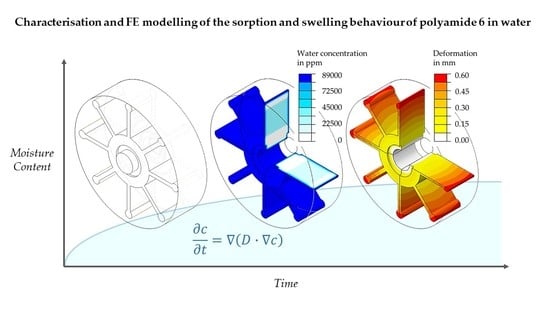
Characterisation and FE Modelling of the Sorption and Swelling Behaviour of Polyamide 6 in Water
Abstract
1. Introduction
2. Calculation Basics on the Sorption and Swelling Processes
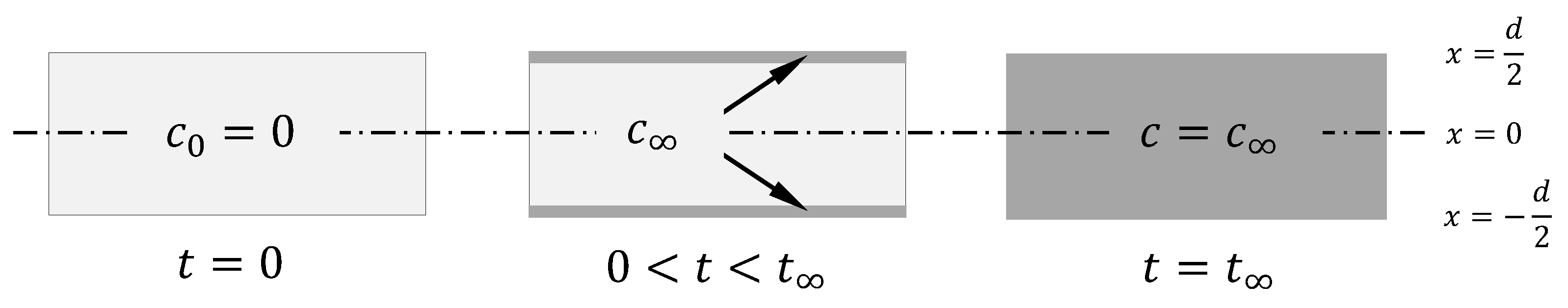
2.1. Calculation Basics on the Sorption Process
| (1) | for | at | |
| (2) | for | at | |
| (3) | for | at |
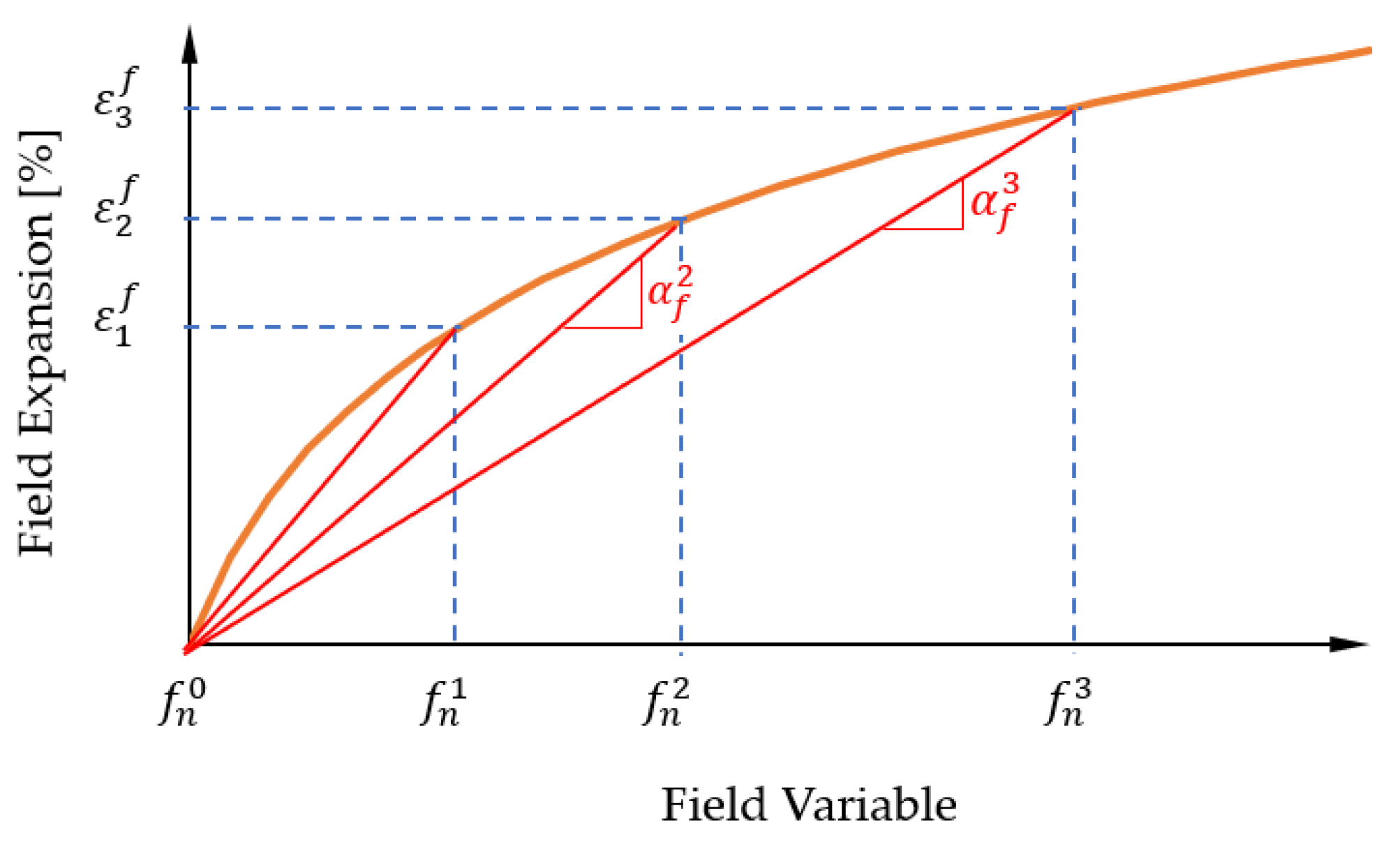
2.2. Calculation Basics on the Swelling Process
2.3. Overview of Modelling the Sorption and Swelling Behaviour
3. FE Modelling of the Sorption and Swelling Behaviour
3.1. Introduction of the FE Model Used
3.2. Modelling of the Sorption Behaviour Using Mass Diffusion Analysis
3.3. Modelling of the Swelling Behaviour Using Static Stress Analysis
3.4. Determination of the Relevant Values for the FE Model
4. Materials and Experimental Approaches
4.1. Introduction of the Developed Specimen Geometry
4.2. Determination of Implemented FE Model Parameters
5. Results and Discussion
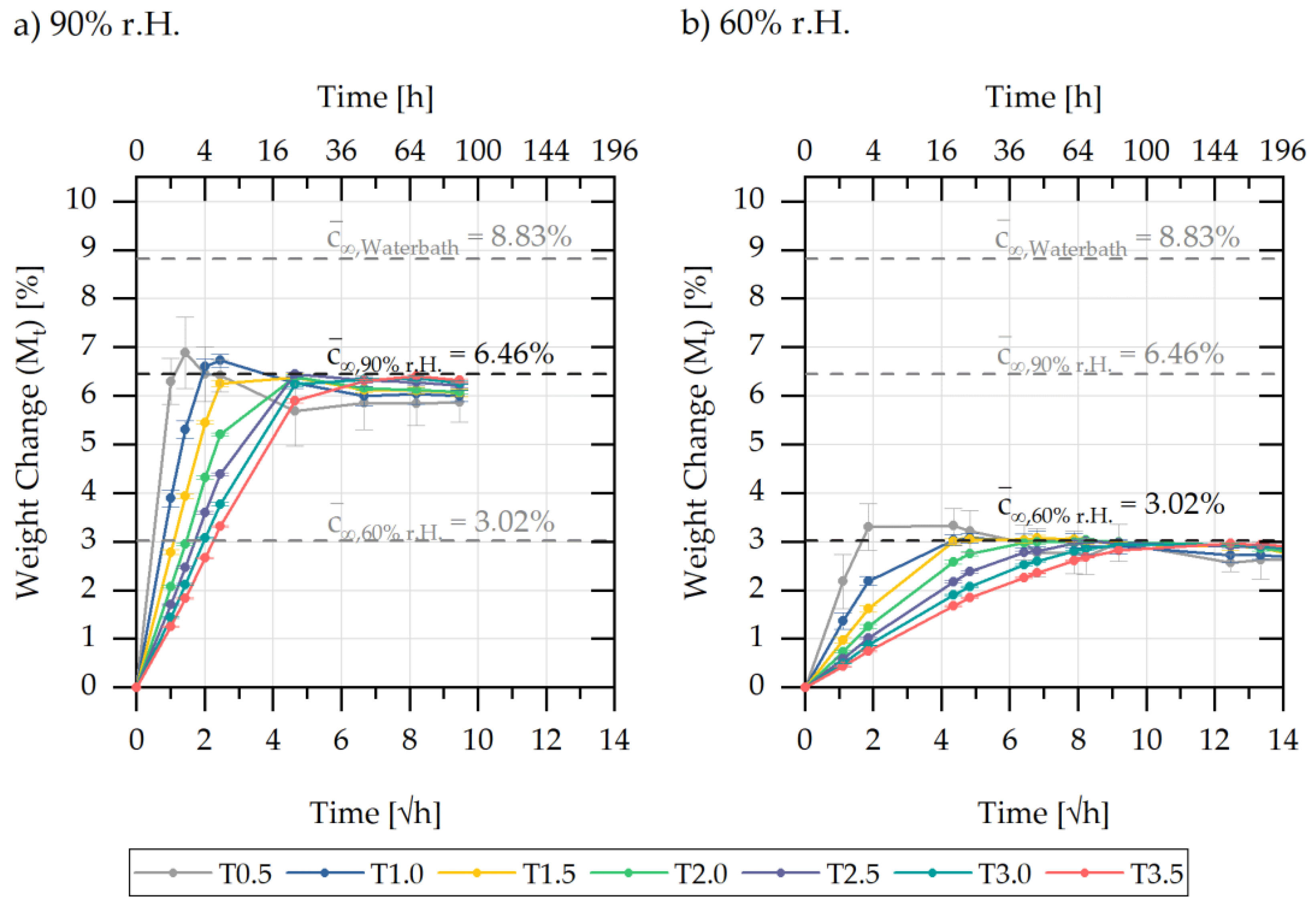
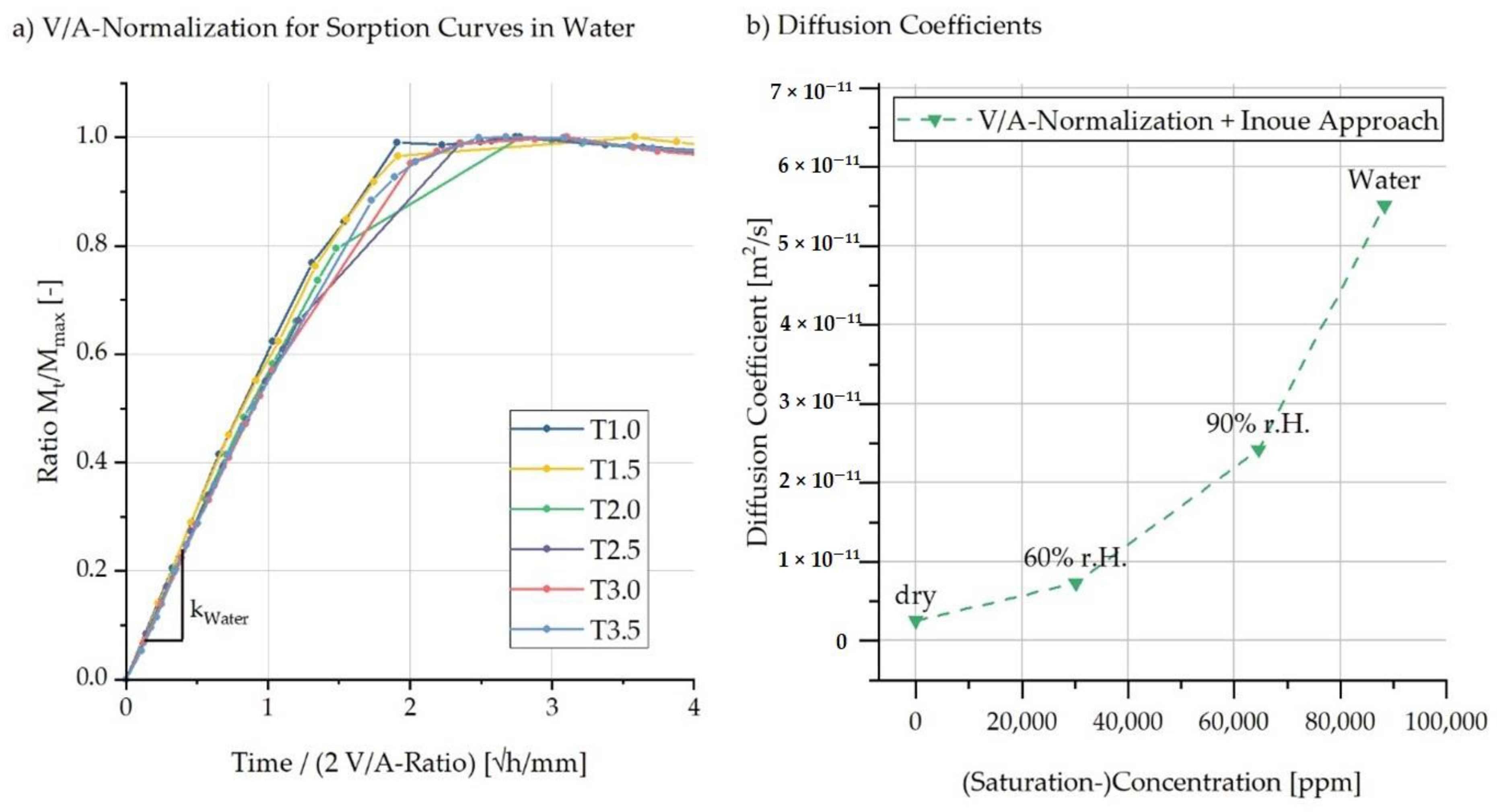
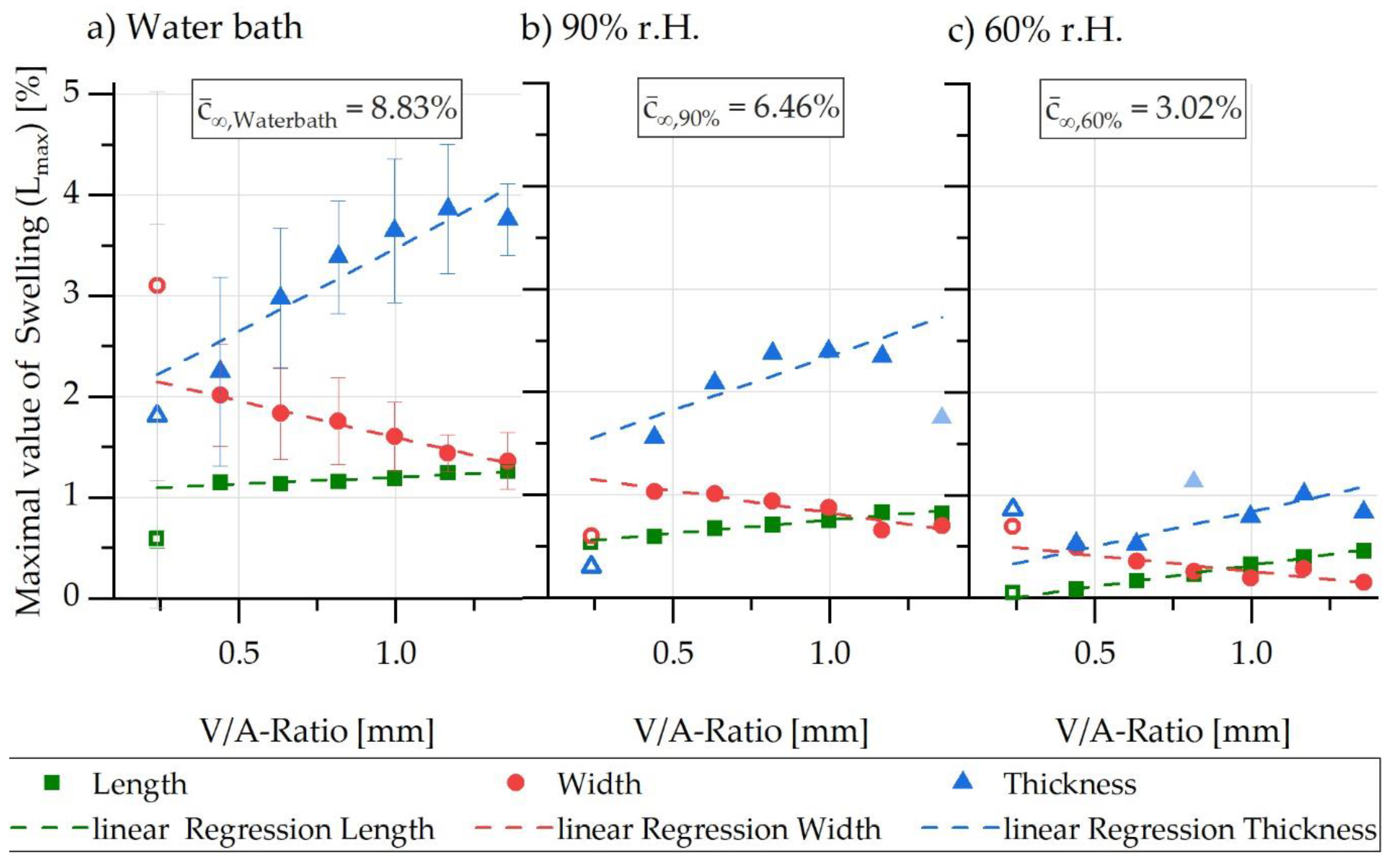
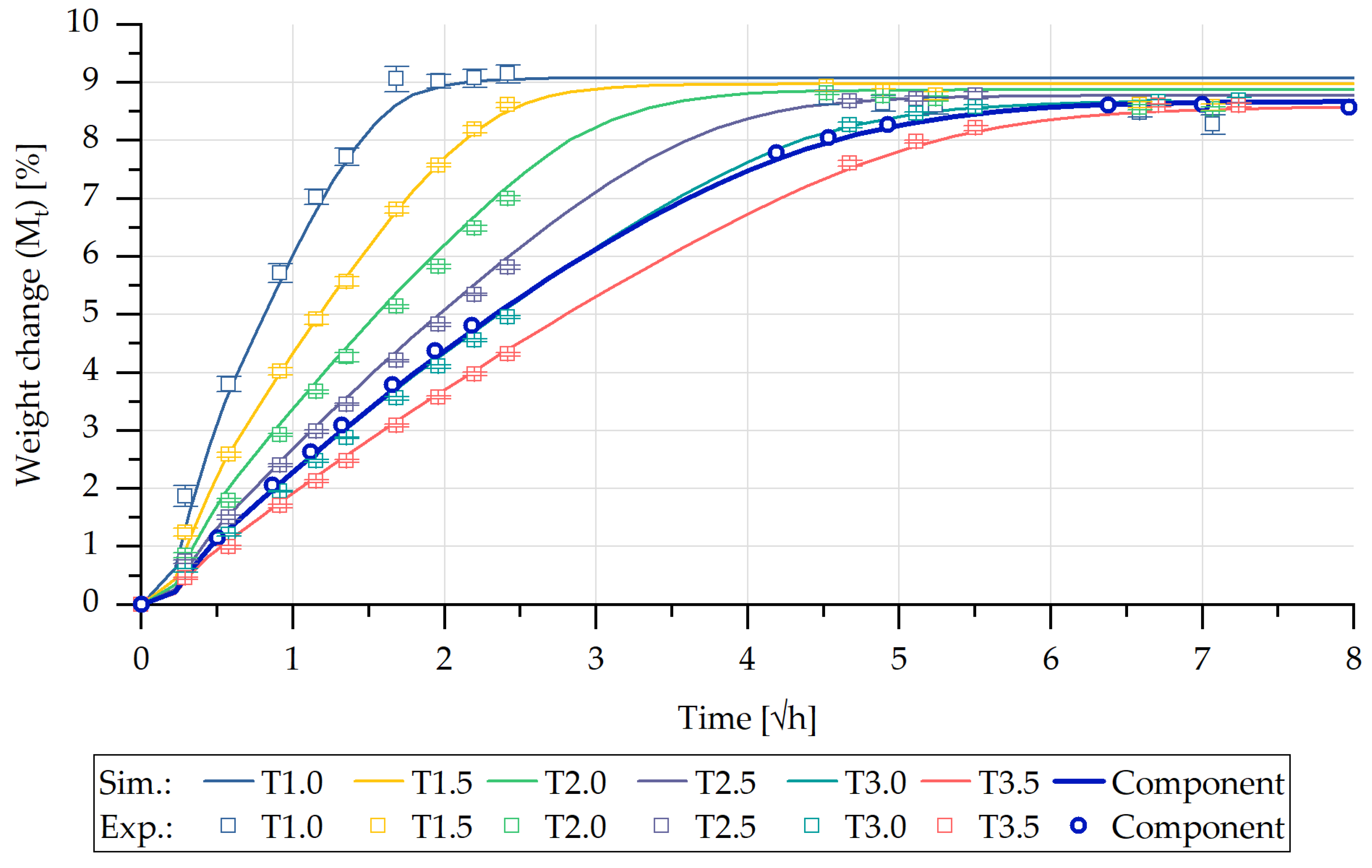
5.1. Experimental Characterisation of Water Absorption and Swelling
5.2. FE-Based Simulation of Water Absorption and Swelling
6. Conclusions
- Development of a method for the characterisation and FE modelling of the sorption and swelling behaviour of PA6 parts in water using Abaqus.
- Development of a step-shaped test specimen that can be subdivided into simple bar specimens by means of separation and then characterised individually.
