Self-Organization in Dilute Aqueous Solutions of Thermoresponsive Star-Shaped Six-Arm Poly-2-Alkyl-2-Oxazines and Poly-2-Alkyl-2-Oxazolines
Abstract
1. Introduction
2. Materials and Methods
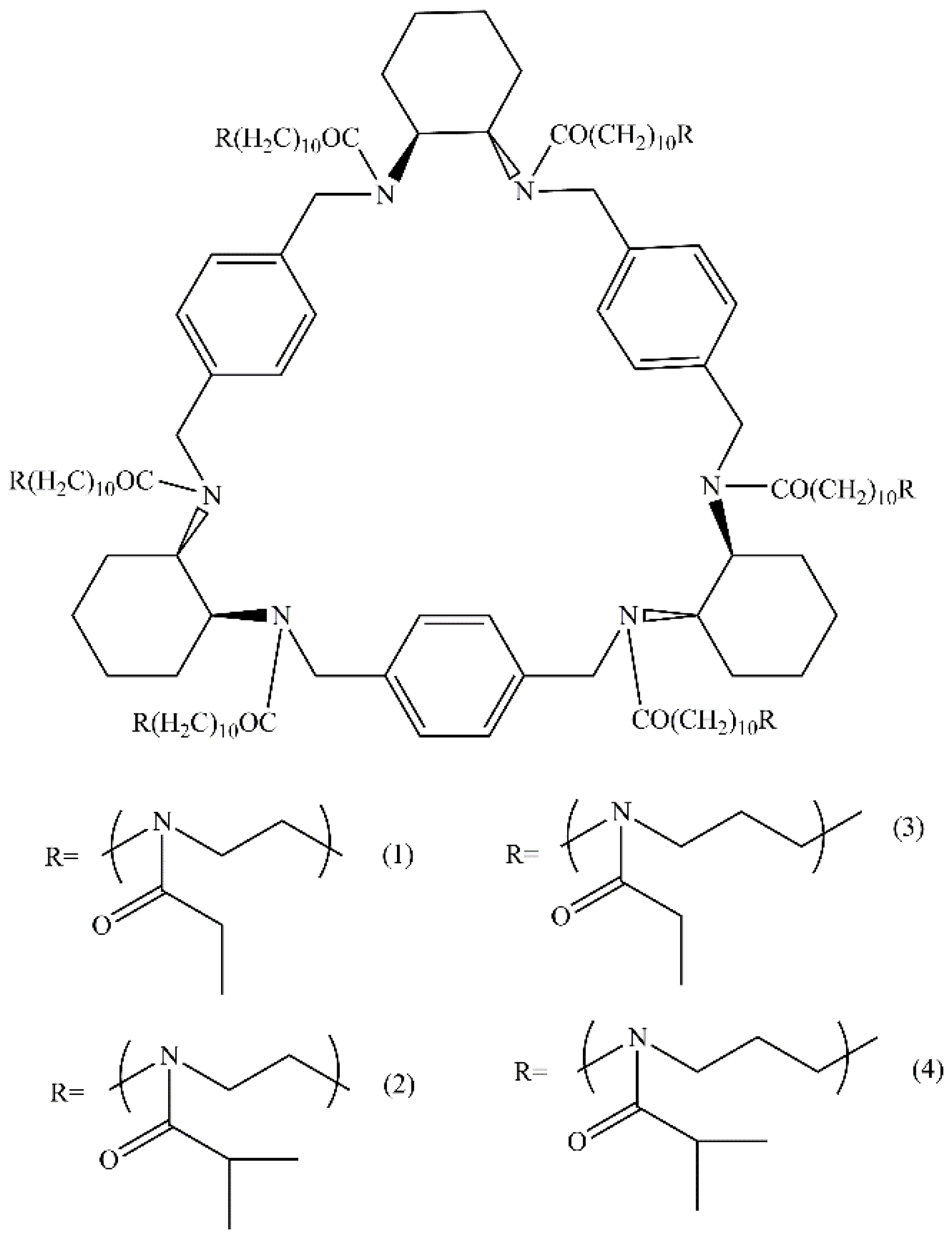
2.1. Polymer Star Synthesis
2.2. Solution Investigation
3. Results and Discussion
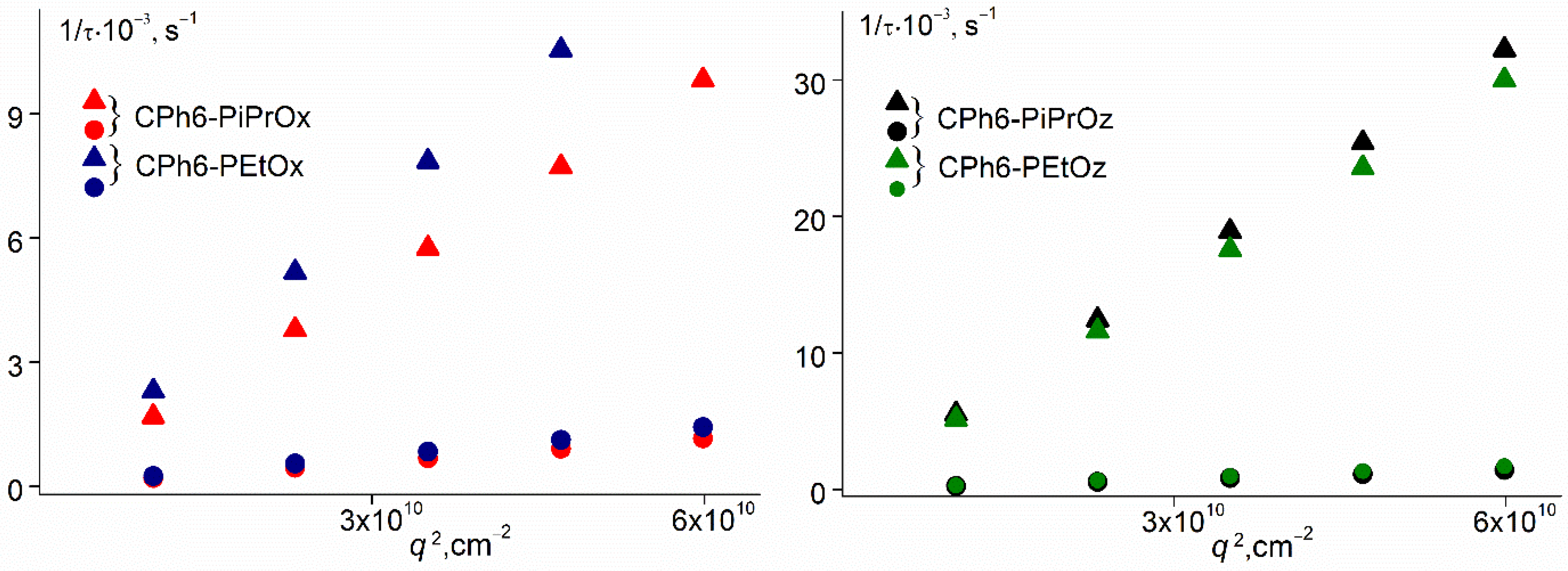
3.1. Behavior of Star-Shaped Six-Arm Pseudo-Polypeptoids in Aqueous Solutions at Low Temperatures
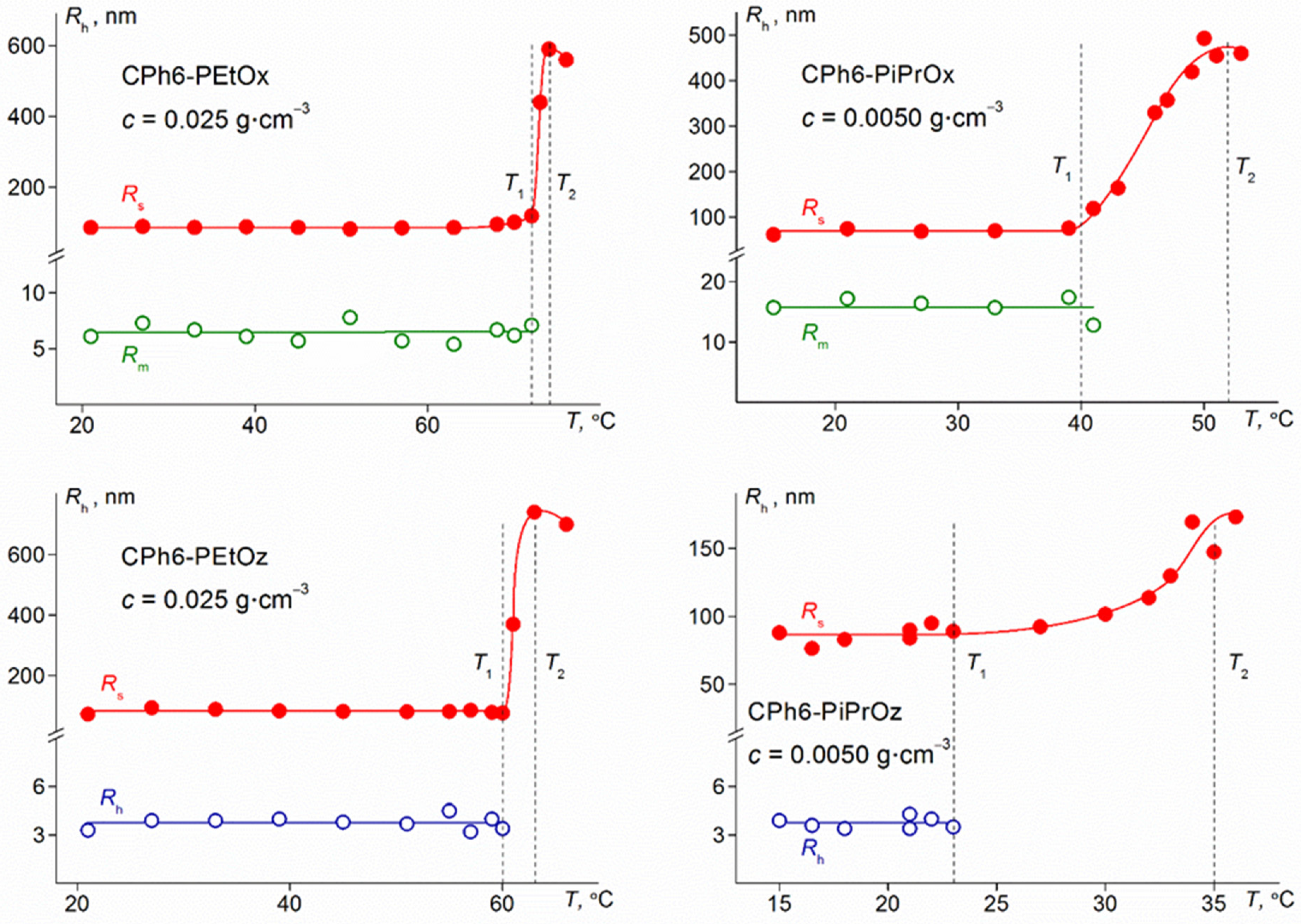
3.2. Behavior of Star-Shaped Six-Arm Pseudo-Polypeptoids in Aqueous Solutions on Heating
3.3. Concentration Dependences of Phase Separation Temperatures for Aqueous Solutions of Investigated Polymer Stars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2017, 67, 32–45. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Zahoranova, A.; Luxenhofer, R. Poly(2-oxazoline)- and poly(2-oxazine)-based self-assemblies, polyplexes, and drug nanoformulations—An update. Adv. Healthcare Mater. 2021, 10, 2001382. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Jarrett, K.; Saunders, M.; Roth, P.J.; Buckley, C.E.; Lowe, A.B. Triply responsive soft matter nanoparticles based on poly[oligo(ethylene glycol) methyl ether methacrylate-block-3-phenylpropyl methacrylate] copolymers. Polym. Chem. 2016, 7, 2740–2750. [Google Scholar] [CrossRef]
- Verbraeken, B.; Monnery, B.D.; Lava, K.; Hoogenboom, R. The chemistry of poly(2-oxazoline)s. Eur. Polym. J. 2017, 88, 451–469. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, T.; Terao, K.; Qiu, X.-P.; Winnik, F.M. Self-association of a thermosensitive poly(alkyl-2-oxazoline) block copolymer in aqueous solution. Macromolecules 2012, 45, 6111–6119. [Google Scholar] [CrossRef]
