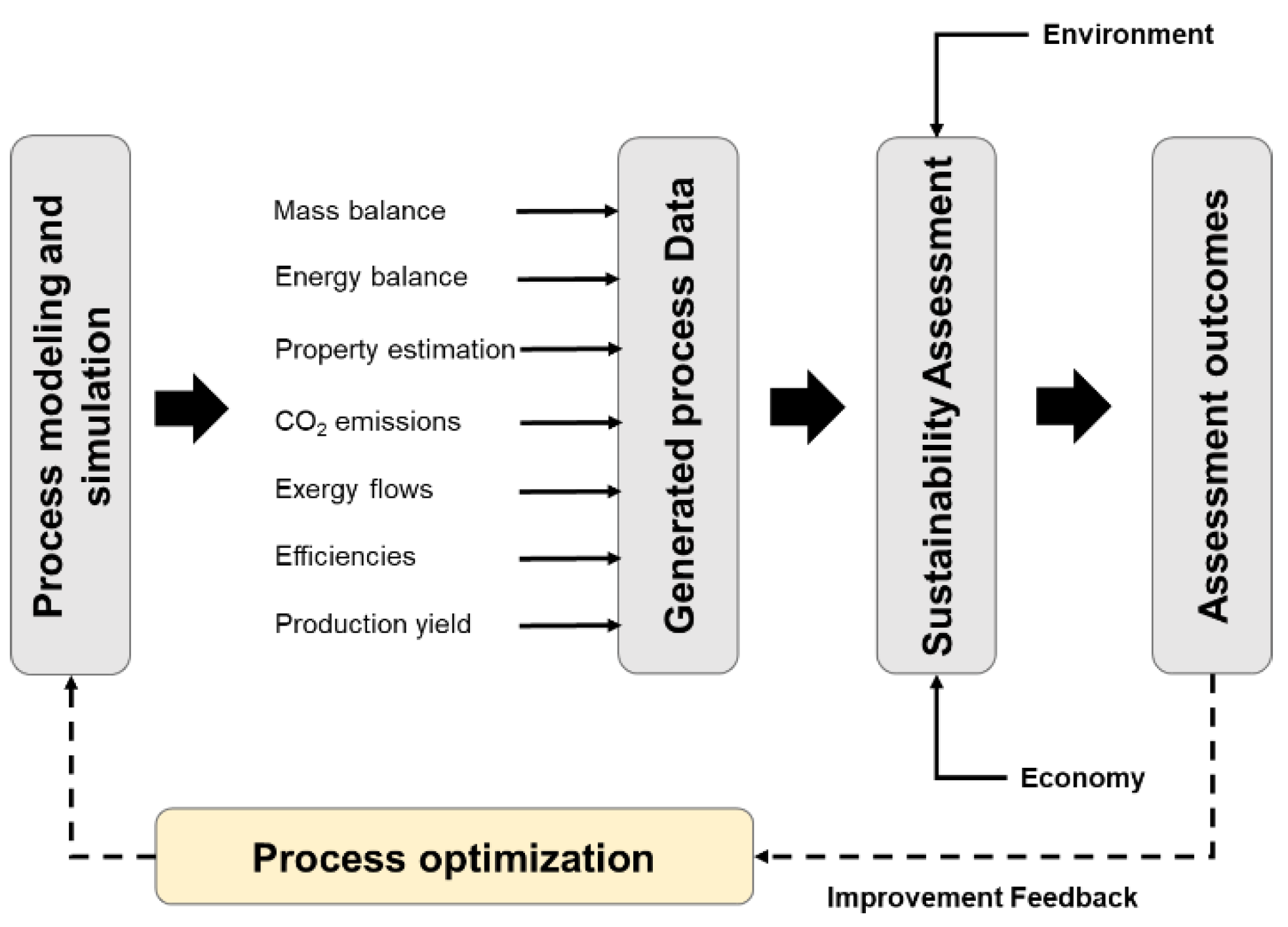
2.1. General Procedure for Modeling Bioprocesses
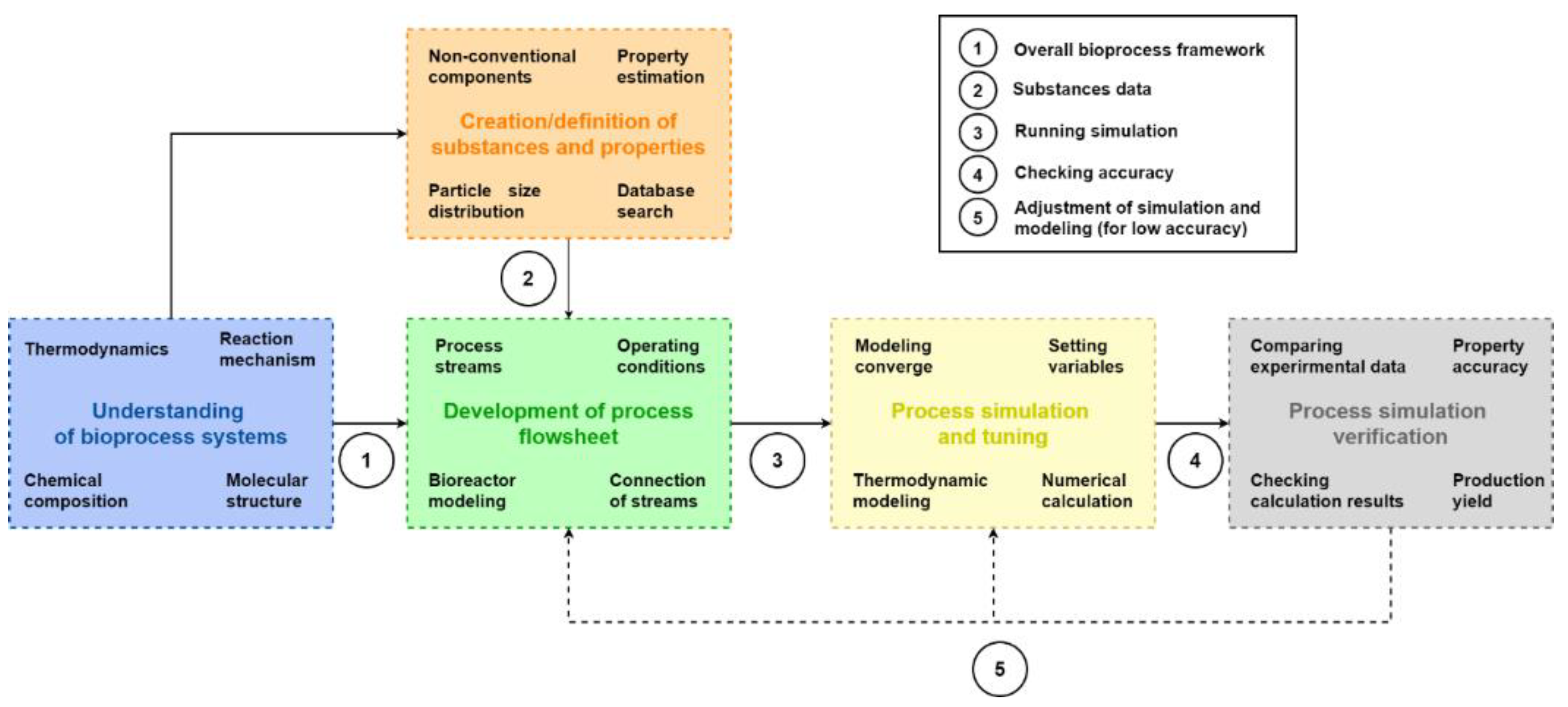
2.2. A Step-Wise Framework for Simulating Bioprocesses
2.3. Sustainability Assessment
2.3.1. Technical Indicators
2.3.2. Life Cycle Assessment
2.4. Process Optimization