- Using simple characterisation methods, such as the determination of the sorption behaviour based on the mass increase, as well as the swelling behaviour based on the measurement of the dimensional change with microscopy or thickness measurement using a mechanical dial gauge.
- Use of concentration-dependent diffusion coefficients as well as expansion coefficients for FE model development.
- Development of an FE model with good accuracy, which initially separated the coupled processes of sorption and swelling for simplification and calculated them successively.
- Conclusions from the sorption and swelling behaviour of individual sample geometries with different V/A ratios on the part geometry behaviour with varying wall thicknesses.
- Possibility to extend the simulation method to real parts with a complex geometry and wall thickness distribution and also the possibility to extend the method to different surrounding humidities instead of direct contact with water by changing the simulations’ boundary conditions.
Author Contributions
Funding
Conflicts of Interest
References
- Puffr, R.; Šebenda, J. On the Structure and Properties of Polyamides. XXVII. The Mechanism of Water Sorption in Polyamides. J. Polym. Sci. C Polym. Symp. 1967, 16, 79–93. [Google Scholar] [CrossRef]
- Bessler, V.E.; Bier, G. Über Wasserstofffbrücken in Polyamiden. Makromol. Chem. 1969, 122, 30–37. [Google Scholar] [CrossRef]
- Brill, V.R. Beziehungen zwischen Wasserstoffbindung und einigen Eigenschaften von Polyamiden. Makromol. Chem. 1956, 18, 294–309. [Google Scholar] [CrossRef]
