Chemical Imaging of Single Anisotropic Polystyrene/Poly (Methacrylate) Microspheres with Complex Hierarchical Architecture
Abstract
1. Introduction
2. Materials and Methods
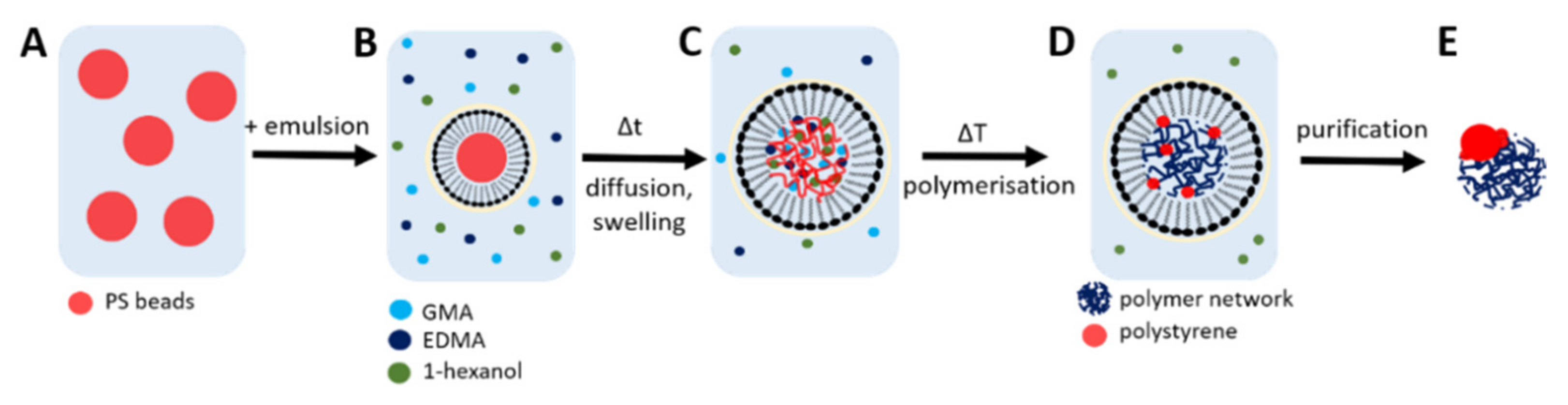
2.1. Particle Synthesis
2.2. Particle Characterization
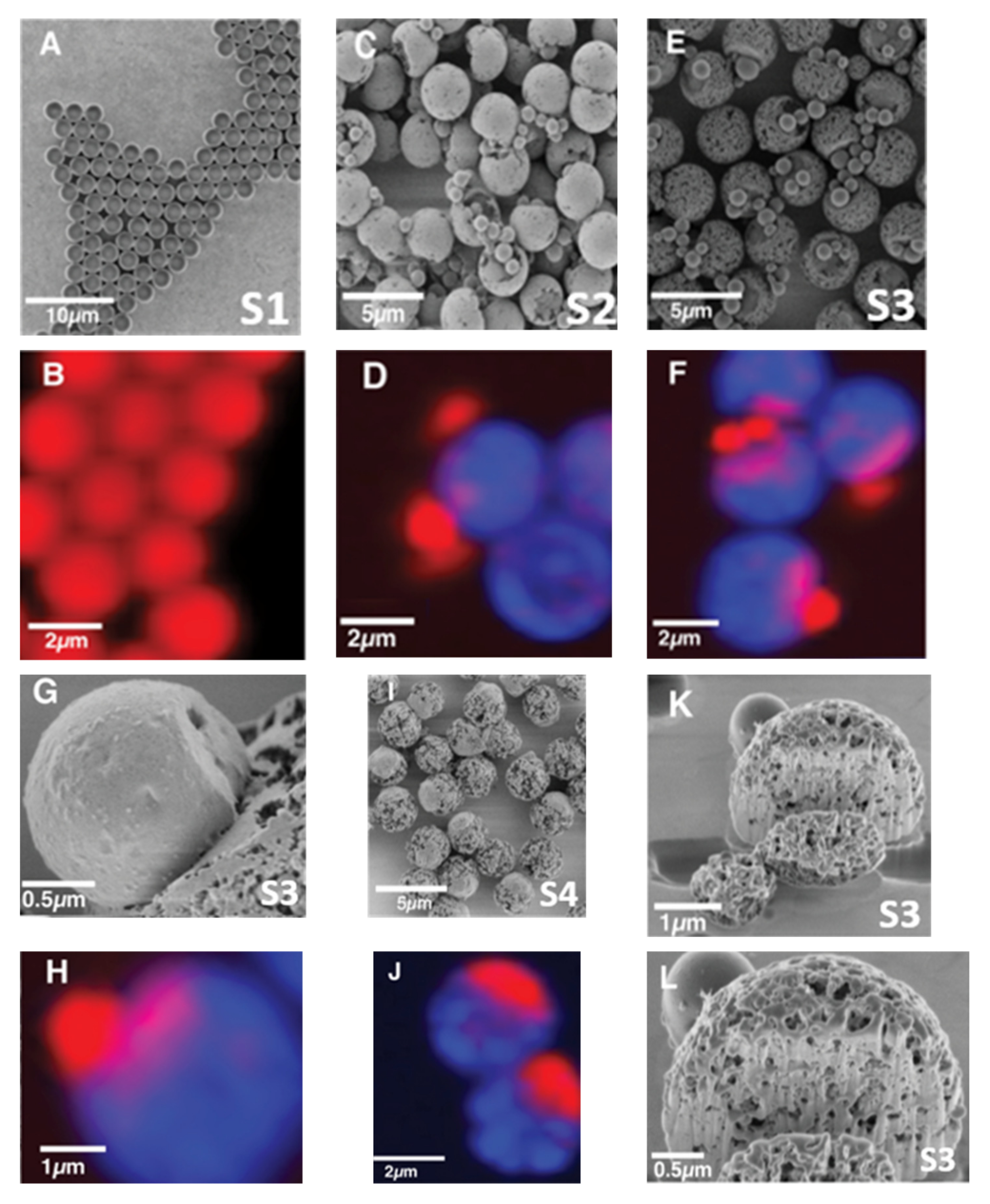
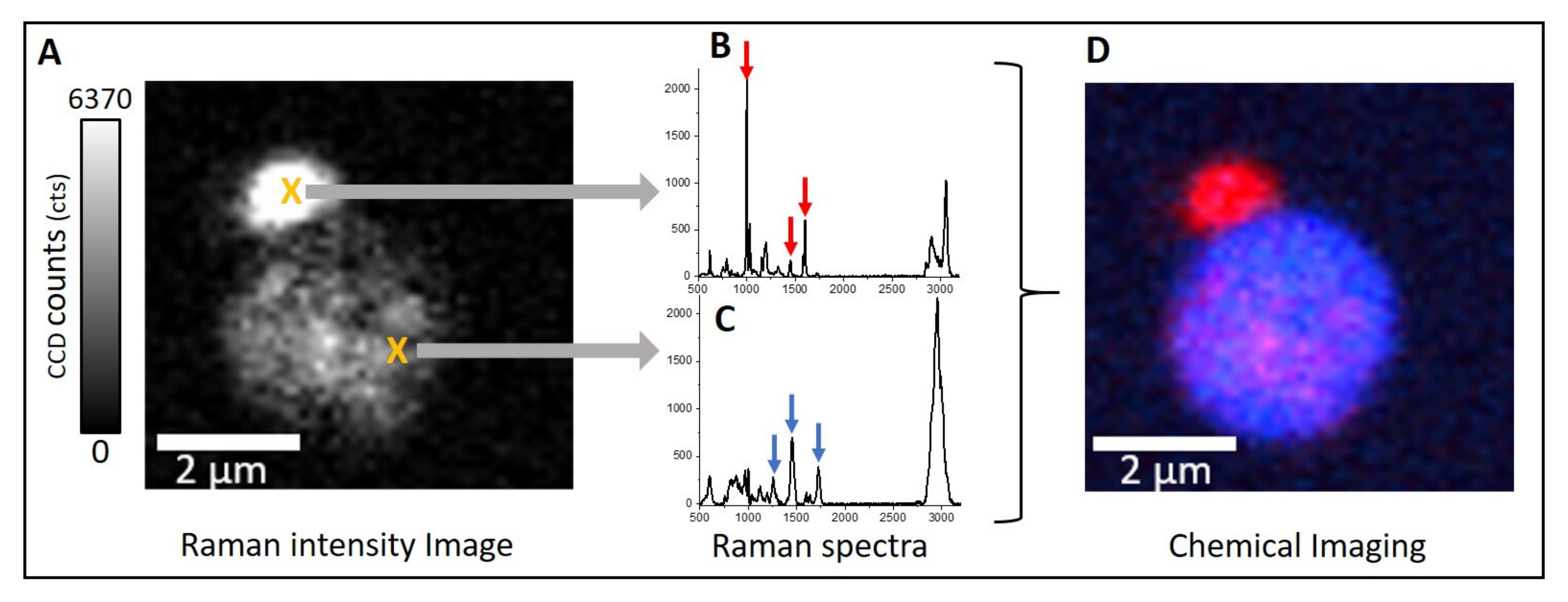
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulka, C.P.; Swartz, J.D.; Trantum, J.R.; Davis, K.M.; Peak, C.M.; Denton, A.J.; Haselton, F.R.; Wright, D.W. Coffee rings as low-resource diagnostics: Detection of the malaria biomarker Plasmodium falciparum histidine-rich protein-II using a surface-coupled ring of Ni(II)NTA gold-plated polystyrene particles. ACS Appl. Mater. Interfaces 2014, 6, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.-A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an ex Vivo Human Placental Perfusion Model. Environ. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, V.; Musyanovych, A.; Landfester, K.; Lorenz, M.R.; Mailänder, V. Preparation of Fluorescent Carboxyl and Amino Functionalized Polystyrene Particles by Miniemulsion Polymerization as Markers for Cells. Macromol. Chem. Phys. 2005, 206, 2440–2449. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Xue, Y.; Xia, W.; Ray, T.R.; Reeder, J.T.; Bandodkar, A.J.; Kang, D.; Xu, S.; Huang, Y.; Rogers, J.A. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip 2017, 17, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-B.; Song, Y.; Liu, H.; Lu, Z.; Zhang, F.; Liu, H.; Meng, J.; Gu, L.; Wang, S.; Jiang, L. A general strategy to synthesize chemically and topologically anisotropic Janus particles. Sci. Adv. 2017, 3, e1603203. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cao, L.; Tan, L. Synthesis of sea urchin-like polystyrene/polyaniline microspheres by seeded swelling polymerization and their catalytic application. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 678–684. [Google Scholar] [CrossRef]