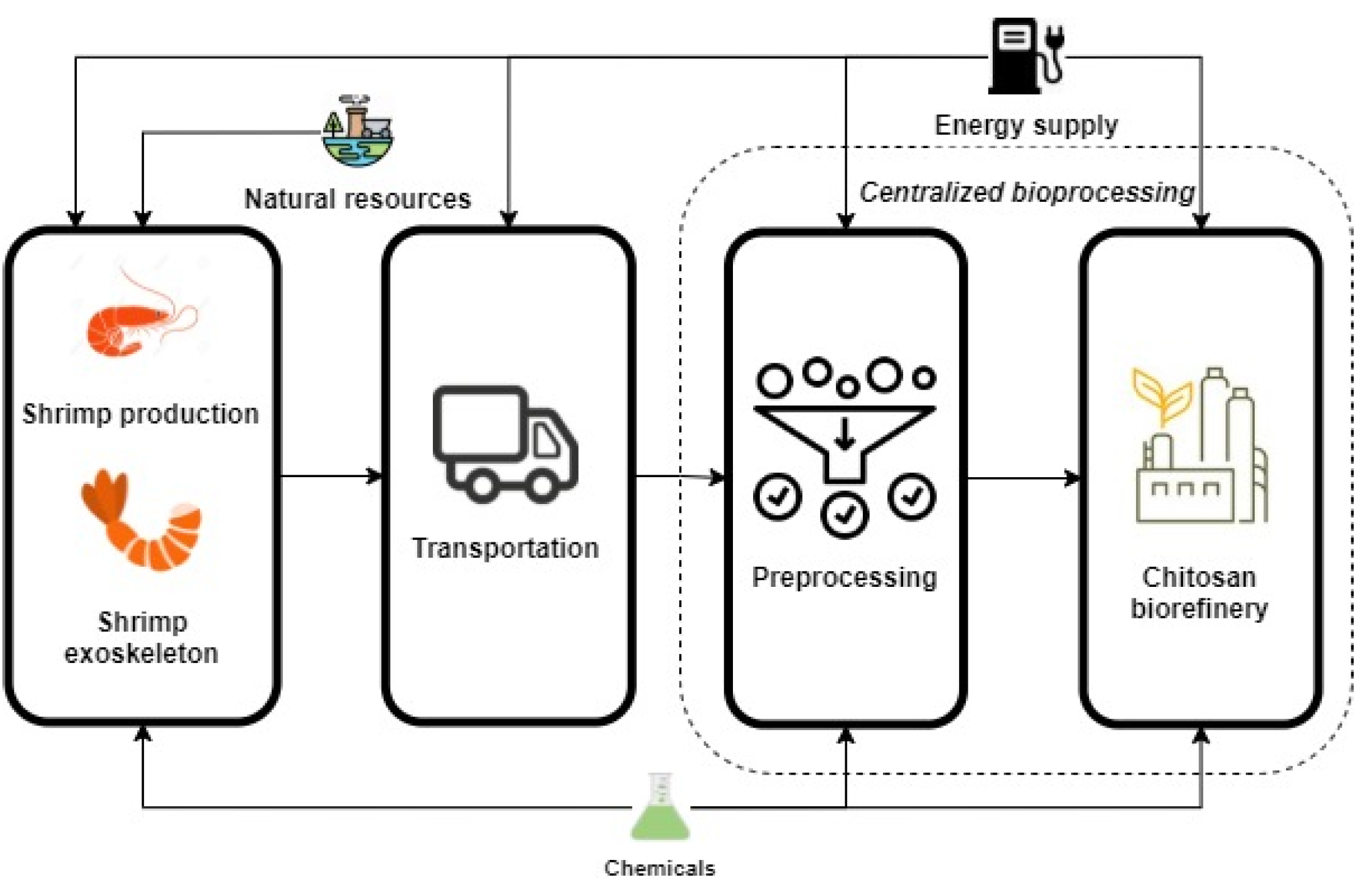
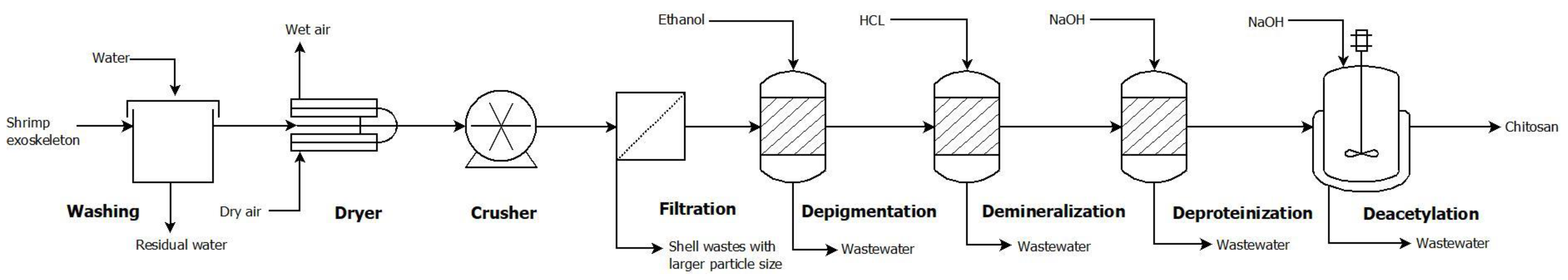
3. Case Study: Chitosan Production from Shrimp Exoskeleton
4. Sustainability Assessment
4.1. Base Design
4.2. Optimized Design: Including a WRN
5. Comparison of Alternatives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Jong, E.; Stichnothe, H.; Bell, G.; Jorgensen, H. Bio-Based Chemicals: A 2020 Update. 2020. Available online: https://task42.ieabioenergy.com/wp-content/uploads/sites/10/2020/02/Bio-based-chemicals-a-2020-update-final-200213.pdf (accessed on 15 October 2021).
