Organophosphorus Polyurethane Ionomers as Water Vapor Permeable and Pervaporation Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Synthetic Procedures
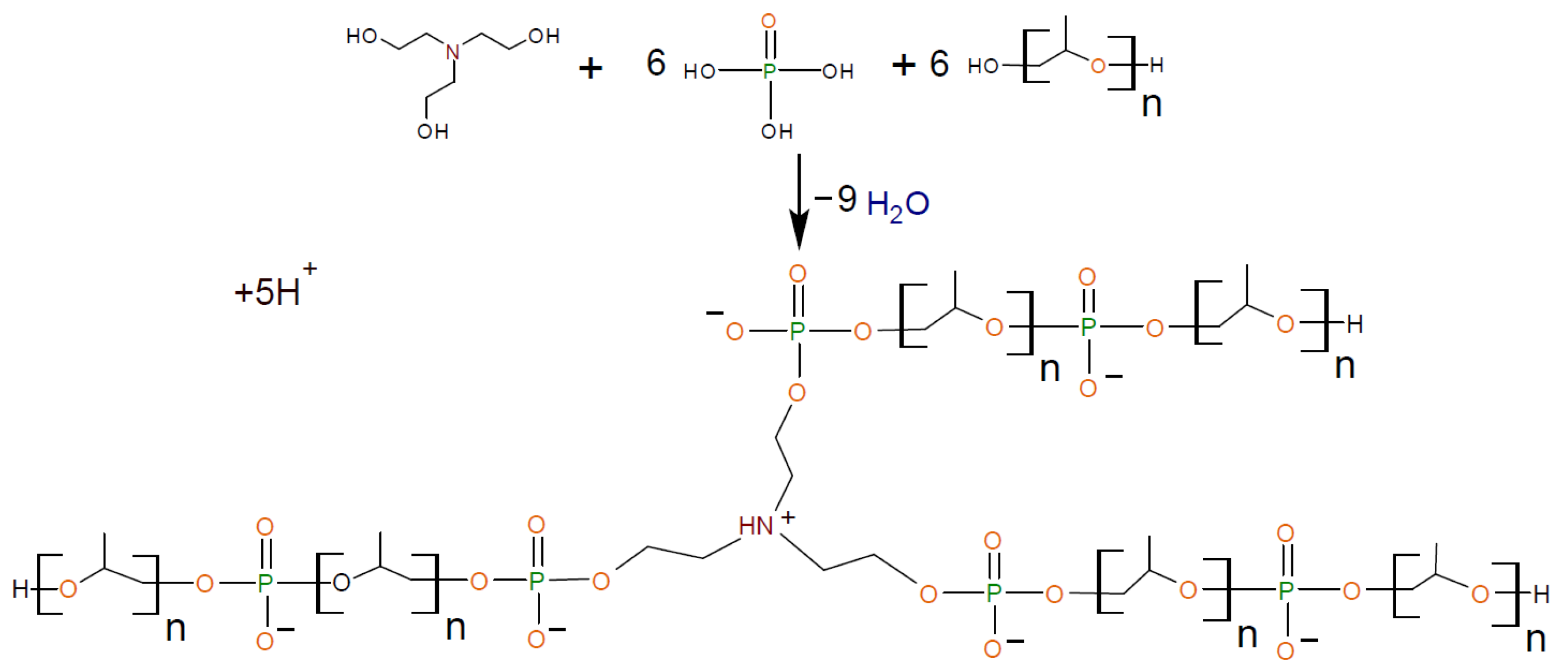
2.2.1. General Procedure for Synthesis of Amino Ethers of Ortho-Phosphoric Acid (AEPA-PEG-400)
2.2.2. General Procedure for Synthesis of Amino Ethers of Ortho-Phosphoric Acid (AEPA-(3 ÷ 6)-PPG-400/1000/2000)
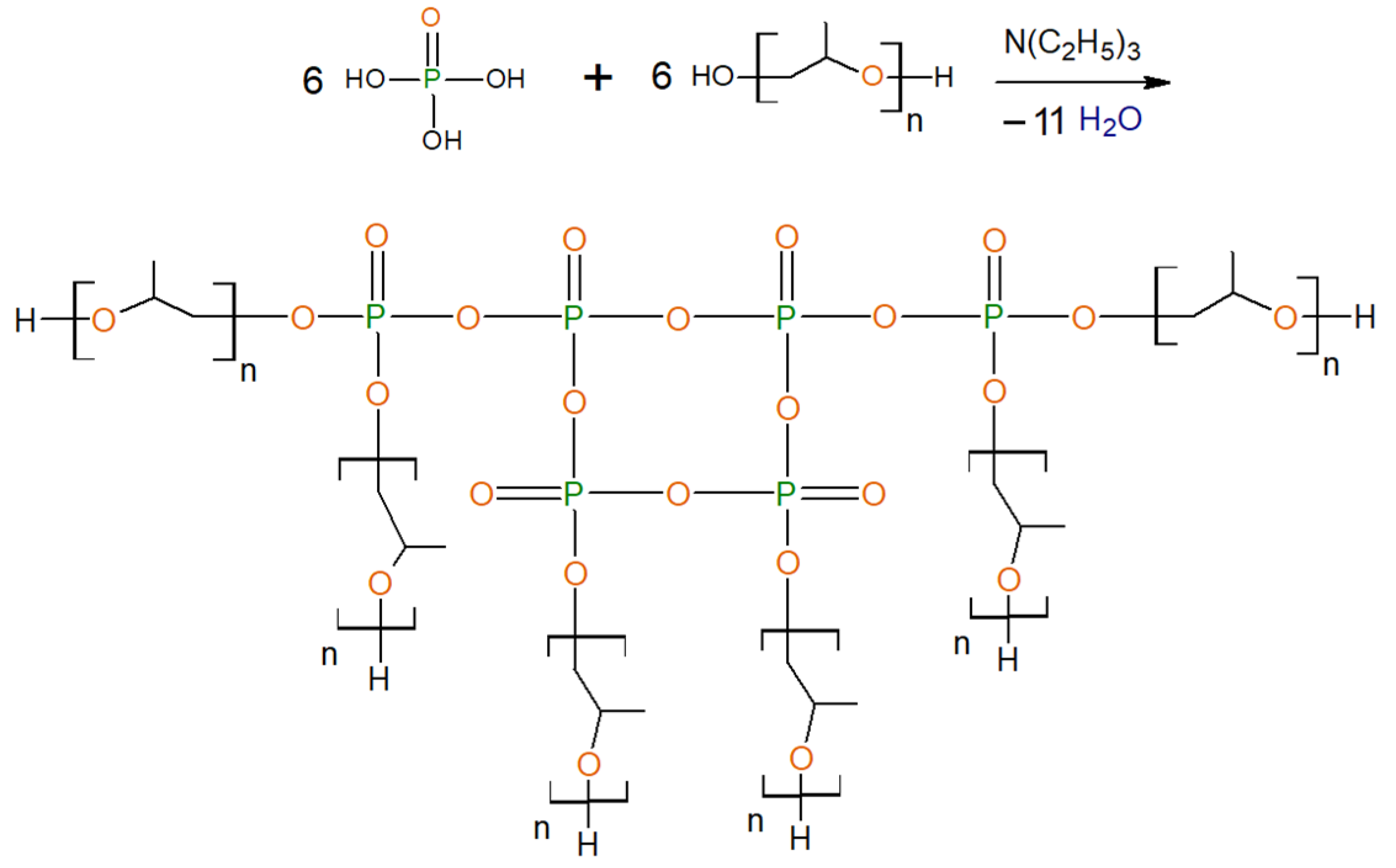
2.2.3. General Procedure for Synthesis of Ethers of Ortho-Phosphoric Acid (EPA-(3 ÷ 6)-PPG-1000)
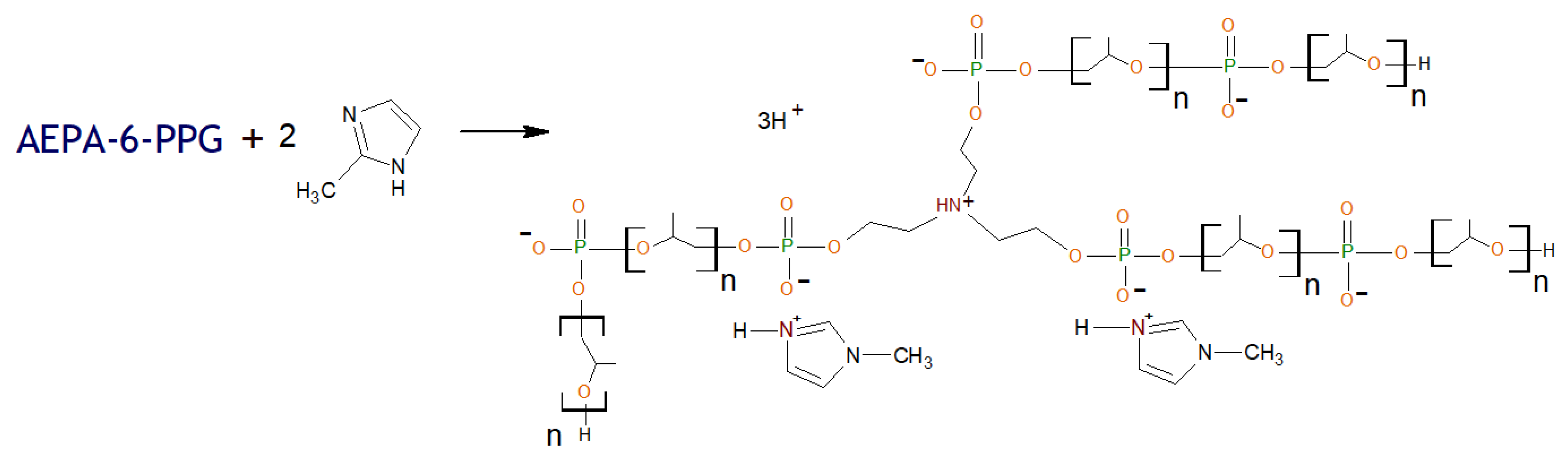
2.2.4. General Procedure for Synthesis of Polyurethanes Based on Amino Ethers of Ortho-Phosphoric Acid and Ethers of Ortho-Phosphoric Acid (AEPA-(3 ÷ 6)-PPG-1000/2000-PU, EPA-(3 ÷ 6)-PPG-1000-PU, AEPA-PEG-400-PU).