- Caponi, P.F.; Qiu, X.P.; Vilela, F.; Winnik, F.M.; Ulijn, R.V. Phosphatase/temperature responsive poly(2-isopropyl-2-oxazoline). Polym. Chem. 2011, 2, 306–308. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A.; Pispas, S. Amphiphilic poly(2-oxazoline) copolymers as self-assembled carriers for drug delivery applications. Eur. Polym. J. 2017, 88, 516–523. [Google Scholar] [CrossRef]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive poly(2-oxazoline) molecular brushes by living ionic polymerization: Modulation of the cloud point by random and block copolymer pendant chains. Macromol. Chem. Phys. 2012, 213, 1963–1969. [Google Scholar] [CrossRef]
- Amirova, A.I.; Blokhin, A.N.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. The behavior of thermoresponsive star-shaped poly-2-isopropyl-2-oxazoline in saline media. Mendeleev Commun. 2019, 29, 472–474. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, V.R. Poly(2-oxazoline)s as materials for biomedical applications. J. Mater. Sci. Mater. 2013, 25, 1211–1225. [Google Scholar] [CrossRef]
- Drakalska, E.; Momekova, D.; Manolova, Y.; Budurova, D.; Momekov, G.; Genova, M.; Antonov, L.; Lambov, N.; Rangelov, S. Hybrid liposomal PEGylated calix[4]arene systems as drug delivery platforms for curcumin. Int. J. Pharm. 2014, 472, 165–174. [Google Scholar] [CrossRef]
- Morgese, G.; Verbraeken, B.; Ramakrishna, S.N.; Gombert, Y.; Cavalli, E.; Rosenboom, J.G.; Zenobi-Wong, M.; Spencer, N.D.; Hoogenboom, R. Chemical design of non-ionic polymer brushes as biointerfaces: Poly(2-oxazine)s outperform both poly(2-oxazoline)s and PEG. Angew. Chem. Int. Ed. 2018, 57, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Bloksma, M.M.; Schubert, U.S.; Hoogenboom, R. Poly(cyclic imino ether)s beyond 2-substituted-2-oxazolines. Macromol. Rapid Commun. 2011, 32, 1419–1441. [Google Scholar] [CrossRef]
- Sinnwell, S.; Ritter, H. Microwave accelerated polymerization of 2-phenyl-5,6-dihydro-4H-1,3-oxazine: Kinetics and influence of end-groups on glass transition temperature. Macromol. Rapid Commun. 2006, 27, 1335–1340. [Google Scholar] [CrossRef]