- Lokko, Y.; Heijde, M.; Schebesta, K.; Scholtès, P.; Van Montagu, M.; Giacca, M. Biotechnology and the bioeconomy—Towards inclusive and sustainable industrial development. New Biotechnol. 2018, 40, 5–10. [Google Scholar] [CrossRef]
- Vieira, H.; Leal, M.C.; Calado, R. Fifty Shades of Blue: How Blue Biotechnology is Shaping the Bioeconomy. Trends Biotechnol. 2020, 38, 940–943. [Google Scholar] [CrossRef]
- Brandão, A.S.; Gonçalves, A.; Santos, J.M.R.C.A. Circular bioeconomy strategies: From scientific research to commercially viable products. J. Clean. Prod. 2021, 295, 126407. [Google Scholar] [CrossRef]
- Ruiz-Mercado, G.J.; Carvalho, A.; Cabezas, H. Using green chemistry and engineering principles to design, assess, and retrofit chemical processes for sustainability. ACS Sustain. Chem. Eng. 2016, 4, 6208–6221. [Google Scholar] [CrossRef]
- de Lorenzo, V.; Couto, J. The important versus the exciting: Reining contradictions in contemporary biotechnology. Microb. Biotechnol. 2019, 12, 32–34. [Google Scholar] [CrossRef] [Green Version]
- Turton, R.; Bailie, R.; Whiting, W.; Shaeiwitz, J.; Bhattacharyya, D. Analysis, Synthesis, and Design of Chemical Processes, 4th ed.; Prentice Hall: Ann Arbor, MI, USA, 2013; Volume 36, ISBN 9780135129661. [Google Scholar]
- Huccetogullari, D.; Luo, Z.W.; Lee, S.Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Fact. 2019, 18, 41. [Google Scholar] [CrossRef]
- Cramer, S.M.; Holstein, M.A. Downstream bioprocessing: Recent advances and future promise. Curr. Opin. Chem. Eng. 2011, 1, 27–37. [Google Scholar] [CrossRef]
- Kopsahelis, A.; Kourmentza, C.; Zafiri, C.; Kornaros, M. Gate-to-gate life cycle assessment of biosurfactants and bioplasticizers production via biotechnological exploitation of fats and waste oils. J. Chem. Technol. Biotechnol. 2018, 93, 2833–2841. [Google Scholar] [CrossRef]
- Santibañez-Aguilar, J.E.; González-Campos, J.B.; Ponce-Ortega, J.M.; Serna-González, M.; El-Halwagi, M.M. Optimal planning of a biomass conversion system considering economic and environmental aspects. Ind. Eng. Chem. Res. 2011, 50, 8558–8570. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Figueroa-Torres, G.; Azapagic, A. The extent of food waste generation in the UK and its environmental impacts. Sustain. Prod. Consum. 2021, 26, 532–547. [Google Scholar] [CrossRef]
- Caduff, M.; Huijbregts, M.A.J.; Althaus, H.J.; Hendriks, A.J. Power-law relationships for estimating mass, fuel consumption and costs of energy conversion equipments. Environ. Sci. Technol. 2011, 45, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, F.; Benavides, J.; Rito-Palomares, M. Scaling-up of a B-phycoerythrin production and purification bioprocess involving aqueous two-phase systems: Practical experiences. Process Biochem. 2013, 48, 738–745. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the environmental impact of a future nanocellulose production at industrial scale: Application of the life cycle assessment scale-up framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; González-Valdez, J.; González-González, M.; Benavides, J.; Rito-Palomares, M. Practical experiences from the bench-scale implementation of a bioprocess for fucoxanthin production. J. Chem. Technol. Biotechnol. 2018, 93, 2033–2039. [Google Scholar] [CrossRef]