2.3. Manufacturing of Pervaporation Membranes
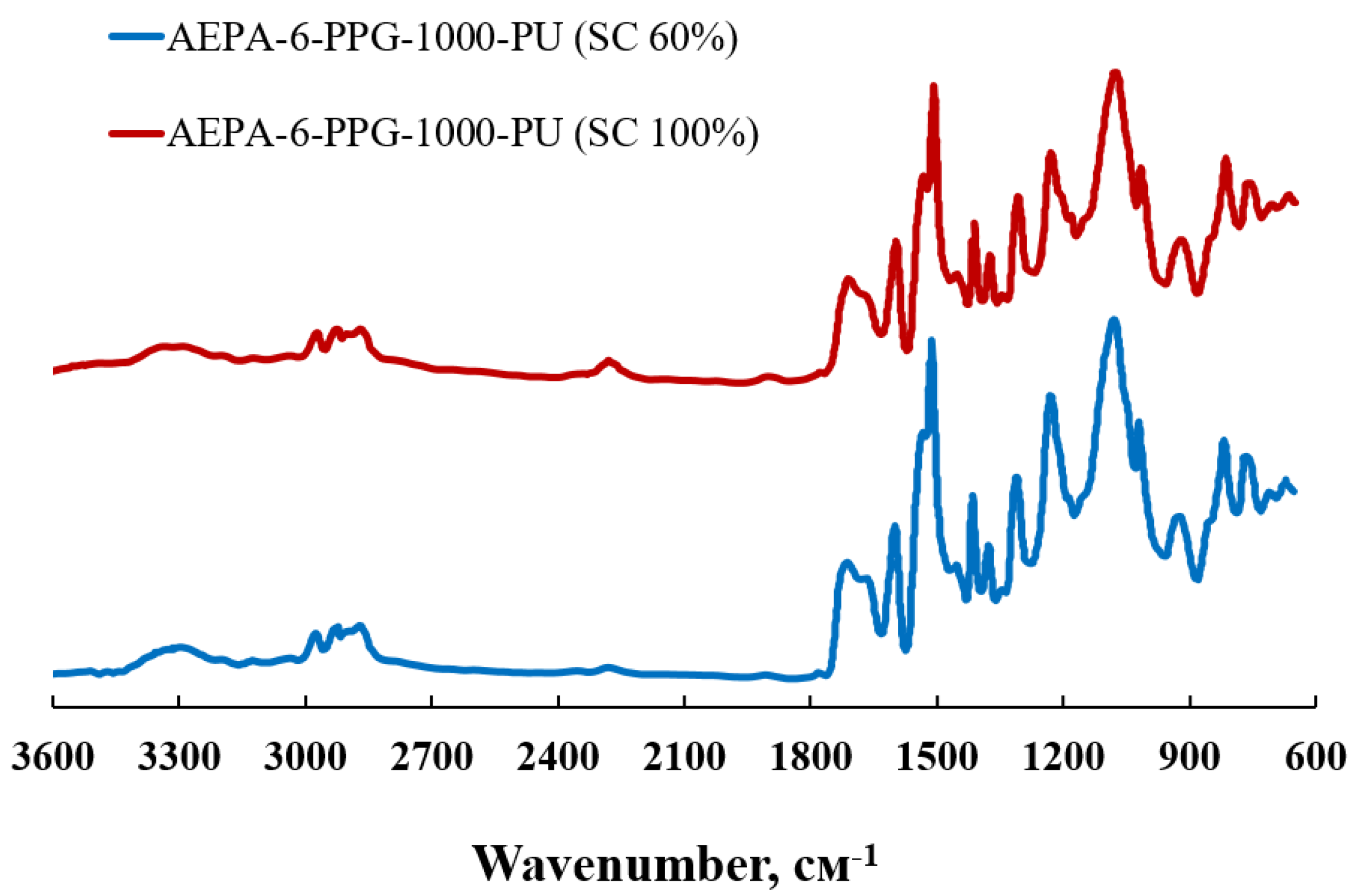
2.4. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
2.5. Viscosity and Density Measurements
2.6. Measurements of the Surface Tension
2.7. Tensile Stress–Strain Measurements
2.8. Thermomechanical Analysis (TMA)
2.9. Mechanical Loss Tangent Measurements (MLT)
2.10. Thermal Gravimetric Analysis (TGA)
2.11. The Densitometer
2.12. Water Adsorption
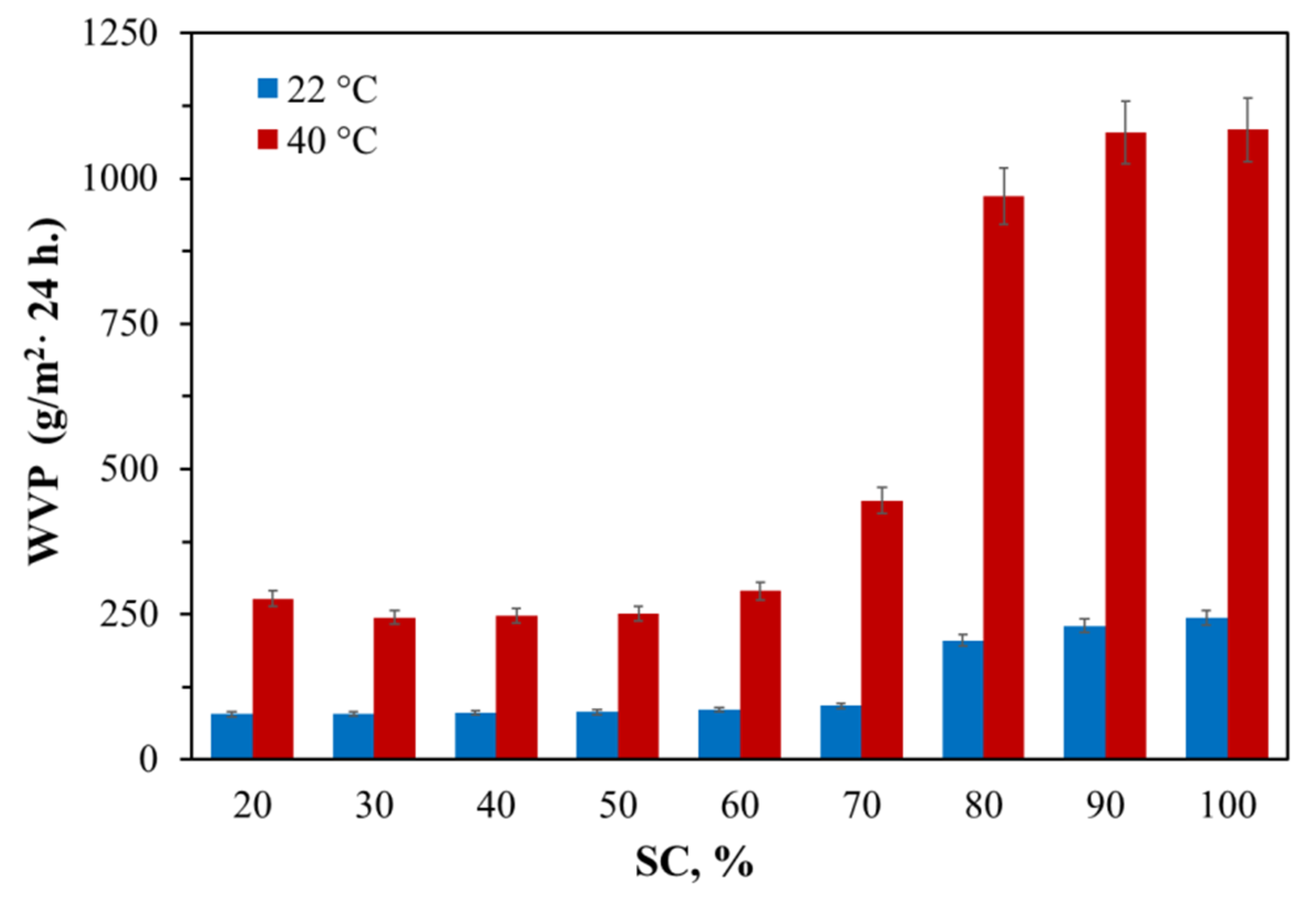
2.13. Water Vapor Permeability (WVP) Measurements
2.14. Pervaporation Processes
3. Results
3.1. Membranes Based on AEPA-PPG-1000/2000-PU
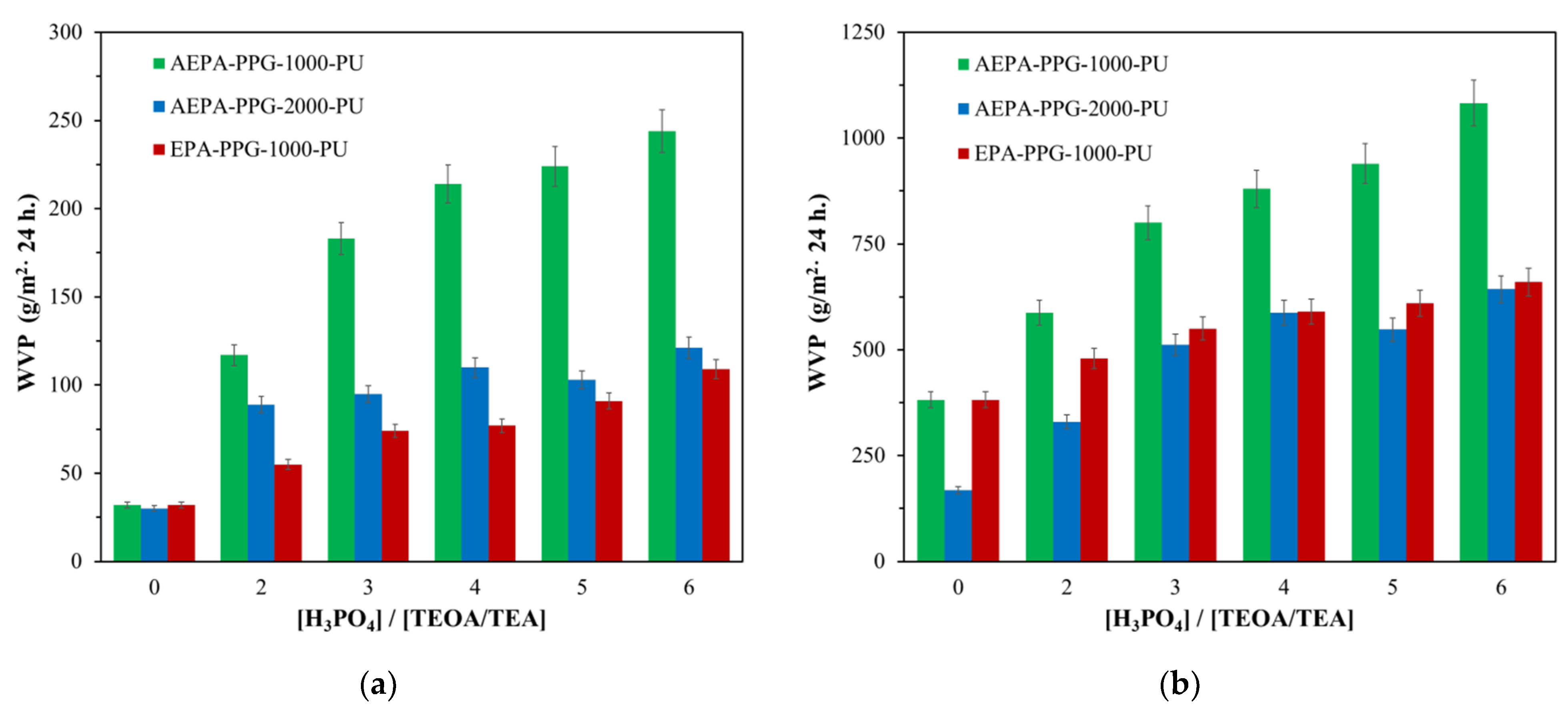
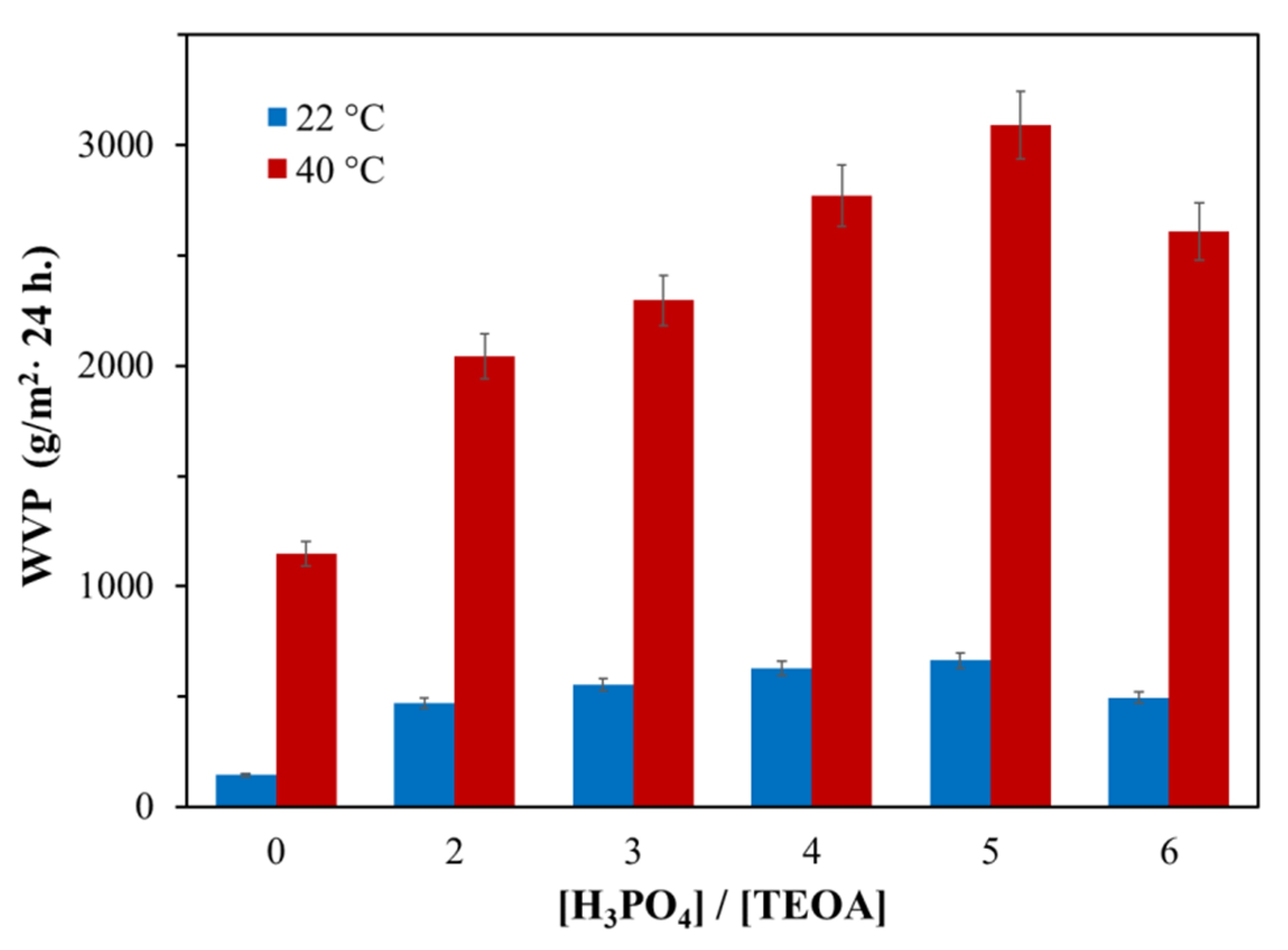
3.1.1. Water vapor permeability of AEPA-PPG-1000/2000-PU
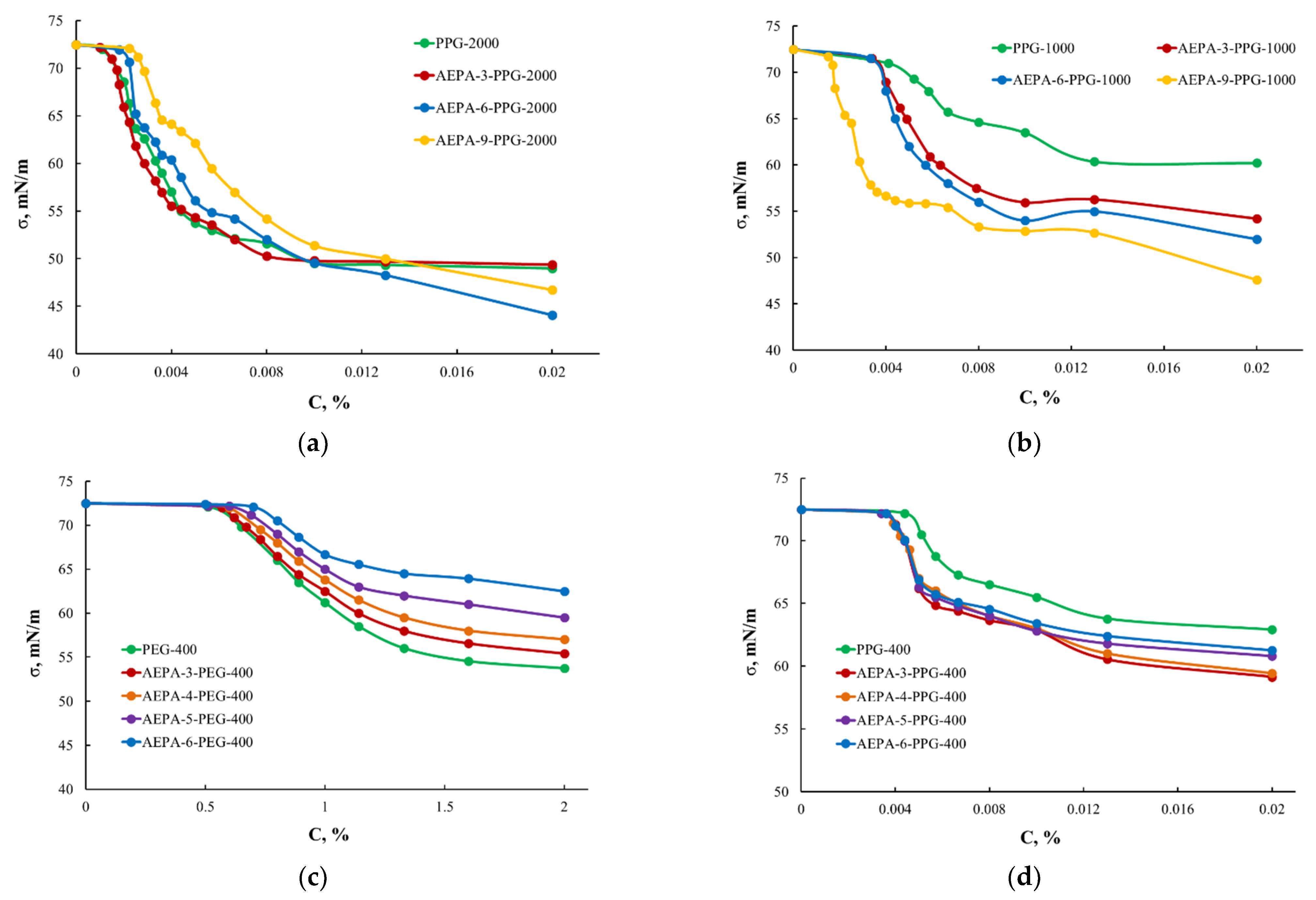
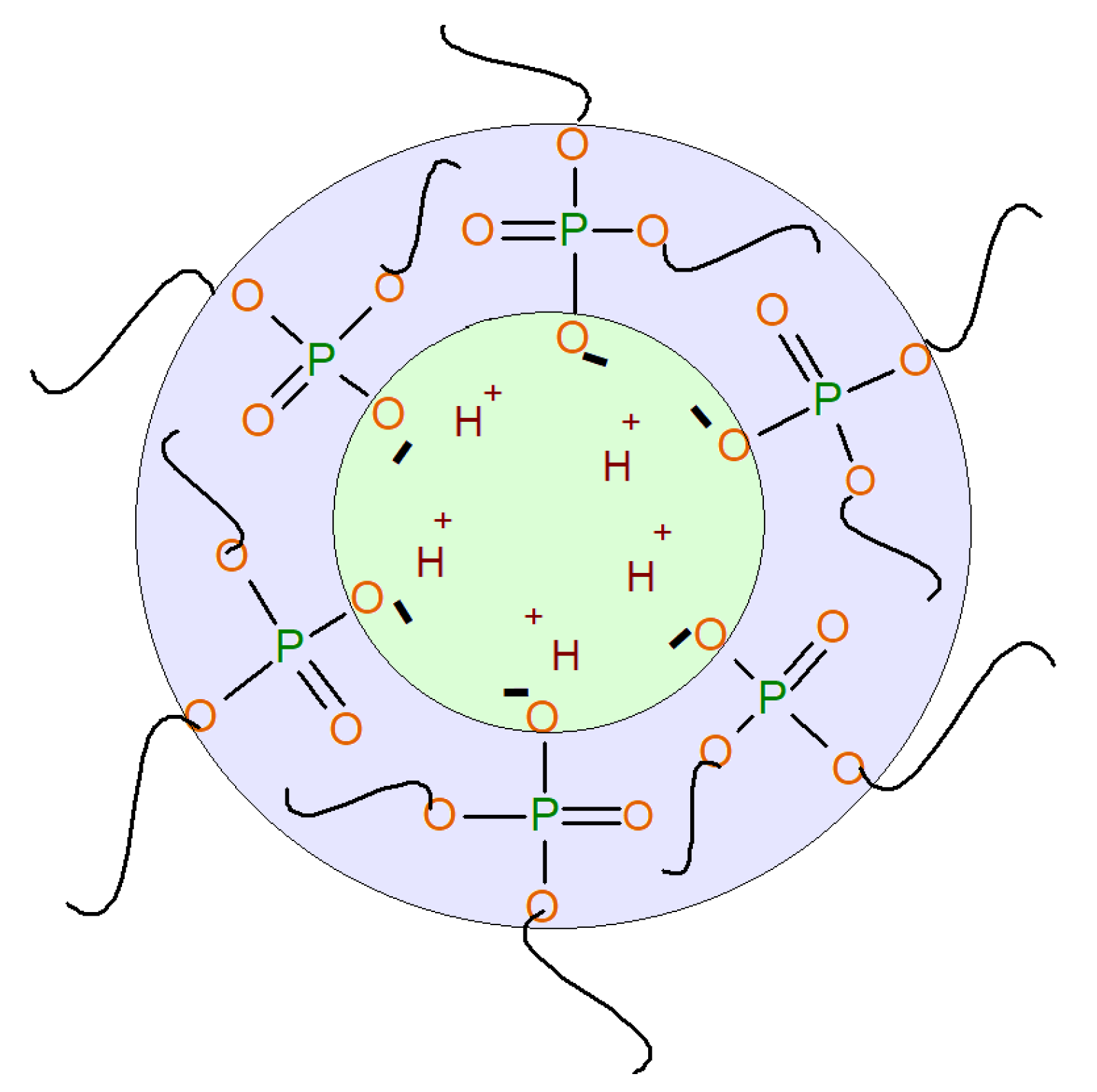
3.1.2. Surface-Active Properties of AEPA-PPG-1000/2000
3.1.3. Study of AEPA-PPG-1000-PU as Pervaporation Membranes for the Separation of Isopropanol/Water Mixtures
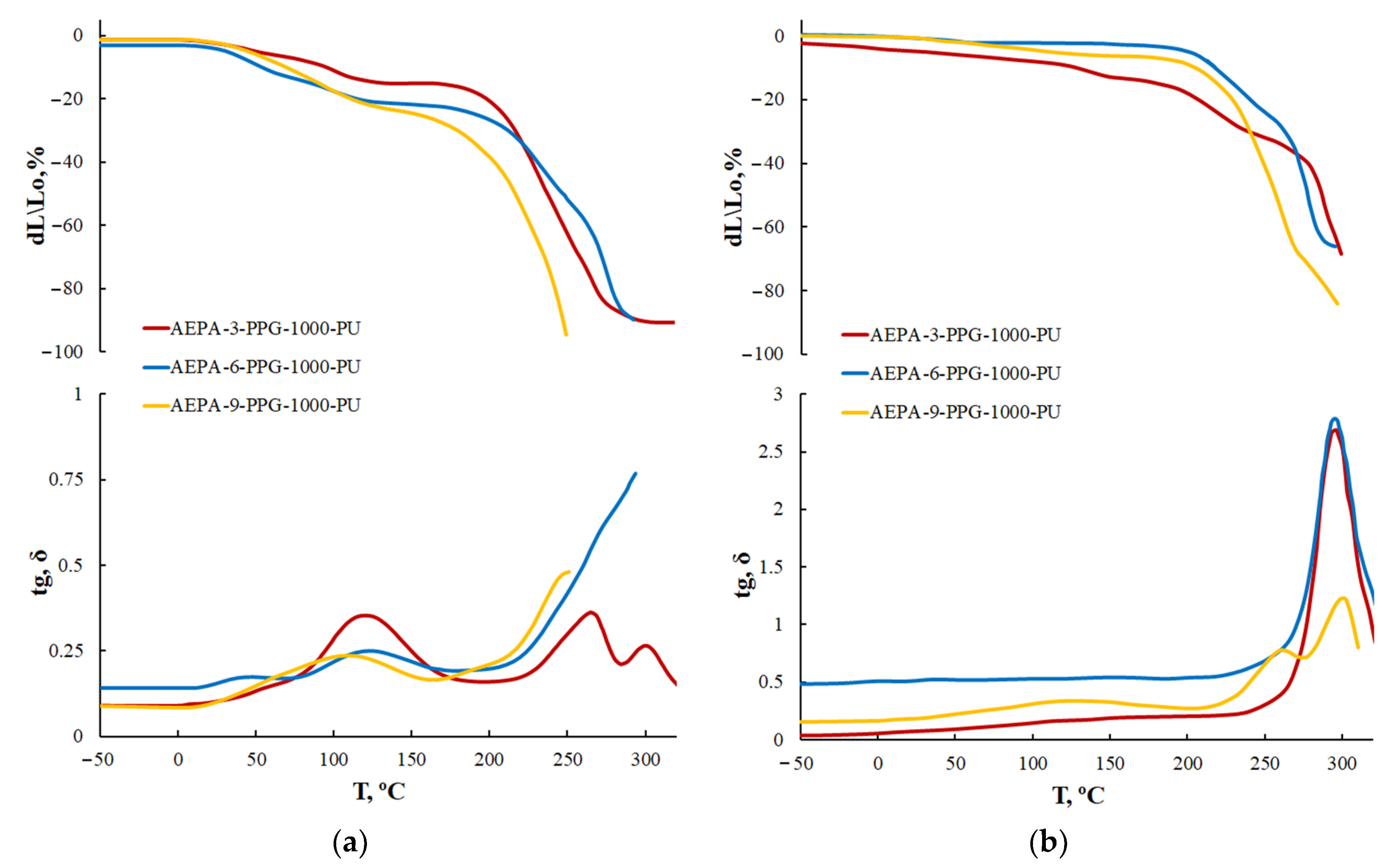
3.1.4. Thermogravimetric Analysis of AEPA-PU
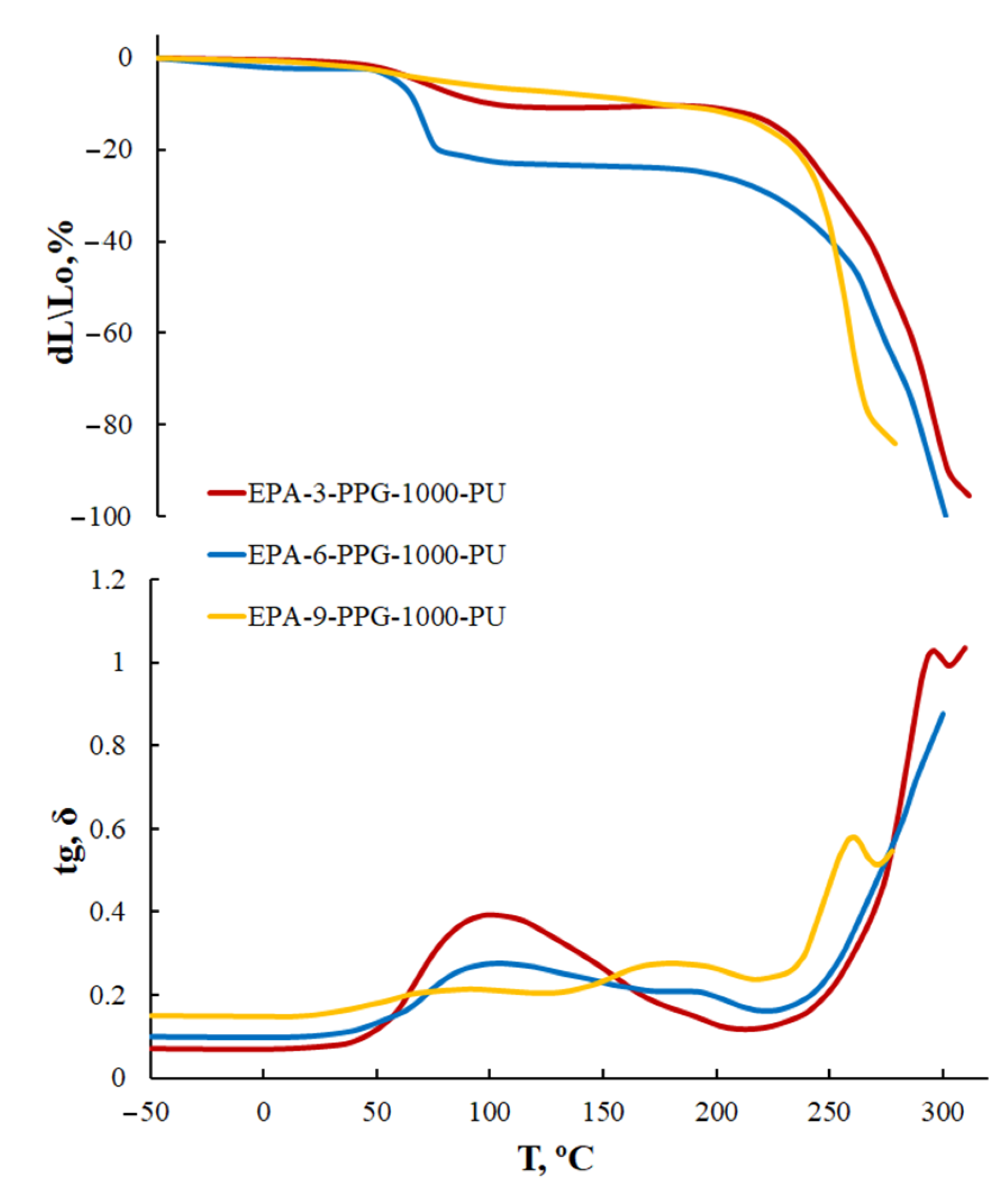
3.1.5. Thermomechanical Analysis of AEPA-PPG-1000-PU and EPA-PPG-1000-PU
3.2. Membranes Based on AEPA-PEG-400-PU
3.2.1. Surface-Active Properties of AEPA-PPG-400/1000 and AEPA-PEG-400
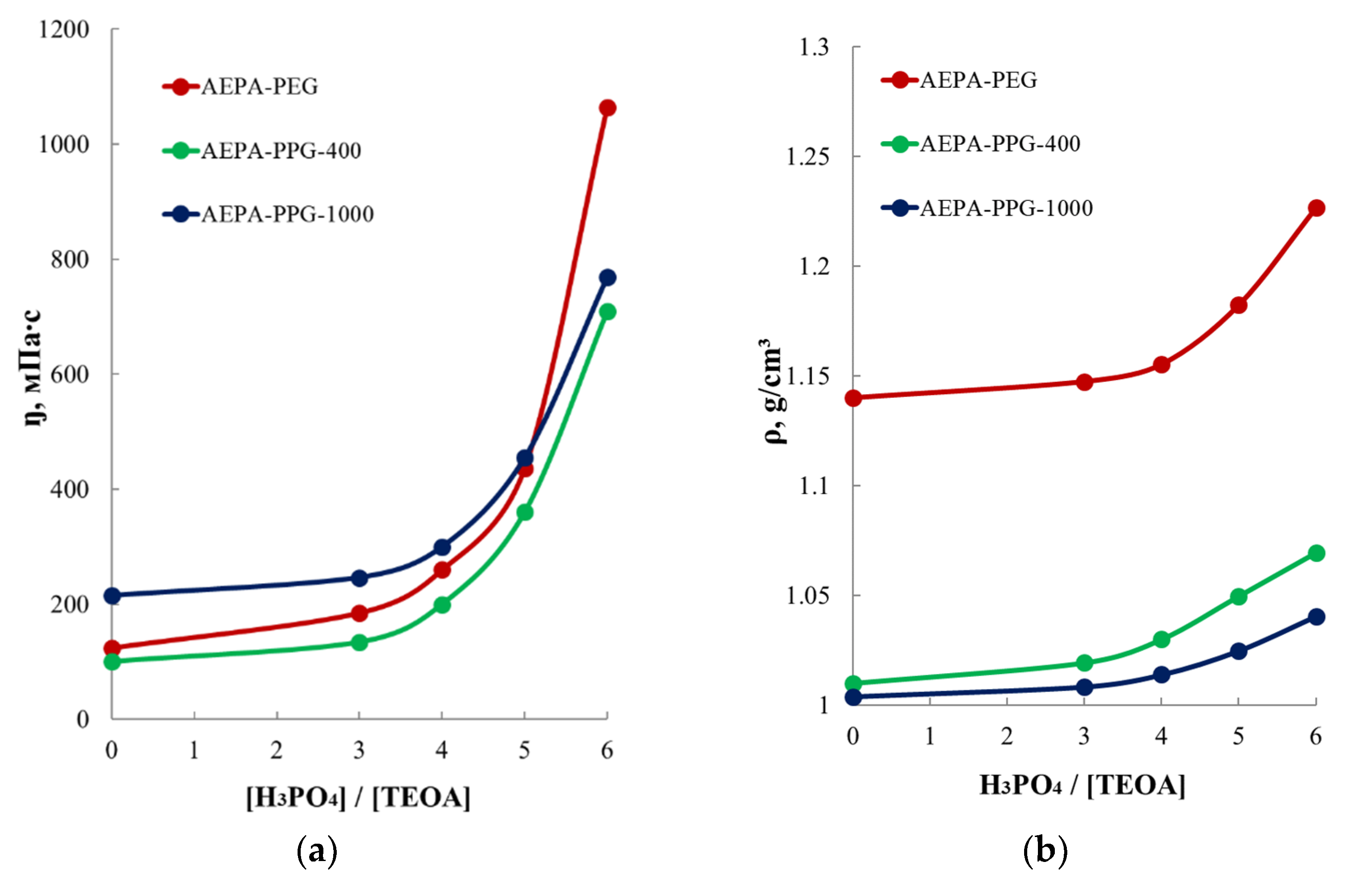
3.2.2. Study of the Viscosity and Density of AEPA-PPG-400/1000 and AEPA-PEG-400
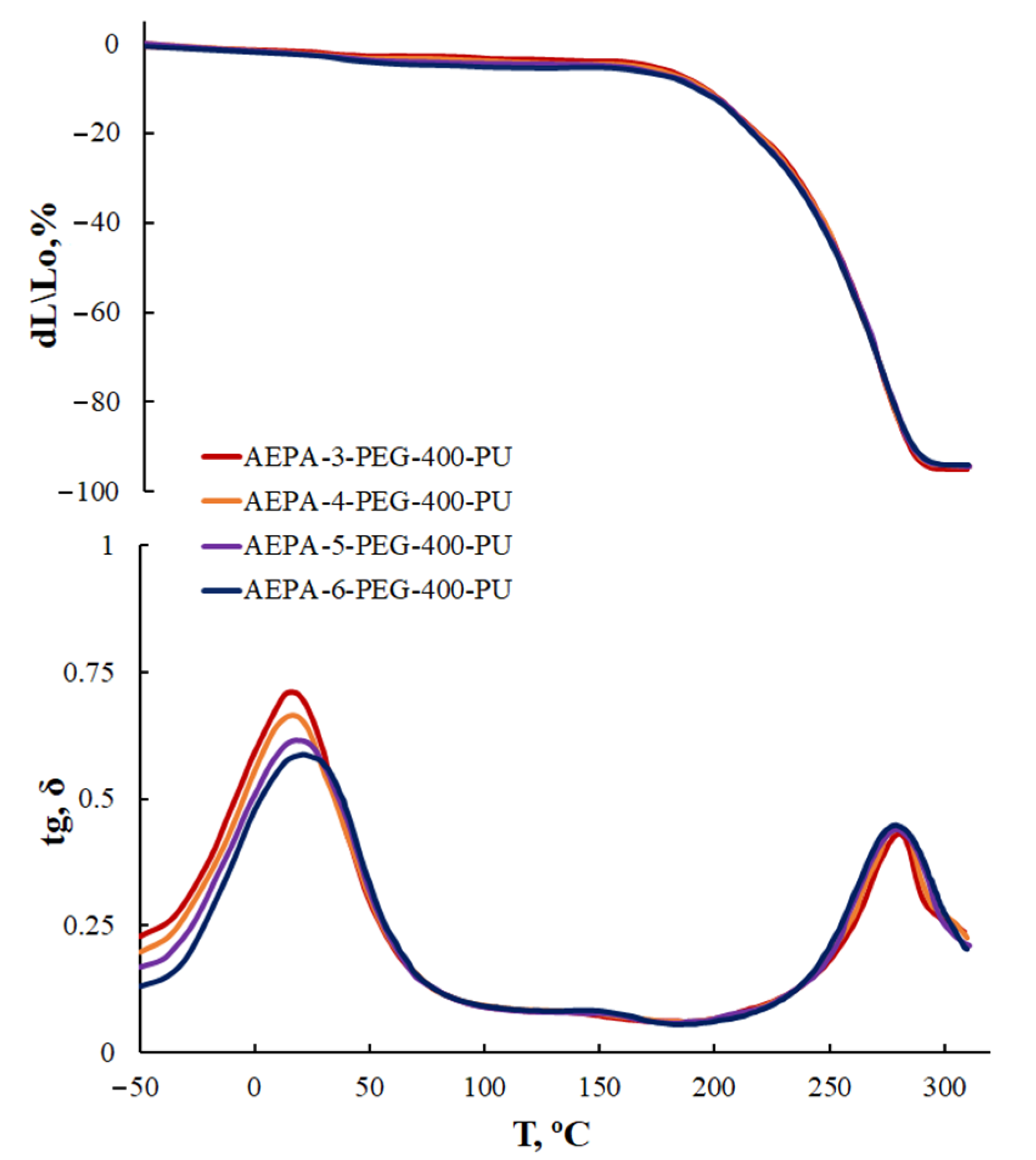
3.2.3. Thermogravimetric Analysis of AEPA-PEG-400-PU
3.2.4. Thermomechanical Analysis of AEPA-PEG-400-PU
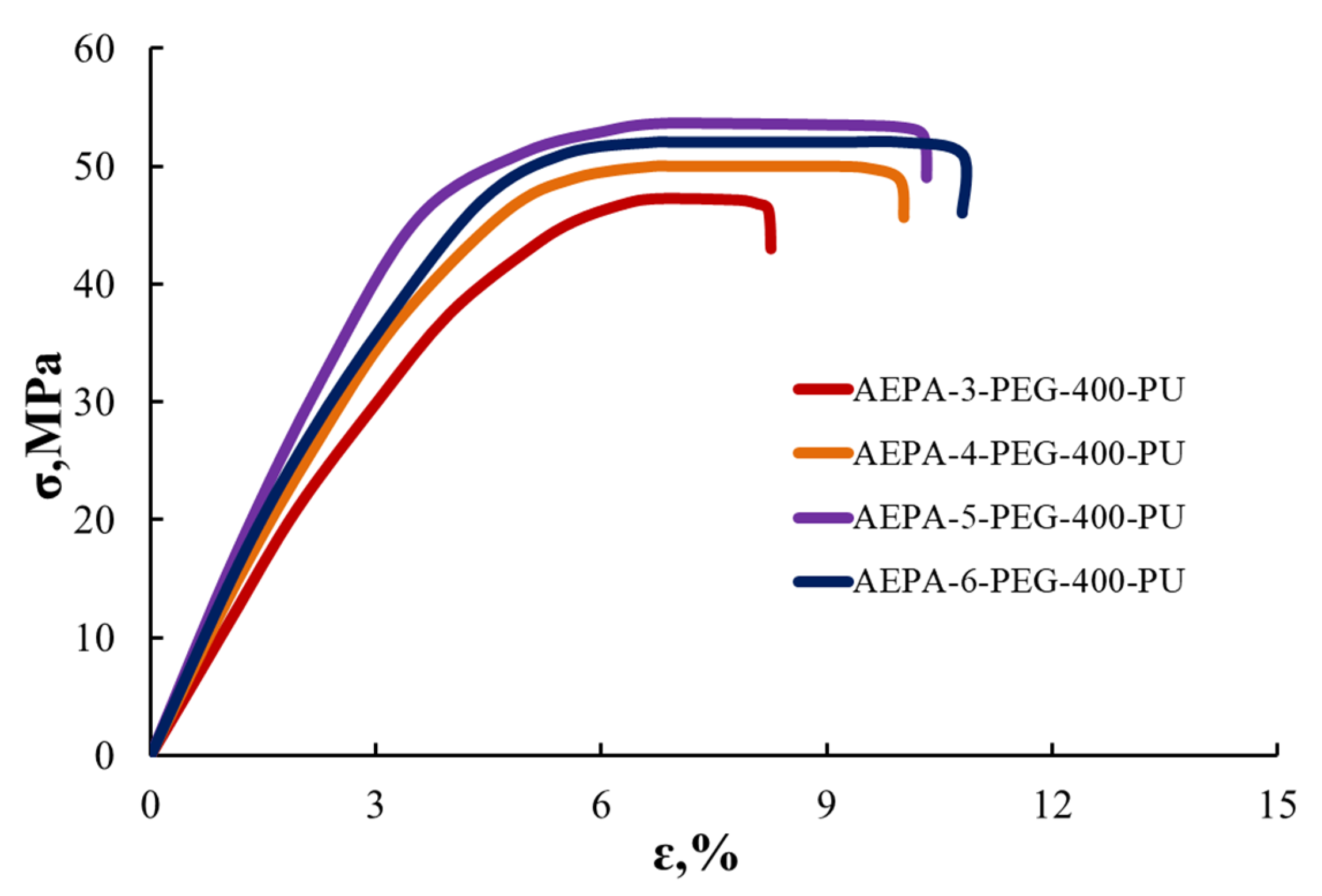
3.2.5. Tensile Stress–Strain Analysis of AEPA-PEG-400-PU