- Shim, S.-E.; Cha, Y.-J.; Byun, J.-M.; Choe, S. Size control of polystyrene beads by multistage seeded emulsion polymerization. J. Appl. Polym. Sci. 1999, 71, 2259–2269. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Zhong, J.; Li, W.; Wei, Q.; Wang, K. Molecularly imprinted polymer microspheres prepared via the two-step swelling polymerization for the separation of lincomycin. J. Appl. Polym. Sci. 2019, 136, 47938. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, C.; Zhao, Z.; Xue, G. Controlling the morphology of micrometre-size polystyrene/polyaniline composite particles by Swelling–Diffusion–Interfacial-Polymerization Method. Polymer 2011, 52, 409–414. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.; Lee, W.-E.; Byun, D.-J.; Kim, M.H. One-Step Synthesis of Hollow Dimpled Polystyrene Microparticles by Dispersion Polymerization. Langmuir 2017, 33, 2275–2282. [Google Scholar] [CrossRef]
- Omer-Mizrahi, M.; Margel, S. Synthesis and characterization of spherical and hemispherical polyepoxide micrometer-sized particles of narrow size distribution by a single-step swelling of uniform polystyrene template microspheres with glycidyl methacrylate. J. Polym. Sci. A Polym. Chem. 2007, 45, 4612–4622. [Google Scholar] [CrossRef]
- Galperin, A.; Margel, S. Synthesis and characterization of new micrometer-sized radiopaque polymeric particles of narrow size distribution by a single-step swelling of uniform polystyrene template microspheres for X-ray imaging applications. Biomacromolecules 2006, 7, 2650–2660. [Google Scholar] [CrossRef]
- Boguslavsky, L.; Margel, S. Synthesis and characterization of micrometer-sized homo and composite polyacrylonitrile particles of narrow size distribution on the basis of single-step swelling of uniform polystyrene template microspheres. J. Polym. Sci. A Polym. Chem. 2004, 42, 4847–4861. [Google Scholar] [CrossRef]
- Bryce, D.A.; Kitt, J.P.; Harris, J.M. Confocal Raman Microscopy Investigation of Molecular Transport into Individual Chromatographic Silica Particles. Anal. Chem. 2017, 89, 2755–2763. [Google Scholar] [CrossRef]
- Wang, W.-P.; Pan, C.-Y. Preparation and characterization of polystyrene/graphite composite prepared by cationic grafting polymerization. Polymer 2004, 45, 3987–3995. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Stachowiak, G.W. Characterization of surface topography of wear particles by SEM stereoscopy. Wear 1997, 206, 39–52. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829. [Google Scholar] [CrossRef]