- Koutinas, M.; Kiparissides, A.; Pistikopoulos, E.N.; Mantalaris, A. Bioprocess systems engineering: Transferring traditional process engineering principles to industrial biotechnology. Comput. Struct. Biotechnol. J. 2012, 3, e201210022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int. J. Hydrogen Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, D.; Barrera-Zapata, R.; Ríos-Estepa, R. Comparison of process technologies for chitosan production from shrimp shell waste: A techno-economic approach using Aspen Plus®. Food Bioprod. Process. 2017, 103, 49–57. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, C.; Gillet, D.; Moerschbacher, B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Meramo-Hurtado, S.; Alarcón-Suesca, C.; González-Delgado, Á.D. Exergetic sensibility analysis and environmental evaluation of chitosan production from shrimp exoskeleton in Colombia. J. Clean. Prod. 2020, 248, 119285. [Google Scholar] [CrossRef]
- Cogollo-Herrera, K.; Bonfante-Álvarez, H.; De Ávila-Montiel, G.; Barros, A.H.; González-Delgado, Á.D. Techno-economic sensitivity analysis of large scale chitosan production process from shrimp shell wastes. Chem. Eng. Trans. 2018, 70, 2179–2184. [Google Scholar]
- Zuorro, A.; Moreno-Sader, K.A.; González-Delgado, Á.D. Evaluating the feasibility of a pilot-scale shrimp biorefinery via techno-economic analysis. J. Clean. Prod. 2021, 320, 128740. [Google Scholar] [CrossRef]
- Arteaga-Díaz, S.J.; Meramo-Hurtado, S.I.; León-Pulido, J.; Zuorro, A.; González-Delgado, A.D. Environmental assessment of large scale production of magnetite (Fe3O4) nanoparticles via coprecipitation. Appl. Sci. 2019, 9, 1682. [Google Scholar] [CrossRef] [Green Version]
- Wooley, R.J.; Putsche, V. Development of an ASPEN PLUS Physical Property Database for Biofuels Components; National Renewable Energy Lab.: Golden, CO, USA, 1996. [Google Scholar]
- Dang, Q.; Yu, C.; Luo, Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 2014, 131, 36–42. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Dussan, K.; Dooley, S.; Monaghan, R. Integrating compositional features in model compounds for a kinetic mechanism of hemicellulose pyrolysis. Chem. Eng. J. 2017, 328, 943–961. [Google Scholar] [CrossRef]
- Darkwah, K.; Nokes, S.E.; Seay, J.R.; Knutson, B.L. Mechanistic simulation of batch acetone–butanol–ethanol (ABE) fermentation with in situ gas stripping using Aspen PlusTM. Bioprocess Biosyst. Eng. 2018, 41, 1283–1294. [Google Scholar] [CrossRef]
- Smith, R.L.; Tan, E.C.D.; Ruiz-Mercado, G.J. Applying Environmental Release Inventories and Indicators to the Evaluation of Chemical Manufacturing Processes in Early Stage Development. ACS Sustain. Chem. Eng. 2019, 7, 10937–10950. [Google Scholar] [CrossRef] [PubMed]
- AlNouss, A.; McKay, G.; Al-Ansari, T. A techno-economic-environmental study evaluating the potential of oxygen-steam biomass gasification for the generation of value-added products. Energy Convers. Manag. 2019, 196, 664–676. [Google Scholar] [CrossRef]