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vane, L.M. Membrane materials for the removal of water from industrial solvents by pervaporation and vapor permeation. J. Chem. Technol. Biot. 2019, 94, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, W.Z.A.W.; Rahman, S.A.; Ahmad, A.L.; Mokhtar, N.M. Modifications on Polymeric Membranes for Isopropanol Dehydration Using Pervaporation: A Review. In Applications of Nanotechnology for Green Synthesis. Nanotechnology in the Life Sciences; Inamuddin, M., Asiri, A.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; Chapter 5; pp. 97–124. [Google Scholar]
- Jyothi, M.S.; Reddy, K.R.; Soontarapa, K.; Naveen, S.; Raghu, A.V.; Kulkarni, R.V.; Suhas, D.P.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Membranes for dehydration of alcohols via pervaporation. J. Environ. Manag. 2019, 242, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Hua, D.; Maricar, V.; Ong, Y.K.; Chung, T.-S. Dehydration of industrial isopropanol (IPA) waste by pervaporation and vapor permeation membranes. J. Appl. Polym. Sci. 2017, 45086. [Google Scholar] [CrossRef]
- Andre, A.; Nagy, T.; Toth, A.J.; Haaz, E.; Fozer, D.; Tarjani, J.A.; Mizsey, P. Distillation contra pervaporation: Comprehensive investigation of isobutanol-water separation. J. Clean. Prod. 2018, 187, 804–818. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Chem. Eng. 2018, 35, 565–590. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of membrane science development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef]
- Zoubeik, M.; Ismail, M.; Salama, A.; Henni, A. New developments in membrane technologies used in the treatment of produced water. Arab. J. Sci. Eng. 2017, 43, 2093–2118. [Google Scholar] [CrossRef]
- Ray, S.S.; Iroegbu, A.O.C.; Bordado, J.C. Polymer-Based membranes and composites for safe, potable, and usable water: A survey of recent advances. Chem. Afr. 2020, 3, 593–608. [Google Scholar] [CrossRef]
- Malakhov, A.O.; Volkov, A.V. Modification of polymer membranes for use in organic solvents. Russ. J. Appl. Chem. 2020, 93, 14–24. [Google Scholar] [CrossRef]