- Terashima, T.; Kojima, H.; Sawamoto, M. Core-imprinted star polymers via living radical polymerization: Precision cavity microgels for selective molecular recognition. Chem. Lett. 2014, 43, 1690–1692. [Google Scholar] [CrossRef]
- Kobayashi, S.; Igarashi, T.; Moriuchi, Y.; Saegusa, T. Block copolymers from cyclic imino ethers: A new class of nonionic polymer surfactant. Macromolecules 1986, 19, 535–541. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Paulus, R.M.; van Kuringen, H.P.C.; van der Woerdt, F.; Lambermont-Thijs, H.M.L.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive poly(2-oxazine)s. Macromol. Rapid Commun. 2011, 33, 92–96. [Google Scholar] [CrossRef]
- Lambermont-Thijs, H.M.L.; Fijten, M.W.M.; van der Linden, A.J.; van Lankvelt, B.M.; Bloksma, M.M.; Schubert, U.S.; Hoogenboom, R. Efficient cationic ring-opening polymerization of diverse cyclic imino ethers: Unexpected copolymerization behavior. Macromolecules 2011, 44, 4320–4325. [Google Scholar] [CrossRef]
- Meyer, M.; Antonietti, M.; Schlaad, H. Unexpected thermal characteristics of aqueous solutions of poly(2-isopropyl-2-oxazoline). Soft Matter 2007, 3, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Demirel, A.L.; Meyer, M.; Schlaad, H. Formation of polyamide nanofibers by directional crystallization in aqueous solution. Angew. Chem. Int. Ed. 2007, 46, 8622–8778. [Google Scholar] [CrossRef] [PubMed]
- Lübtow, M.M.; Hahn, L.; Haider, M.S.; Luxenhofer, R. Drug specificity, synergy and antagonism in ultrahigh capacity poly(2-oxazoline)/poly(2-oxazine) based formulations. J. Am. Chem. Soc. 2017, 139, 10980–10983. [Google Scholar] [CrossRef] [PubMed]
- Kirila, T.; Smirnova, A.; Razina, A.; Tenkovtsev, A.; Filippov, A. Synthesis and conformational characteristics of thermosensitive star-shaped six-arm polypeptoids. Polymers 2020, 12, 800. [Google Scholar] [CrossRef]