- Chatterjee, J.; Haik, Y.; Chen, C.-J. Modification and characterization of polystyrene-based magnetic microspheres and comparison with albumin-based magnetic microspheres. J. Magn. Magn. Mater. 2001, 225, 21–29. [Google Scholar] [CrossRef]
- Nagy, J.B. Multinuclear NMR characterization of microemulsions: Preparation of monodisperse colloidal metal boride particles. Colloids Surf. 1989, 35, 201–220. [Google Scholar] [CrossRef]
- Leu, G.; Liu, Y.; Werstler, D.D.; Cory, D.G. NMR Characterization of Elastomer−Carbon Black Interactions. Macromolecules 2004, 37, 6883–6891. [Google Scholar] [CrossRef]
- Stinner, C.; Tang, Z.; Haouas, M.; Weber, T.; Prins, R. Preparation and 31P NMR Characterization of Nickel Phosphides on Silica. J. Catal. 2002, 208, 456–466. [Google Scholar] [CrossRef]
- Santamaria, A.; Mondragon, F.; Molina, A.; Marsh, N.; Eddings, E.; Sarforim, A. FT-IR and 1H NMR characterization of the products of an ethylene inverse diffusion flame. Combust. Flame 2006, 146, 52–62. [Google Scholar] [CrossRef]
- Joshi, R.; Feldmann, V.; Koestner, W.; Detje, C.; Gottschalk, S.; Mayer, H.A.; Sauer, M.G.; Engelmann, J. Multifunctional silica nanoparticles for optical and magnetic resonance imaging. Biol. Chem. 2013, 394, 125–135. [Google Scholar] [CrossRef]
- Lica, G.C.; Zelakiewicz, B.S.; Tong, Y.Y. Electrochemical and NMR characterization of octanethiol-protected Au nanoparticles. J. Electroanal. Chem. 2003, 554–555, 127–132. [Google Scholar] [CrossRef]
- Markervich, E.; Salitra, G.; Levi, M.D.; Aurbach, D. Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods, Raman spectroscopy and SEM. J. Power Sources 2005, 146, 146–150. [Google Scholar] [CrossRef]
- Santini, A.; Miletic, V. Comparison of the hybrid layer formed by Silorane adhesive, one-step self-etch and etch and rinse systems using confocal micro-Raman spectroscopy and SEM. J. Dent. 2008, 36, 683–691. [Google Scholar] [CrossRef]
- Jin, Q.; Li, M.; Polat, B.; Paidi, S.K.; Dai, A.; Zhang, A.; Pagaduan, J.V.; Barman, I.; Gracias, D.H. Mechanical Trap Surface-Enhanced Raman Spectroscopy for Three-Dimensional Surface Molecular Imaging of Single Live Cells. Angew. Chem. 2017, 129, 3880–3884. [Google Scholar] [CrossRef]