- Fen Yong, W.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2020, 116, 100713–100745. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, F.; Wang, M.; Li, W.; Song, Y.; Liu, G.; Yang, P.; Gao, B.; Wu, H.; Jiang, Z. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Liu, H.-X.; Wang, N.; Zhao, C.; Ji, S.; Li, J.-R. Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures. Chin. J. Chem. Eng. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Uragami, T. Selective Membranes for Purification and Separation of Organic Liquid Mixtures. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier B. V.: Oxford, UK, 2017; Volume 2, pp. 256–331. [Google Scholar]
- Mulder, M. Basic Principals of Membrane Technology; Kluwer Academic Publishers: London, UK, 1996. [Google Scholar]
- Dharupaneedi, S.P.; Kotrappanavar, N.S.; Nadagouda, M.; Raghava, R.K.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Lipnizki, F.; Field, R.W.; Ten, P.-K. Pervaporation-based hybrid process: A review of process design, applications and economics. J. Membr. Sci. 1999, 153, 183–210. [Google Scholar] [CrossRef]
- Huang, S.; Chang, P.; Tsai, M.; Chang, H. Properties and pervaporation performances of crosslinked HTPB-based polyurethane membranes. Sep. Purif. Technol. 2007, 56, 63–70. [Google Scholar] [CrossRef]
- Chao, M.-S.; Huang, S.-L. Epoxidized HTPB-based polyurethane membranes for pervaporation separation. J. Chin. Chem. Soc. 2005, 52, 287–294. [Google Scholar] [CrossRef]
- Hu, M.; Gao, L.; Fu, W.; Liu, X.; Huang, F.; Luo, Y.; Huang, C. High-performance interpenetrating polymer network polyurethane pervaporation membranes for butanol recovery. J. Chem. Technol. Biot. 2014, 90, 2195–2207. [Google Scholar] [CrossRef]
- Schauer, J.; Bartz, D.; Maroušek, V. Polyurethane pervaporation membranes. Die Angew. Makromol. Chem. 1999, 268, 41–45. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Huang, S.-L.; Chang, P.-H.; Chen, C.