- Sambale, A.; Kurkowski, M.; Stommel, M. Determination of moisture gradients in polyamide 6 using StepScan DSC. Thermochim. Acta 2018. [Google Scholar] [CrossRef]
- Le Gac, P.-Y.; Arhant, M.; Le Gall, M.; Davies, P. Yield stress changes induced by water in polyamide 6: Characterization and modeling. Polym. Degrad. Stab. 2017, 137, 272–280. [Google Scholar] [CrossRef]
- Dlubek, G.; Redmann, F.; Krause-Rehberg, R. Humidity-induced plasticization and antiplasticization of polyamide 6: A positron lifetime study of the local free volume. J. Appl. Polym. Sci. 2002, 84, 244–255. [Google Scholar] [CrossRef]
- Reimschuessel, H.K. Relationships on the effect of water on glass transition temperature and young’s modulus of nylon 6. J. Polym. Sci. Part A Polym. Chem. 1978, 16, 1229–1236. [Google Scholar] [CrossRef]
- Launay, A.; Marco, Y.; Maitournam, M.H.; Raoult, I. Modelling the influence of temperature and relative humidity on the time-dependent mechanical behaviour of a short glass fibre reinforced polyamide. Mech. Mater. 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Kettle, G.J. Variation of the glass transition temperature of nylon-6 with changing water content. Polymer 1977, 18, 742–743. [Google Scholar] [CrossRef]
- Miri, V.; Persyn, O.; Lefebvre, J.-M.; Seguela, R. Effect of water absorption on the plastic deformation behavior of nylon 6. Eur. Polym. J. 2009, 45, 757–762. [Google Scholar] [CrossRef]
- Benaarbia, A.; Chrysochoos, A.; Robert, G. Influence of relative humidity and loading frequency on the PA6.6 cyclic thermomechanical behavior: Part I. mechanical and thermal aspects. Polym. Test. 2014, 40, 290–298. [Google Scholar] [CrossRef]