- Gao, X.; Abdul Raman, A.A.; Hizaddin, H.F.; Bello, M.M. Systematic review on the implementation methodologies of inherent safety in chemical process. J. Loss Prev. Process Ind. 2020, 65, 104092. [Google Scholar] [CrossRef]
- Medina-Herrera, N.; Tututi-Avila, S.; Jiménez-Gutierrez, A. A new index for chemical process design considering risk analysis and controllability. Comput. Aided Chem. Eng. 2019, 46, 373–378. [Google Scholar]
- Sikdar, S.K. Fractured state of decisions on sustainability: An assessment. Sustain. Prod. Consum. 2019, 19, 231–237. [Google Scholar] [CrossRef]
- Sikdar, S.K.; Sengupta, D.; Harten, P. More on aggregating multiple indicators into a single index for sustainability analyses. Clean Technol. Environ. Policy 2012, 14, 765–773. [Google Scholar] [CrossRef]
- Sikdar, S.K.; Sengupta, D.; Mukherjee, R. Measuring Progress towards Sustainability: A Treatise for Engineers; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Mattes, M.D.; Sloane, M.A. Reflections on Hope and Its Implications for End-of-Life Care. J. Am. Geriatr. Soc. 2015, 63, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mercado, G.J.; Smith, R.L.; Gonzalez, M.A. Sustainability indicators for chemical processes: I. Taxonomy. Ind. Eng. Chem. Res. 2012, 51, 2309–2328. [Google Scholar] [CrossRef]
- Chalermthai, B.; Giwa, A.; Schmidt, J.E.; Taher, H. Life cycle assessment of bioplastic production from whey protein obtained from dairy residues. Bioresour. Technol. Rep. 2021, 15, 100695. [Google Scholar] [CrossRef]
- Ekvall, T.; Azapagic, A.; Finnveden, G.; Rydberg, T.; Weidema, B.P.; Zamagni, A. Attributional and consequential LCA in the ILCD handbook. Int. J. Life Cycle Assess. 2016, 21, 293–296. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Patiño-Ruiz, D.A.; Meramo-Hurtado, S.I.; González-Delgado, Á.D.; Herrera, A. Environmental Sustainability Evaluation of Iron Oxide Nanoparticles Synthesized via Green Synthesis and the Coprecipitation Method: A Comparative Life Cycle Assessment Study. ACS Omega 2021, 6, 12410–12423. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Zhang, X.; Rodriguez, L.; Khanna, M.; de Jong, S.; Ting, K.C.; Ying, Y.; Lin, T. Multi-objective optimization for sustainable renewable jet fuel production: A case study of corn stover based supply chain system in Midwestern U.S. Renew. Sustain. Energy Rev. 2019, 115, 109403. [Google Scholar] [CrossRef]
- Moreno, J.; Iglesias, J.; Blanco, J.; Montero, M.; Morales, G.; Melero, J.A. Life-cycle sustainability of biomass-derived sorbitol: Proposing technological alternatives for improving the environmental profile of a bio-refinery platform molecule. J. Clean. Prod. 2020, 250. [Google Scholar] [CrossRef]
- van Zelm, R.; de Paiva Seroa da Motta, R.; Lam, W.Y.; Menkveld, W.; Broeders, E. Life cycle assessment of side stream removal and recovery of nitrogen from wastewater treatment plants. J. Ind. Ecol. 2020, 24, 913–922. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Herrgård, M.J.; Forster, J.; Hauschild, M.Z.; Fantke, P. Addressing environmental sustainability of biochemicals. Nat. Sustain. 2020, 3, 167–174. [Google Scholar] [CrossRef]