-J. Properties and pervaporation separation of hydroxyl-terminated polybutadiene-based polyurethane/poly(methyl metharcylate) interpenetrating networks membranes. J. Appl. Polym. Sci. 2007, 106, 4277–4286. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, S.; Basak, P.; Adhikari, B. Dehydration of alcohols by pervaporation using hydrophilic polyether urethane membranes. J. Sci. Ind. Res. 2008, 67, 219–227. [Google Scholar]
- Yao, L.; Wu, C.; Yang, Z.; Qiu, W.; Cui, P.; Xu, T. Waterborne Polyurethane/Poly(vinyl alcohol) membranes: Preparation, characterization, and potential application for pervaporation. J. Appl. Polym. Sci. 2012, 124, E216–E224. [Google Scholar] [CrossRef]
- Xi, T.; Lu, Y.; Ai, X.; Tang, L.; Yao, L.; Hao, W.; Cui, P. Ionic liquid copolymerized polyurethane membranes for pervaporation separation of benzene/cyclohexane mixtures. Polymer 2019, 185, 121948. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Shi, X.; Wu, L.; Zhang, X.; Zhang, S. Polyurethane hybrid membranes with confined mass transfer channels: The effect of functionalized multi-walled carbon nanotubes on permeation properties. Chem. Eng. Sci. 2019, 201, 191–200. [Google Scholar] [CrossRef]
- Wolińska-Grabczyk, A. Effect of the hard segment domains on the permeation and separation ability of the polyurethane-based membranes in benzene/cyclohexane separation by pervaporation. J. Membr. Sci. 2006, 282, 225–236. [Google Scholar] [CrossRef]
- Das, S.; Banthia, A.K.; Adhikari, B. Porous polyurethane urea membranes for pervaporation separation of phenol and chlorophenols from water. Chem. Eng. J. 2008, 138, 215–223. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Dou, S.; Ralph, H.C.; Runt, J. Molecular mobility, ion mobility, and mobile ion concentration in poly(ethylene oxide)-based polyurethane ionomers. Macromolecules 2008, 41, 5723–5728. [Google Scholar] [CrossRef]
- Król, P.; Król, B. Structures, properties and applications of the polyurethane ionomers. J. Mater. Sci. 2019, 55, 73–87. [Google Scholar] [CrossRef]