- Jia, N.; Kagan, V.A. Mechanical Performance of Polyamides with Influence of Moisture and Temperature—Accurate Evaluation and Better Understanding. In Plastics Failure Analysis and Prevention; Plastics Design Library: Norwich, UK, 2001; pp. 95–104. ISBN 9781884207921. [Google Scholar]
- Boukal, I. Effect of water on the mechanism of deformation of nylon 6. J. Appl. Polym. Sci. 1967, 11, 1483–1494. [Google Scholar] [CrossRef]
- Vlasveld, D.P.N.; Groenewold, J.; Bersee, H.E.N.; Picken, S.J. Moisture absorption in polyamide-6 silicate nanocomposites and its influence on the mechanical properties. Polymer 2005, 46, 12567–12576. [Google Scholar] [CrossRef]
- Abacha, N.; Kubouchi, M.; Sakai, T. Diffusion behavior of water in polyamide 6 organoclay nanocomposites. Express Polym. Lett. 2009, 3, 245–255. [Google Scholar] [CrossRef]
- Monson, L.; Braunwarth, M.; Extrand, C.W. Moisture absorption by various polyamides and their associated dimensional changes. J. Appl. Polym. Sci. 2008, 107, 355–363. [Google Scholar] [CrossRef]
- Sar, B.E.; Fréour, S.; Davies, P.; Jacquemin, F. Coupling moisture diffusion and internal mechanical states in polymers—A thermodynamical approach. Eur. J. Mech. A/Solids 2012, 36, 38–43. [Google Scholar] [CrossRef]
- Jia, N.; Fraenkel, H.A.; Kagan, V.A. Effects of Moisture Conditioning Methods on Mechanical Properties of Injection Molded Nylon 6. J. Reinf. Plast. Compos. 2004, 23, 729–737. [Google Scholar] [CrossRef]
- Deutsche Norm. Kunststoffe: Polyamide—Beschleunigte Konditionierung von Probekörpern (1110). Available online: https://www.beuth.de/de/norm/din-en-iso-1110/310232274 (accessed on 3 May 2021).