- Sezonenko, T.; Qiu, X.P.; Winnik, F.M.; Sato, T. Dehydration, micellization, and phase separation of thermosensitive polyoxazoline star block copolymers in aqueous solution. Macromolecules 2019, 52, 935–944. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Kronek, J.; Bosowska, K.; Trzebicka, B.; Dworak, A. Star poly(2-ethyl-2-oxazoline)s-synthesis and thermosensitivity. Polym. Int. 2011, 60, 1001–1009. [Google Scholar] [CrossRef]
- Angot, S.; Murthy, K.S.; Taton, D.; Gnanou, Y. Scope of the copper halide/bipyridyl system associated with calixarene-based multihalides for the synthesis of well-defined polystyrene and poly(meth)acrylate stars. Macromolecules 2000, 33, 7261–7274. [Google Scholar] [CrossRef]
- Strandman, S.; Hietala, S.; Aseyev, V.; Koli, B.; Butcher, S.J.; Tenhu, H. Supramolecular assemblies of amphiphilic PMMA-block-PAA stars in aqueous solutions. Polymer 2006, 47, 6524–6535. [Google Scholar] [CrossRef]
- Smith, G.W. Crystal Structure of a Nitrogen Isostere of Pentacyclo-Octacosadodecaene. Nature 1963, 198, 879. [Google Scholar] [CrossRef]
- Gawroński, J.; Kołbon, H.; Kwit, M.; Katrusiak, A. Designing large triangular chiral macrocycles: Efficient [3 + 3] diamine−dialdehyde condensations based on conformational bias. J. Org. Chem. 2000, 65, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Kirila, T.; Smirnova, A.; Razina, A.; Tenkovtsev, A.; Filippov, A. Influence of salt on the self-organization in solutions of star-shaped poly-2-alkyl-2-oxazoline and poly-2-alkyl-2-oxazine on heating. Polymers 2021, 13, 1152. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Influence of a hydrophobic core on thermoresponsive behavior of dendrimer-based star-shaped poly(2-isopropyl-2-oxazoline) in aqueous solutions. J. Polym. Res. 2017, 24, 124. [Google Scholar] [CrossRef]
- Salzinger, S.; Huber, S.; Jaksch, S.; Busch, P.; Jordan, R.; Papadakis, C.M. Aggregation behavior of thermo-responsive poly(2-oxazoline)s at the cloud point investigated by FCS and SANS. Colloid. Polym. Sci. 2012, 290, 385–400. [Google Scholar] [CrossRef]