- Daniela, F.; Doina, M.; Stelian, V.; Gabriela, L.; Mariana, C.; Mirela, F.Z. Structure-property relationship of sodium deoxycholate based poly(ester ether)urethane ionomers for biomedical applications. J. Appl. Polym. Sci. 2016, 133, 42921. [Google Scholar]
- Bottino, A.; Capannelli, G.; Comite, A.; Costa, C. Synthesis and characterization of polyurethanic proton exchange membranes. J. Fuel Cell Sci. Tech. 2011, 8, 051011. [Google Scholar] [CrossRef]
- Chen, K.; Liu, R.; Zou, C.; Shao, Q.; Lan, Y.; Cai, X.; Zhai, L. Linear polyurethane ionomers as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. C 2014, 130, 466–473. [Google Scholar] [CrossRef]
- Król, B.; Pielichowska, K.; Król, P.; Kędzierski, M. Polyurethane cationomer films as ecological membranes for building industry. Prog. Org. Coat. 2019, 130, 83–92. [Google Scholar] [CrossRef]
- Jaudouin, O.; Robin, J.-J.; Lopez-Cuesta, J.-M.; Perrin, D.; Imbert, C. Ionomer-based polyurethanes: A comparative study of properties and applications. Polym. Int. 2012, 61, 495–510. [Google Scholar] [CrossRef]
- Luo, J.L.; Liou, Y.J.; Chin, T.M.; Kuo, Y.M.; Chao, D.Y. Water vapor-permeable polyurethane ionomer. J. Appl. Polym. Sci. 2006, 101, 3767–3773. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Dulmaev, S.E.; Sazonov, O.O.; Gumerov, A.M.; Davletbaev, R.S.; Valiullin, L.R.; Ibragimov, R.G. Polyurethane based on modified aminoethers of boric acid. Polym. Sci. Ser. B 2020, 20, 295–305. [Google Scholar]
- Davletbaeva, I.M.; Dulmaev, S.E.; Sazonov, O.O.; Klinov, A.V.; Davletbaev, R.S.; Gumerov, A.M. Water vapor permeable polyurethane films based on the hyperbranched aminoethers of boric acid. RSC Adv. 2019, 9, 23535–23544. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Polyurethane ionomers based on amino ethers of orto-phosphoric acid. RSC Adv. 2019, 9, 18599–18608. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Sazonov, O.O.; Fazlyev, A.R.; Zakirov, I.N.; Davletbaev, R.S.; Efimov, S.V.; Klochkov, V.V. Thermal behavior of polyurethane ionomers based on amino ethers of ortho-phosphoric acid. Polym. Sci. Ser. A 2020, 62, 337–349. [Google Scholar] [CrossRef]