- Diban, P.; El-Halwagi, M.M.; Foo, D.C.Y. A Decomposition-Based Approach for the Optimum Integration of Heating Utility and Phase Separation Systems in Oil and Gas Platform. Ind. Eng. Chem. Res. 2019, 58, 21584–21601. [Google Scholar] [CrossRef]
- Kamat, S.; Bandyopadhyay, S.; Sahu, G.C.; Foo, D.C.Y. Optimal Synthesis of Heat-Integrated Water Regeneration Network. Ind. Eng. Chem. Res. 2019, 58, 1310–1321. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef]
- Zahedi, S.; Safaei Ghomi, J.; Shahbazi-Alavi, H. Preparation of chitosan nanoparticles from shrimp shells and investigation of its catalytic effect in diastereoselective synthesis of dihydropyrroles. Ultrason. Sonochem. 2018, 40, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, D.; Navarro, G.; Monsalve, P.; Barrera-Zapata, R.; Ríos-Estepa, R. Aspen plus simulation strategies applied to the study of chitin ioextraction from shrimp waste. Food Technol Biotechnol. 2019, 57, 238–248. [Google Scholar] [CrossRef]
- Paz, N.; Pérez, D.; Fernández, M.; López, O.; Nogueira, A.; Rapado Paneque, M.; Altanés Valentín, S.; García, C. Evaluación viscosimétrica del quitosano derivado de la quitina de langosta. Rev. Iberoam. Polímeros 2013, 14, 84–91. [Google Scholar]
- Ibrahim, M.; Osman, O.; Mahmoud, A.A. Spectroscopic analyses of cellulose and chitosan: FTIR and modeling approach. J. Comput. Theor. Nanosci. 2011, 8, 117–123. [Google Scholar] [CrossRef]
- Bonfante-Alvarez, H.; De Avila-Montiel, G.; Cogollo-Herrera, K.; Torrenegra-Alarcón, M.; Gonzalez-Delgado, A. Evaluation of five chitosan production routes with astaxanthin recovery from shrimp exoskeletons. Chem. Eng. Trans. 2018, 70, 1969–1974. [Google Scholar]
- Cheng, W.H.; Adi, V.S.K. Simultaneous Optimization of Non-Isothermal Design of Water Networks with Regeneration and Recycling. Process Integr. Optim. Sustain. 2018, 2, 183–203. [Google Scholar] [CrossRef]
- Chin, H.H.; Foo, D.C.Y.; Lam, H.L. Simultaneous water and energy integration with isothermal and non-isothermal mixing—A P-graph approach. Resour. Conserv. Recycl. 2019, 149, 687–713. [Google Scholar] [CrossRef]


| Indicator | Formula | Area | Description |
|---|---|---|---|
| Energy intensity (kW/kg p.) | Energy | The amount of energy needed for production | |
| Material intensity (kg/kg p.) | Process efficiency | The total input material for producing the product | |
| Water consumption (m3/kg p.) | Water management | The total water consumed in production | |
| Total liquid waste (m3/kg p.) | Water management | Total liquid waste flows in production | |
| Treatment cost ($USD/kg p.) | Economy | Total cost of water management in production |
| Property | Unit | Simulation | Experimental | Accuracy (%) |
|---|---|---|---|---|
| Molecular weight | g/mol | 322.32 | 310.00 | 96.17 |
| Heat capacity | Cal/mol K | 132.80 | 135.00 | 98.37 |
| Production yield | kg/kg | 0.21 | 0.212 | 98.59 |
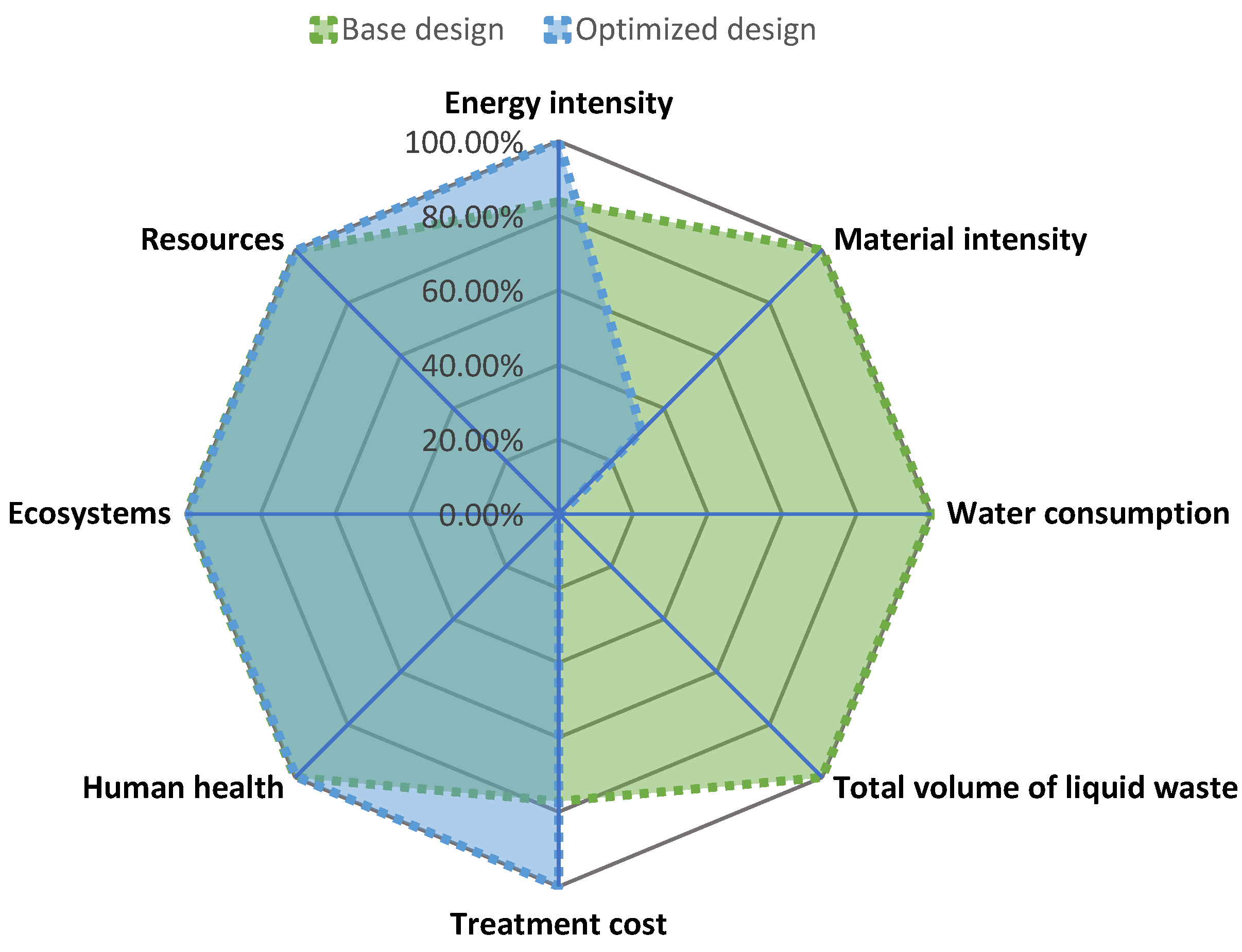
| Process Alternative | Energy Intensity (kW/kg p.) | Material Intensity (kg/h/kg p.) | Water Consumption (kg/h/kg p.) | Total Volume of Liquid Waste (m3/kg p.) | Treatment Cost (USD/kg p.) |
|---|---|---|---|---|---|