- Krumm, C.; Fik, C.P.; Meuris, M.; Dropalla, G.J.; Geltenpoth, H.; Sickmann, A.; Tiller, J.C. Well-defined amphiphilic poly(2-oxazoline) ABA-triblock copolymers and their aggregation behavior in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, S.; Shi, W.; Liu, S. Two-stage collapse of unimolecular micelles with double thermoresponsive coronas. Langmuir 2006, 22, 989–997. [Google Scholar] [CrossRef]
- Kyriakos, K.; Aravopoulou, D.; Augsbach, L.; Sapper, J.; Ottinger, S.; Psylla, C.; Aghebat Rafat, A.; Benitez-Montoya, C.A.; Miasnikova, A.; Di, Z.; et al. Novel thermoresponsive block copolymers having different architectures—Structural, rheological, thermal, and dielectric investigations. Colloid. Polym. Sci. 2014, 292, 1757–1774. [Google Scholar] [CrossRef][Green Version]
- Witte, H.; Seeliger, W. Cyclische imidsäureester aus nitrilen und aminoalkoholen. Lieb. Ann. 1974, 6, 996–1009. [Google Scholar] [CrossRef]
- Trinh, L.T.T.; Lambermont-Thijs, H.M.L.; Schubert, U.S.; Hoogenboom, R.; Kjoniksen, A.L. Thermoresponsive poly(2-oxazoline) block copolymers exhibiting two cloud points: Complex multistep assembly behavior. Macromolecules 2012, 45, 4337–4345. [Google Scholar] [CrossRef]
- Steinschulte, A.A.; Schulte, B.; Rutten, S.; Eckert, T.; Okuda, J.; Moller, M.; Schneider, S.; Borisov, O.V.; Plamper, F.A. Effects of architecture on the stability of thermosensitive unimolecular micelles. Phys. Chem. Chem. Phys. 2014, 16, 4917–4932. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Amirova, A.; Golub, O.; Kirila, T.; Razina, A.; Tenkovtsev, A.; Filippov, A. Influence of arm length on aqueous solution behavior of thermosensitive poly(2-isopropyl-2-oxazoline) stars. Colloid Polym. Sci. 2017, 295, 117–124. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Self-organization in aqueous solutions of thermosensitive star-shaped and linear gradient copolymers of 2-ethyl-2-oxazoline and 2-isopropyl-2-oxazoline. Colloid Polym. Sci. 2020, 298, 535–546. [Google Scholar] [CrossRef]