| Polyurethane | Water in Permeate, wt.% | Flux, g/m2·h | Separation Factor | PSI, g/m2·h |
|---|---|---|---|---|
| Feed temperature, 40 °C | ||||
| AEPA-3-PPG-1000-PU | 84.4 | 880 | 51 | 44 |
| AEPA-4-PPG-1000-PU | 83.2 | 1021 | 49 | 49 |
| AEPA-5-PPG-1000-PU | 82.8 | 1145 | 52 | 58.4 |
| AEPA-6-PPG-1000-PU | 80.9 | 1250 | 45 | 52.8 |
| AEPA-3-PEG-400-PU | 96.4 | 653 | 151 | 98.0 |
| AEPA-4-PEG-400-PU | 96.3 | 805 | 148 | 118.3 |
| AEPA-5-PEG-400-PU | 90.9 | 2039 | 56 | 112.2 |
| AEPA-6-PEG-400-PU | 90.1 | 1580 | 65 | 101.1 |
| Feed temperature, 60 °C | ||||
| AEPA-3-PPG-1000-PU | 83.0 | 1800 | 28 | 48.6 |
| AEPA-4-PPG-1000-PU | 82.0 | 2551 | 26 | 56.3 |
| AEPA-5-PPG-1000-PU | 81.1 | 2655 | 24 | 74.3 |
| AEPA-6-PPG-1000-PU | 79.1 | 2853 | 21 | 57.1 |
| AEPA-3-PEG-400-PU | 92.7 | 1859 | 72 | 132.0 |
| AEPA-4-PEG-400-PU | 92.6 | 2298 | 71 | 161.0 |
| AEPA-5-PEG-400-PU | 90.5 | 3734 | 54 | 197.9 |
| AEPA-6-PEG-400-PU | 89.2 | 2624 | 62 | 160.0 |
| Polyurethane * | T5% (°C) | T10% (°C) | T50% (°C) | Coke Content at 600 °C, wt.% |
|---|---|---|---|---|
| AEPA-3-PPG-1000-PU | 278/271 | 292/284 | 369/394 | 5/2 |
| AEPA-4-PPG-1000-PU | 275/266 | 292/283 | 381/414 | 3.5/6 |
| AEPA-5-PPG-1000-PU | 277/268 | 293/282 | 378/410 | 4/6 |
| AEPA-6-PPG-1000-PU | 280/263 | 294/284 | 383/401 | 5/6 |
| Polyurethane * | T5% (°C) | T10% (°C) | T50% (°C) | Coke Content at 600 °C, wt.% |
|---|---|---|---|---|
| AEPA-3-PPG-1000-PU | 308/296 | 322/313 | 362/364 | 17/20 |
| AEPA-4-PPG-1000-PU | 235/295 | 268/309 | 310/352 | 16/19.1 |
| AEPA-5-PPG-1000-PU | 220/291 | 243/308 | 300/349 | 14/18.5 |
| AEPA-6-PPG-1000-PU | 275/294 | 300/312 | 350/350 | 15/16 |
| EPA-3-PPG-1000-PU | 300/298 | 317/318 | 365/372 | 18/26 |