- Liu, B.; Möhwald, H.; Wang, D. Synthesis of Janus particles via kinetic control of phase separation in emulsion droplets. Chem. Commun. 2013, 49, 9746–9748. [Google Scholar] [CrossRef]
- Chang, F.; Ouhajji, S.; Townsend, A.; Sanogo Lacina, K.; van Ravensteijn, B.G.P.; Kegel, W.K. Controllable synthesis of patchy particles with tunable geometry and orthogonal chemistry. J. Colloid Interface Sci. 2021, 582, 333–341. [Google Scholar] [CrossRef]
- Maisch, J.; Jafarli, F.; Chassé, T.; Blendinger, F.; Konrad, A.; Metzger, M.; Meixner, A.J.; Brecht, M.; Dähne, L.; Mayer, H.A. One-pot synthesis of micron partly hollow anisotropic dumbbell shaped silica core-shell particles. Chem. Commun. 2016, 52, 14392–14395. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Gibson, C.T.; Yoshida, M.; Nandivada, H.; Deng, X.; Voelcker, N.H.; Lahann, J. Engineering, characterization and directional self-assembly of anisotropically modified nanocolloids. Small 2011, 7, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, Y.; Harada, Y.; Takamatsu, T.; Tanaka, H. Label-free Molecular Imaging and Analysis by Raman Spectroscopy. Acta Histochem. Cytochem. 2018, 51, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Walkenfort, B.; Schlücker, S. Label-free SERS monitoring of chemical reactions catalyzed by small gold nanoparticles using 3D plasmonic superstructures. J. Am. Chem. Soc. 2013, 135, 1657–1660. [Google Scholar] [CrossRef]
- Socrates, G. Polymers—Macromolecules. In Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons LTD: Chichester, UK, 2015; ISBN 0470093072. [Google Scholar]
- Anema, J.R.; Brolo, A.G.; Felten, A.; Bittencourt, C. Surface-enhanced Raman scattering from polystyrene on gold clusters. J. Raman Spectrosc. 2010, 41, 745–751. [Google Scholar] [CrossRef]
- Palm, A. Raman Spectrum of Polystyrene. J. Phys. Chem. 1951, 55, 1320–1324. [Google Scholar] [CrossRef]
- Filipecka, K.; Miedziński, R.; Sitarz, M.; Filipecki, J.; Makowska-Janusik, M. Optical and vibrational properties of phosphorylcholine-based contact lenses-Experimental and theoretical investigations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 176, 83–90. [Google Scholar] [CrossRef]