- Smirnova, A.V.; Kirila, T.U.; Kurlykin, M.P.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of aqueous solutions of polymer star with block copolymer poly(2-ethyl-2-oxazoline) and poly(2-isopropyl- 2-oxazoline) arms. Int. J. Polym. Anal. Charact. 2017, 22, 677–684. [Google Scholar] [CrossRef]
- Kirila, T.U.; Smirnova, A.V.; Filippov, A.S.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. Thermosensitive star-shaped poly-2-ethyl-2-oxazine. Synthesis, structure characterization, conformation, and self-organization in aqueous solutions. Eur. Polym. J. 2019, 120, 109215. [Google Scholar] [CrossRef]
- Daoud, M.; Cotton, J.P. Star shaped polymers: A model for the conformation and its concentration dependence. J. Phys. 1982, 43, 531–538. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarasova, E.V.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix [8] arene core. Int. J. Polym. Anal. Charact. 2019, 24, 87–95. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solution, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions, 1st ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Øgendal, L.H. Light Scattering Demystified Theory and Practice; University of Copenhagen: Copenhagen, Danmark, 2017. [Google Scholar]
- Rodchenko, S.; Amirova, A.; Kurlykin, M.; Tenkovtsev, A.; Milenin, S.; Filippov, A. Amphiphilic molecular brushes with regular polydimethylsiloxane backbone and poly-2-isopropyl-2-oxazoline side chains. 2. Self-organization in aqueous solutions on heating. Polymers 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Kirile, T.Y.; Tobolina, A.I.; Elkina, A.A.; Kurlykin, M.P.; Ten’kovtsev, A.V.; Filippov, A.P. Self-assembly processes in aqueous solutions of heat-sensitive star-shaped poly-2-ethyl-2-oxazoline. Fibre Chem. 2018, 50, 248–251. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Mendrek, B.; Żymełka-Miara, I.; Libera, M.; Marcinkowski, A.; Trzebicka, B.; Smet, M.; Dworak, A. Solution behavior of star polymers with oligo(ethylene glycol) methyl ether methacrylate arms. Polymer 2012, 53, 5619–5631. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, D.; Wang, R.; Huan, X.; Tong, G.; Zhu, Q.; Yan, D.; Zhu, X. Temperature-induced emission enhancement of star conjugated copolymers with poly(2-(dimethylamino) ethyl methacrylate) coronas for detection of bacteria. Biomacromolecules 2013, 14, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Le Dévédec, F.; Strandman, S.; Baille, W.E.; Zhu, X.X. Functional star block copolymers with a cholane core: Thermo-responsiveness and aggregation behavior. Polymer 2013, 54, 3898–3903. [Google Scholar] [CrossRef]
- Privalov, P.L. Physical basis of the stability of the folded conformations of proteins. In Protein Folding; Creighton, T.E., Ed.; W.H. Freeman and Co.: New York, NY, USA, 1992; pp. 83–126. [Google Scholar]
- Tiktopulo, E.I.; Bychkova, V.E.; Ricka, J.; Ptitsyn, O.B. Cooperativity of the coil-globule transition in a homopolymer: Microcalorimetric study of Poly(N-sopropylacrylamide). Macromolecules 1994, 27, 2879–2882. [Google Scholar] [CrossRef]
- Ladbury, J.E.; Chowdhry, B.Z. Biocalorimetry: Applications of Calorimetry in the Biological Sciences; John Wiley and Sons: Chichester, UK, 1998. [Google Scholar]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Milenin, S.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Self-organization of thermosensitive star-shaped poly(2-isopropyl-2-oxazolines) influenced by arm number and generation of carbosilane dendrimer core in aqueous solutions. Colloid Polym. Sci. 2020, 298, 355–363. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Thermal cloud point fractionation of poly(vinyl alcohol-co-vinyl acetate): Partition of nanogels in the fractions. Polymers 2011, 3, 1065–1075. [Google Scholar] [CrossRef]

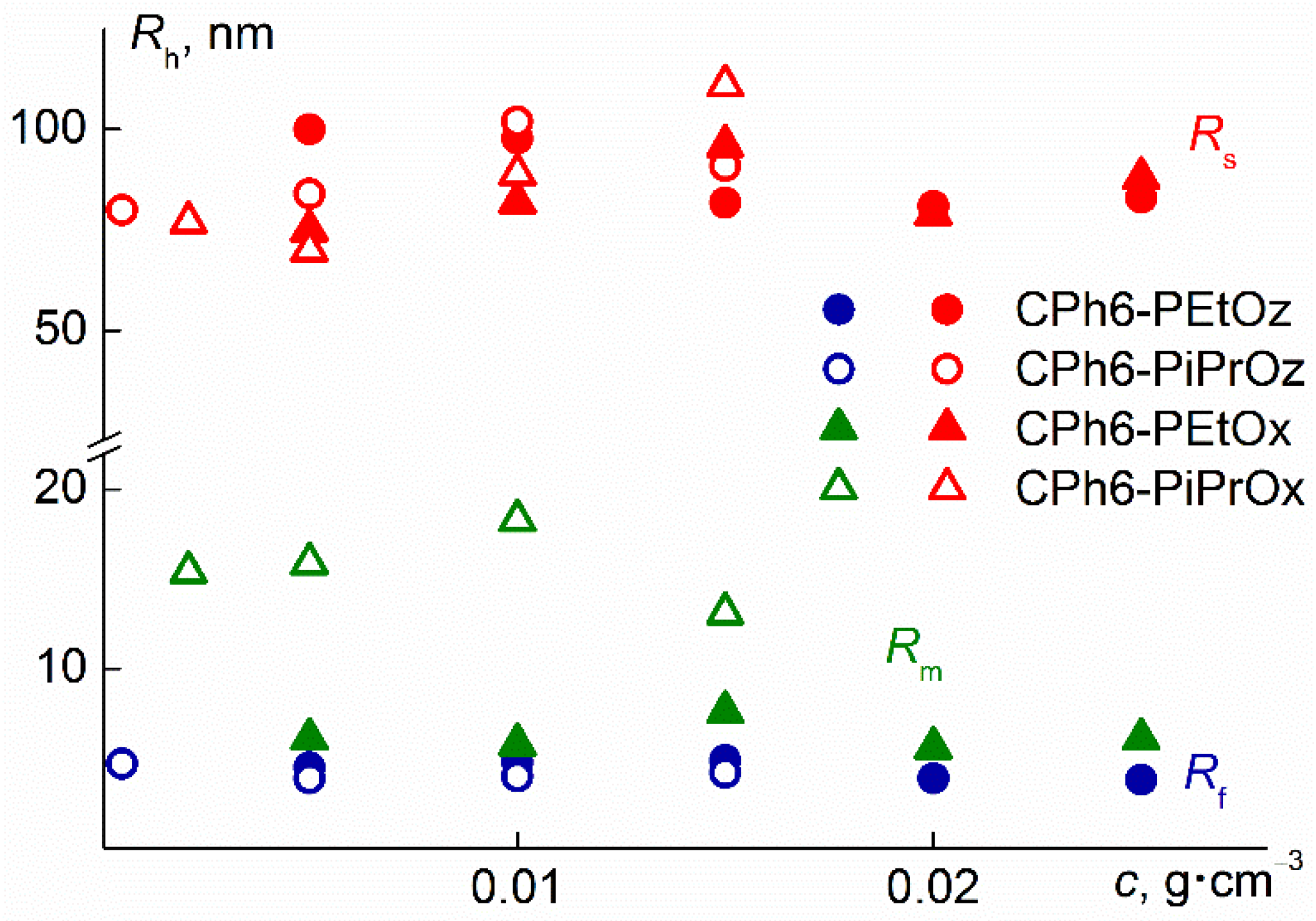




| Polymer | MsD, (1) g mol−1 | Đ(2) | Rh-D, (1) nm | <Rf>, nm | <Rm>, nm | <Rs>, nm | <Sf> | <Sm> | <Ss> | Cs |
|---|---|---|---|---|---|---|---|---|---|---|