| EPA-4-PPG-1000-PU | 295/298 | 311/316 | 355/368 | 17/21 |
| EPA-5-PPG-1000-PU | 280/296 | 301/318 | 350/359 | 16/16.7 |
| EPA-6-PPG-1000-PU | 280/282 | 300/300 | 350/350 | 17/16.3 |
| Polyurethane | Density, g/cm3 |
|---|---|
| AEPA-3-PEG-400-PU | 1.193 |
| AEPA-4-PEG-400-PU | 1.194 |
| AEPA-5-PEG-400-PU | 1.223 |
| AEPA-6-PEG-400-PU | 1.229 |
| AEPA-3-PPG-1000-PU | 1.175 |
| AEPA-4-PPG-1000-PU | 1.184 |
| AEPA-5-PPG-1000-PU | 1.185 |
| AEPA-6-PPG-1000-PU | 1.189 |
| Polyurethane | T5% (°C) | T10% (°C) | T50% (°C) | Coke Content at 600 °C, wt.% |
|---|---|---|---|---|
| AEPA-3-PEG-400-PU | 291 | 315 | 383 | 26.5 |
| AEPA-4-PEG-400-PU | 292 | 316 | 385 | 27.5 |
| AEPA-5-PEG-400-PU | 296 | 313 | 390 | 28.5 |
| AEPA-6-PEG-400-PU | 290 | 309 | 398 | 30.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletbaeva, I.M.; Sazonov, O.O.; Zakirov, I.N.; Gumerov, A.M.; Klinov, A.V.; Fazlyev, A.R.; Malygin, A.V. Organophosphorus Polyurethane Ionomers as Water Vapor Permeable and Pervaporation Membranes. Polymers 2021, 13, 1442. https://doi.org/10.3390/polym13091442
Davletbaeva IM, Sazonov OO, Zakirov IN, Gumerov AM, Klinov AV, Fazlyev AR, Malygin AV. Organophosphorus Polyurethane Ionomers as Water Vapor Permeable and Pervaporation Membranes. Polymers. 2021; 13(9):1442. https://doi.org/10.3390/polym13091442
Chicago/Turabian StyleDavletbaeva, Ilsiya M., Oleg O. Sazonov, Ilyas N. Zakirov, Askhat M. Gumerov, Alexander V. Klinov, Azat R. Fazlyev, and Alexander V. Malygin. 2021. "Organophosphorus Polyurethane Ionomers as Water Vapor Permeable and Pervaporation Membranes" Polymers 13, no. 9: 1442. https://doi.org/10.3390/polym13091442
APA StyleDavletbaeva, I. M., Sazonov, O. O., Zakirov, I. N., Gumerov, A. M., Klinov, A. V., Fazlyev, A. R., & Malygin, A. V. (2021). Organophosphorus Polyurethane Ionomers as Water Vapor Permeable and Pervaporation Membranes. Polymers, 13(9), 1442. https://doi.org/10.3390/polym13091442