- Boerio, F.J.; Yuann, J.K. Determination of copolymer composition by Raman spectroscopy. J. Polym. Sci. Polym. Phys. Ed. 1973, 11, 1841–1848. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Du Prez, F.E. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Gong, B.-L.; Ke, C.-Y.; Geng, X.-D. Synthesis of Monodisperse Poly(glycidylmethacrylate-co-ethylene dimethacrylate) Beads and Their Application in Separation of Biopolymers. Chin. J. Chem. 2004, 22, 283–289. [Google Scholar] [CrossRef]
- Gong, B.; Ke, C.; Geng, X. Preparation of weak cation exchange packings based on monodisperse poly(glycidyl methacrylate-co-ethylene dimethacrylate) beads and their chromatographic properties. Anal. Bioanal. Chem. 2003, 375, 769–774. [Google Scholar] [CrossRef]
- Kutuzova, L.; Kandelbauer, A. Self-healing thermosets. In Handbook of Thermoset Plastics, 4th ed.; Dodiuk, H., Ed.; William Andrew Inc. & Elsevier: Norwich, NY, USA, 2021; ISBN 978-0-12-821632-3. [Google Scholar]
- Urdl, K.; Weiss, S.; Karpa, A.; Peric, M.; Zikulnig-Rusch, E.M.; Brecht, M.; Kandelbauer, A.; Müller, U.; Kern, W. Furan-functionalized melamine-formaldehyde particles performing Diels-Alder reactions. Eur. Polym. J. 2018, 108, 225–234. [Google Scholar] [CrossRef]
- Urdl, K.; Weiss, S.; Brodbeck, B.; Kandelbauer, A.; Zikulnig-Rusch, E.M.; Müller, U.; Kern, W. Homogeneous, monodispersed furan-melamine particles performing reversible binding and forming networks. Eur. Polym. J. 2019, 116, 158–168. [Google Scholar] [CrossRef]
- Urdl, K.; Kandelbauer, A.; Kern, W.; Müller, U.; Thebault, M.; Zikulnig-Rusch, E.M. Self-healing of densely crosslinked thermoset polymers—a critical review. Prog. Org. Coat. 2017, 104, 232–249. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Meng, T.; Yang, J.; Huang, B.; Gu, F.L.; Zhang, S.; Meng, C.; Tong, Y. Boosting Electron Transfer with Heterointerface Effect for High-Performance Lithium-Ion Storage. Energy Storage Mater. 2021, 36, 365–375. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Meng, T.; Huang, B.; Hu, L.; Su, H.; Meng, C.; Tong, Y. Engineering Heterostructure-Incorporated Metal Silicates Anchored on Carbon Nanotubes for Highly Durable Lithium Storage. ACS Appl. Energy Mater. 2021, 4, 1548–1559. [Google Scholar] [CrossRef]