- Ellis, T.S. Moisture-induced plasticization of amorphous polyamides and their blends. J. Appl. Polym. Sci. 1988, 36, 451–466. [Google Scholar] [CrossRef]
- Cohen, D.S.; White, J.A.B. Sharp fronts due to diffusion and viscoelastic relaxation in polymers. Siam J. Appl. Math. 1991, 51, 472–483. [Google Scholar] [CrossRef]
- Obeid, H.; Clément, A.; Fréour, S.; Jacquemin, F.; Casari, P. On the identification of the coefficient of moisture expansion of polyamide-6: Accounting differential swelling strains and plasticization. Mech. Mater. 2018, 118, 1–10. [Google Scholar] [CrossRef]
- Inoue, K.; Hoshino, S. Swelling of nylon 6 film due to water sorption. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 1513–1526. [Google Scholar] [CrossRef]
- Lanxess Deutschland GmbH. Datasheet of Durethan B 31 SK 000000: Polyamid 6 Unverstärkt; Lanxess: Köln, Germany, 2017. [Google Scholar]
- Bailakanavar, M.; Fish, J.; Aitharaju, V.; Rodgers, W. Computational coupling of moisture diffusion and mechanical deformation in polymer matrix composites. Int. J. Numer. Meth. Engng 2014, 98, 859–880. [Google Scholar] [CrossRef]
- Osswald, T.A.; Menges, G. (Eds.) Material Science of Polymers for Engineers, 3rd, ed.; Hanser: München, Germany, 2012; ISBN 978-1-56990-514-2. [Google Scholar]
- Crank, J. A theoretical investigation of the influence of molecular relaxation and internal stress on diffusion in polymers. J. Polym. Sci. 1953, 11, 151–168. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979; ISBN 0198534116. [Google Scholar]
- Alfrey, T.; Gurnee, E.F.; Lloyd, W.G. Diffusion in Glassy Polymers. J. Polym. Sci. Part C Polym. Symp. 2007, 12, 249–261. [Google Scholar] [CrossRef]
- Takagi, Y.; Hattori, H. Studies on the drawing of polyamide fibers. IV. Effect of drawing on the degree of longitudinal swelling. J. Appl. Polym. Sci. 1965, 9, 2177–2187. [Google Scholar] [CrossRef]
- Lutterbeck, K. Das Verhalten von Kunststoffen unter dem Einfluss Wechselnder Umgebungsfeuchte und-Temperatur. Ph.D. Thesis, Institut für Kunststoffverarbeitung, RWTH Aachen, Aachen, Germany, 1984. [Google Scholar]
- Naumann, T. Beitrag zur Beschreibung des Mechanischen Langzeitdeformationsverhaltens von Thermoplastischen Kunststoffen. Ph.D. Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2012. [Google Scholar] [CrossRef]
- Arhant, M.; Le Gac, P.Y.; Le Gall, M.; Burtin, C.; Briançon, C.; Davies, P. Modelling the non Fickian water absorption in polyamide 6. Polym. Degrad. Stab. 2016, 133, 404–412. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L.; Ling, H.-C.; Hou, A.-C. Free-volume theories for self-diffusion in polymer–solvent systems. II. Predictive capabilities. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 289–304. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L. Diffusion in polymer—solvent systems. I. Reexamination of the free-volume theory. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 403–416. [Google Scholar] [CrossRef]
- Hong, S.-U. Prediction of Polymer/Solvent Diffusion Behavior Using Free-Volume Theory. Ind. Eng. Chem. Res. 1995, 34, 2536–2544. [Google Scholar] [CrossRef]
- Sharma, P.; Sambale, A.; Stommel, M.; Maisl, M.; Herrmann, H.-G.; Diebels, S. Moisture transport in PA6 and its influence on the mechanical properties. Contin. Mech. Thermodyn. 2019. [Google Scholar] [CrossRef]
- Joannès, S.; Mazé, L.; Bunsell, A.R. A simple method for modeling the concentration-dependent water sorption in reinforced polymeric materials. Compos. Part B Eng. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Dassault Systèmes. Abaqus Analysis User’s Guide (6.14). 2014. Available online: http://wufengyun.com:888/pdf_books/ANALYSIS_1.pdf (accessed on 3 May 2021).
- Dassault Systèmes. Abaqus Theory Guide (6.14). 2014. Available online: http://130.149.89.49:2080/v6.11/pdf_books/THEORY.pdf (accessed on 3 May 2021).