| CPh6-PEtOz | 23,000 | 1.27 | 3.5 | 3.9 ± 0.3 | - | 89 ± 8 | 0.09 | - | 0.91 | 0.07 |
| CPh6-PiPrOz | 20,000 | 1.24 | 3.3 | 4.1 ± 0.3 | - | 89 ± 9 | 0.10 | - | 0.92 | 0.07 |
| CPh6-PEtOx | 15,000 | 1.29 | 3.0 | - | 6.3 ± 0.7 | 84 ± 7 | - | 0.35 | 0.65 | 0.06 |
| CPh6-PiPrOx | 14,000 | 1.19 | 2.6 | - | 15 ± 1 | 86 ± 12 | - | 0.84 | 0.16 | 0.08 |
| Polymer | ΔHvH, kJ/mol | ΔH, kJ/mol | n’ |
|---|---|---|---|
| CPh6-PEtOz | 6.0 | 0.3 | 20 |
| CPh6-PiPrOz | 1.8 | 2.4 | 0.75 |
| CPh6-PEtOx | 10.1 | 0.2 | 50 |
| CPh6-PiPrOx | 3.4 | 2.2 | 1.5 |
| Sample | Tonset, °C | Tp, °C | T1, °C | T2, °C |
|---|---|---|---|---|
| CPh6-PEtOz | 63 | 70 | 65 | 70 |
| CPh6-PiPrOz | 20 | 31 | 20 | 23 |
| CPh6-PEtOx | 75 | 83 | 74 | - |
| CPh6-PiPrOx | 33 | 47 | 34 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirila, T.; Smirnova, A.; Aseyev, V.; Tenkovtsev, A.; Tenhu, H.; Filippov, A. Self-Organization in Dilute Aqueous Solutions of Thermoresponsive Star-Shaped Six-Arm Poly-2-Alkyl-2-Oxazines and Poly-2-Alkyl-2-Oxazolines. Polymers 2021, 13, 1429. https://doi.org/10.3390/polym13091429
Kirila T, Smirnova A, Aseyev V, Tenkovtsev A, Tenhu H, Filippov A. Self-Organization in Dilute Aqueous Solutions of Thermoresponsive Star-Shaped Six-Arm Poly-2-Alkyl-2-Oxazines and Poly-2-Alkyl-2-Oxazolines. Polymers. 2021; 13(9):1429. https://doi.org/10.3390/polym13091429
Chicago/Turabian StyleKirila, Tatyana, Anna Smirnova, Vladimir Aseyev, Andrey Tenkovtsev, Heikki Tenhu, and Alexander Filippov. 2021. "Self-Organization in Dilute Aqueous Solutions of Thermoresponsive Star-Shaped Six-Arm Poly-2-Alkyl-2-Oxazines and Poly-2-Alkyl-2-Oxazolines" Polymers 13, no. 9: 1429. https://doi.org/10.3390/polym13091429
APA StyleKirila, T., Smirnova, A., Aseyev, V., Tenkovtsev, A., Tenhu, H., & Filippov, A. (2021). Self-Organization in Dilute Aqueous Solutions of Thermoresponsive Star-Shaped Six-Arm Poly-2-Alkyl-2-Oxazines and Poly-2-Alkyl-2-Oxazolines. Polymers, 13(9), 1429. https://doi.org/10.3390/polym13091429