- Meng, T.; Li, B.; Wang, Q.; Hao, J.; Huang, B.; Gu, F.L.; Xu, H.; Liu, P.; Tong, Y. Large-Scale Electric-Field Confined Silicon with Optimized Charge-Transfer Kinetics and Structural Stability for High-Rate Lithium-Ion Batteries. ACS Nano 2020, 14, 7066–7076. [Google Scholar] [CrossRef]
- Barbé, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Xia, H.; Wan, G.; Yang, F.; Wang, J.; Bai, Q. Preparation of monodisperse large-porous silica microspheres with polymer microspheres as the templates for protein separation. Mater. Lett. 2016, 180, 19–22. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, A.; Wagner, S.; Bredfeldt, J.-E.; Steinbach, J.C.; Mukherjee, A.; Kronenberger, S.; Braun, K.; Kandelbauer, A.; Mayer, H.A.; Brecht, M. Chemical Imaging of Single Anisotropic Polystyrene/Poly (Methacrylate) Microspheres with Complex Hierarchical Architecture. Polymers 2021, 13, 1438. https://doi.org/10.3390/polym13091438
Wagner A, Wagner S, Bredfeldt J-E, Steinbach JC, Mukherjee A, Kronenberger S, Braun K, Kandelbauer A, Mayer HA, Brecht M. Chemical Imaging of Single Anisotropic Polystyrene/Poly (Methacrylate) Microspheres with Complex Hierarchical Architecture. Polymers. 2021; 13(9):1438. https://doi.org/10.3390/polym13091438
Chicago/Turabian StyleWagner, Alexandra, Stefanie Wagner, Jan-Erik Bredfeldt, Julia C. Steinbach, Ashutosh Mukherjee, Sandra Kronenberger, Kai Braun, Andreas Kandelbauer, Hermann A. Mayer, and Marc Brecht. 2021. "Chemical Imaging of Single Anisotropic Polystyrene/Poly (Methacrylate) Microspheres with Complex Hierarchical Architecture" Polymers 13, no. 9: 1438. https://doi.org/10.3390/polym13091438
APA StyleWagner, A., Wagner, S., Bredfeldt, J.-E., Steinbach, J. C., Mukherjee, A., Kronenberger, S., Braun, K., Kandelbauer, A., Mayer, H. A., & Brecht, M. (2021). Chemical Imaging of Single Anisotropic Polystyrene/Poly (Methacrylate) Microspheres with Complex Hierarchical Architecture. Polymers, 13(9), 1438. https://doi.org/10.3390/polym13091438