- Giencke, E.; Meder, G. Ermittlung der Kriechfunktionen von zweiachsig beanspruchten Harzen und Laminaten aus den Meßwerten für einachsig beanspruchter Harze. Materialprüfung 1981, 23, 75–85. [Google Scholar]
- Beaumont, J. Auslegung von Anguss und Angusskanal: Spritzgießwerkzeuge erfolgreich einsetzen; 1. Auflage. Hanser: München, Germany, 2012; ISBN 9783446427594. [Google Scholar]
- Hopmann, C.; Menges, G.; Michaeli, W.; Mohren, P. Spritzgießwerkzeuge: Auslegung, Bau, Anwendung; 7. Auflage. Hanser: München, Germany, 2018; ISBN 9783446451926. [Google Scholar]
- Deutsche Norm. Kunststoffe—Bestimmung der Zugeigenschaften—Teil 1: Allgemeine Grundsätze (ISO 527—1:2012) Deutsche Fassung EN ISO 527-1:2012 (527-1). Available online: https://www.beuth.de/de/norm/din-en-iso-527-1/147683249 (accessed on 3 May 2021).
- Parodi, E.; Govaert, L.E.; Peters, G.W.M. Glass transition temperature versus structure of polyamide 6: A flash-DSC study. Thermochim. Acta 2017. [Google Scholar] [CrossRef]
- Parodi, E.; Peters, G.W.M.; Govaert, L.E. Structure-properties relations for polyamide 6, Part 2: Influence of processing conditions during injection moulding on deformation and failure kinetics. Polymers 2018, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Hanspach, J.; Pinno, F. Concentration dependent diffusion of water in polyamide 6. Acta Polym. 1992, 43, 210–213. [Google Scholar] [CrossRef]