| Base design | 107.78 | 1175.05 | 809.60 | 805.88 | 704.60 |
| Optimized design | 128.71 | 369.17 | 3.72 | 0.00 | 913.82 |
| Impact Category | Unit | Base Case | Optimized Case |
|---|---|---|---|
| Global warming, Human health | DALY | 7.13 × 10−4 | 7.12 × 10−4 |
| Global warming, Terrestrial ecosystems | species.yr | 1.43 × 10−6 | 1.42 × 10−6 |
| Global warming, Freshwater ecosystems | species.yr | 3.89 × 10−11 | 3.88 × 10−11 |
| Stratospheric ozone depletion | DALY | 6.41 × 10−7 | 6.41 × 10−7 |
| Ionizing radiation | DALY | 6.63 × 10−8 | 6.62 × 10−8 |
| Ozone formation, Human health | DALY | 1.46 × 10−7 | 1.45 × 10−7 |
| Fine particulate matter formation | DALY | 8.71 × 10−5 | 8.70 × 10−5 |
| Ozone formation, Terrestrial ecosystems | species.yr | 2.10 × 10−8 | 2.10 × 10−8 |
| Terrestrial acidification | species.yr | 1.17 × 10−7 | 1.1710−7 |
| Freshwater eutrophication | species.yr | 1.13 × 10−8 | 1.13 × 10−8 |
| Marine eutrophication | species.yr | 9.54 × 10−11 | 9.54 × 10−11 |
| Terrestrial ecotoxicity | species.yr | 2.29 × 10−9 | 2.29 × 10−9 |
| Freshwater ecotoxicity | species.yr | 1.92 × 10−9 | 1.91 × 10−9 |
| Marine ecotoxicity | species.yr | 1.31 × 10−6 | 1.31 × 10−6 |
| Human carcinogenic toxicity | DALY | 4.01 × 10−4 | 3.98 × 10−4 |
| Human non-carcinogenic toxicity | DALY | 2.32 × 10−3 | 2.31 × 10−3 |
| Land use | species.yr | 2.88 × 10−7 | 2.88 × 10−7 |
| Mineral resource scarcity | USD2013 | 0.07 | 0.07 |
| Fossil resource scarcity | USD2013 | 5.92 | 5.91 |
| Water consumption, Human health | DALY | 1.37 × 10−6 | 1.40 × 10−6 |
| Water consumption, Terrestrial ecosystem | species.yr | 1.28 × 10−8 | 1.24 × 10−8 |
| Water consumption, Aquatic ecosystems | species.yr | 8.17 × 10−12 | 6.97 × 10−12 |
| Damage Category | Unit | Base Case | Optimized Design |
|---|---|---|---|
| Human health | DALY | 3.52 × 10−3 | 3.51 × 10−3 |
| Ecosystems | species.yr | 3.19 × 10−6 | 3.18 × 10−6 |
| Resources | USD2013 | 5.99 | 5.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meramo, S.; González-Delgado, Á.D.; Sukumara, S.; Fajardo, W.S.; León-Pulido, J. Sustainable Design Approach for Modeling Bioprocesses from Laboratory toward Commercialization: Optimizing Chitosan Production. Polymers 2022, 14, 25. https://doi.org/10.3390/polym14010025
Meramo S, González-Delgado ÁD, Sukumara S, Fajardo WS, León-Pulido J. Sustainable Design Approach for Modeling Bioprocesses from Laboratory toward Commercialization: Optimizing Chitosan Production. Polymers. 2022; 14(1):25. https://doi.org/10.3390/polym14010025
Chicago/Turabian StyleMeramo, Samir, Ángel Darío González-Delgado, Sumesh Sukumara, William Stive Fajardo, and Jeffrey León-Pulido. 2022. "Sustainable Design Approach for Modeling Bioprocesses from Laboratory toward Commercialization: Optimizing Chitosan Production" Polymers 14, no. 1: 25. https://doi.org/10.3390/polym14010025
APA StyleMeramo, S., González-Delgado, Á. D., Sukumara, S., Fajardo, W. S., & León-Pulido, J. (2022). Sustainable Design Approach for Modeling Bioprocesses from Laboratory toward Commercialization: Optimizing Chitosan Production. Polymers, 14(1), 25. https://doi.org/10.3390/polym14010025