- Stellrecht, E.; Han, B.; Pecht, M. Measurement of the hygroscopic swelling coefficient in mold compounds using moiré interferometry. Exp. Tech. 2003, 27, 40–45. [Google Scholar] [CrossRef]
- Jacquemin, F.; Fréour, S.; Guillén, R. Analytical modeling of transient hygro-elastic stress concentration—Application to embedded optical fiber in a non-uniform transient strain field. Compos. Sci. Technol. 2006, 66, 397–406. [Google Scholar] [CrossRef]






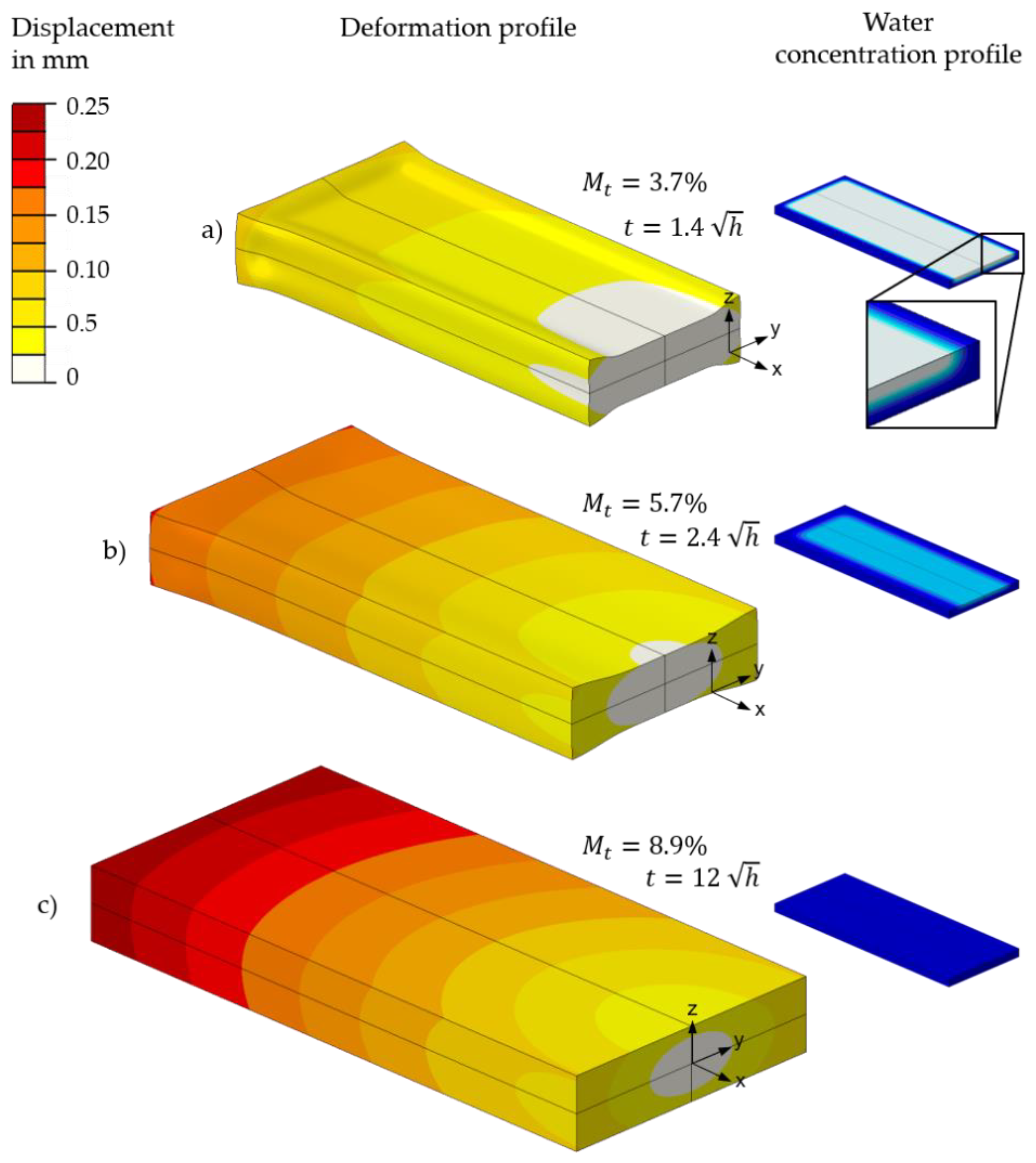
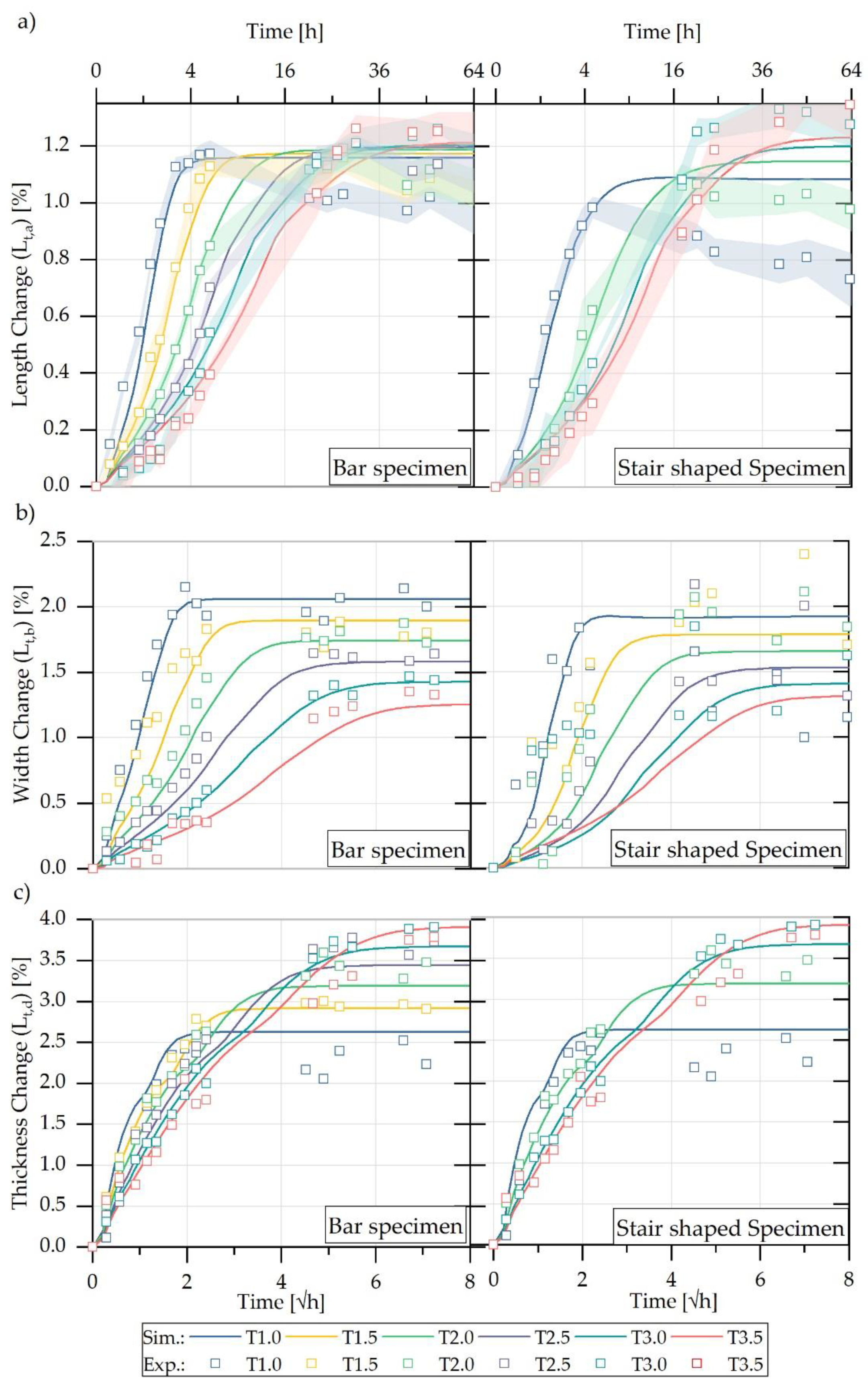
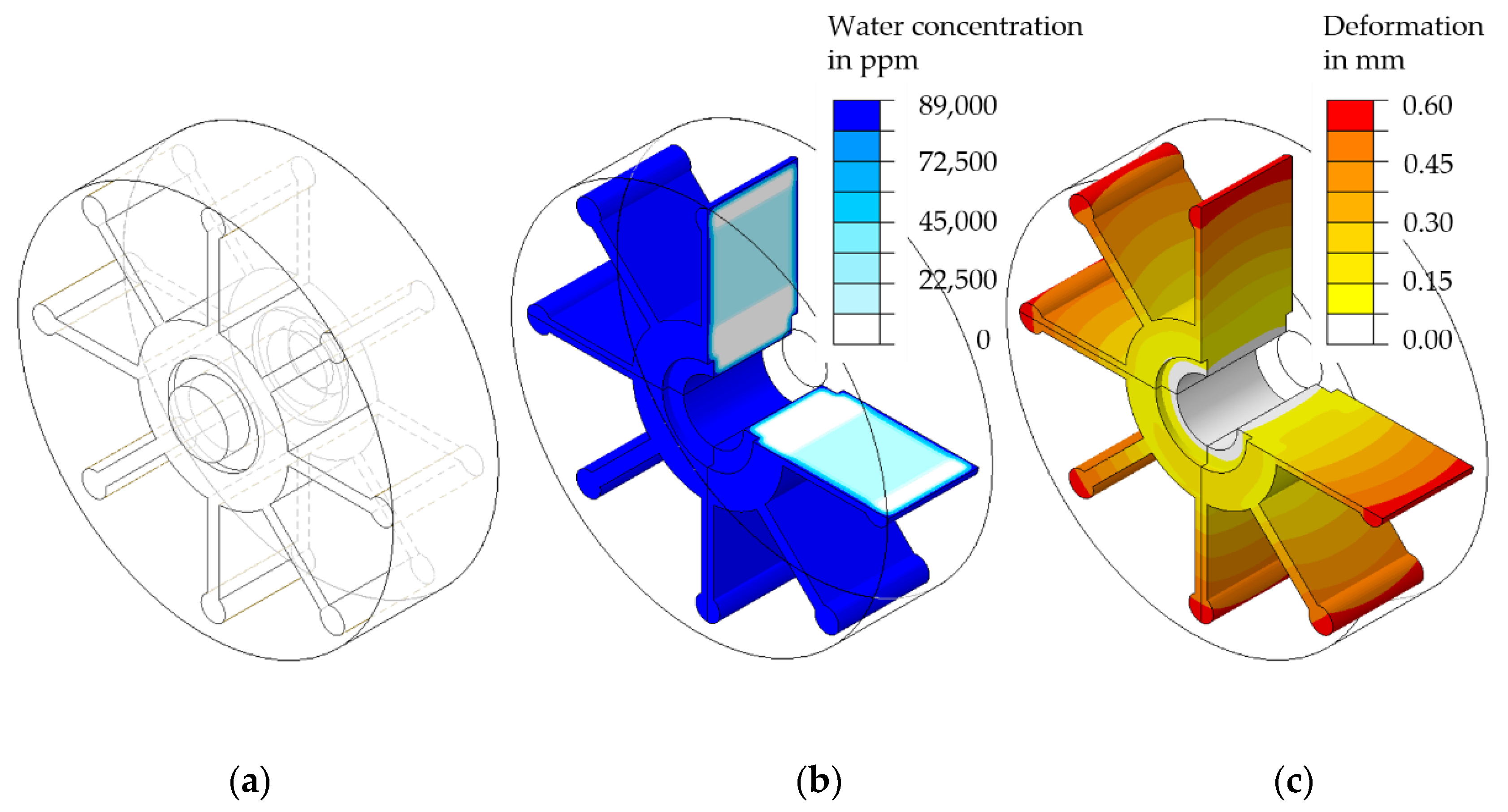
| Sample Name | Thickness t (mm) | Length l (mm) | V/A Ratio Samples (mm) | V/A Ratio Part (mm) | ||
|---|---|---|---|---|---|---|
| T0.5 | 0.5 | 40 | 3 | 2 | 0.24 | 1.18 |
| T1.0 | 1.0 | 40 | 5 | 4 | 0.44 | |
| T1.5 | 1.5 | 40 | 7 | 6 | 0.63 | |
| T2.0 | 2.0 | 40 | 9 | 8 | 0.82 | |
| T2.5 | 2.5 | 40 | 11 | 10 | 1.00 | |
| T3.0 | 3.0 | 40 | 13 | 12 | 1.17 | |
| T3.5 | 3.5 | 40 | 15 | 14 | 1.36 |
| Material Behaviour | |||||
|---|---|---|---|---|---|
| Diffusivity | Swelling-Induced Deformation | ||||
| Water Concentration c | Diffusion Coefficient D | Young’s Modulus E | Expansion Coefficients for O/V = 0.44 | ||
| a11 | a22 | a33 | |||
| [ppm] | [m/s2] | [MPa] | [ppm−1] | [ppm−1] | [ppm−1] |
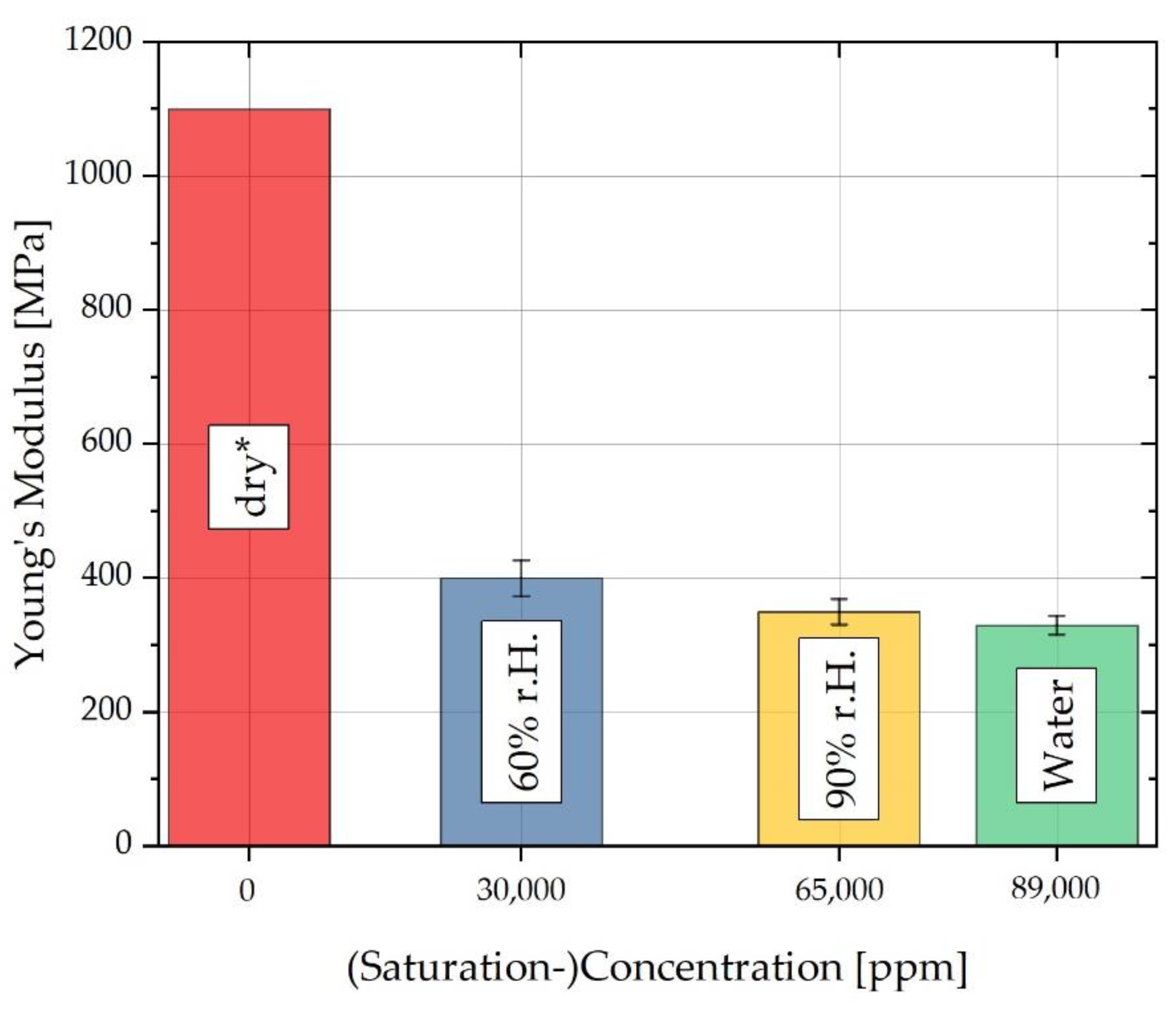
| 0 | 2.58 | 1100 | 0 | 0 | 0 |
| 30,000 | 7.34 | 400 | 0.86 | 2.78 | |
| 65,000 | 2.42 | 350 | 1.17 | 1.28 | 3.64 |
| 89,000 | 5.51 | 330 | 1.36 | 1.81 | 3.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambale, A.K.; Stanko, M.; Emde, J.; Stommel, M. Characterisation and FE Modelling of the Sorption and Swelling Behaviour of Polyamide 6 in Water. Polymers 2021, 13, 1480. https://doi.org/10.3390/polym13091480
Sambale AK, Stanko M, Emde J, Stommel M. Characterisation and FE Modelling of the Sorption and Swelling Behaviour of Polyamide 6 in Water. Polymers. 2021; 13(9):1480. https://doi.org/10.3390/polym13091480
Chicago/Turabian StyleSambale, Anna Katharina, Michael Stanko, Jessica Emde, and Markus Stommel. 2021. "Characterisation and FE Modelling of the Sorption and Swelling Behaviour of Polyamide 6 in Water" Polymers 13, no. 9: 1480. https://doi.org/10.3390/polym13091480
APA StyleSambale, A. K., Stanko, M., Emde, J., & Stommel, M. (2021). Characterisation and FE Modelling of the Sorption and Swelling Behaviour of Polyamide 6 in Water. Polymers, 13(9), 1480. https://doi.org/10.3390/polym13091480