Controlling Growth of Poly (Triethylene Glycol Acrylate-Co-Spiropyran Acrylate) Copolymer Liquid Films on a Hydrophilic Surface by Light and Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dynamic Light Scattering
2.3. QCM-D Experiment
2.4. Data Evaluation
3. Results and Discussions
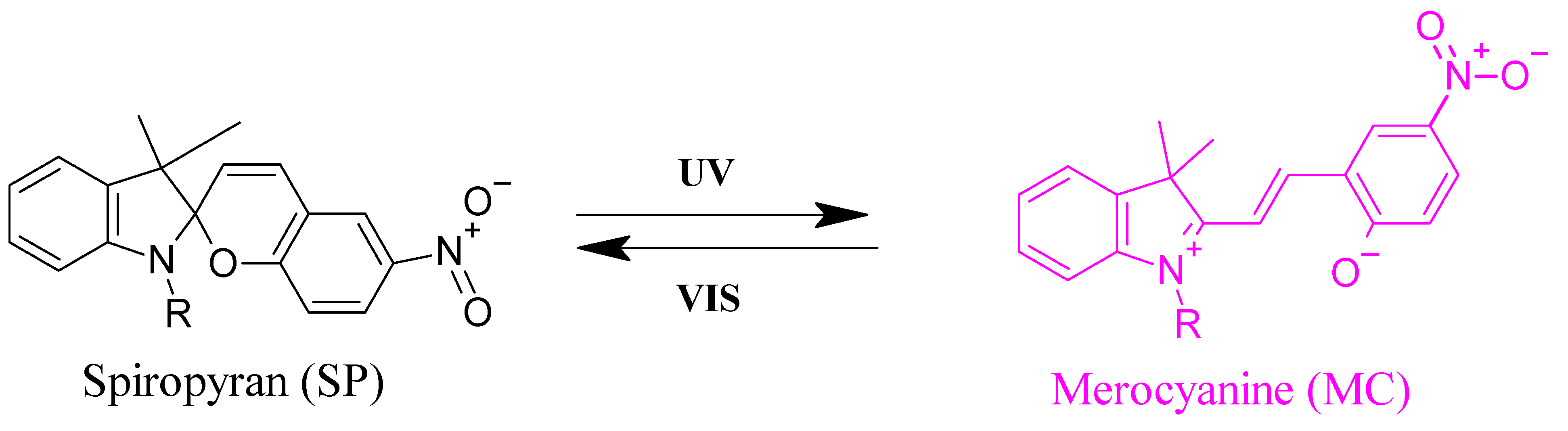
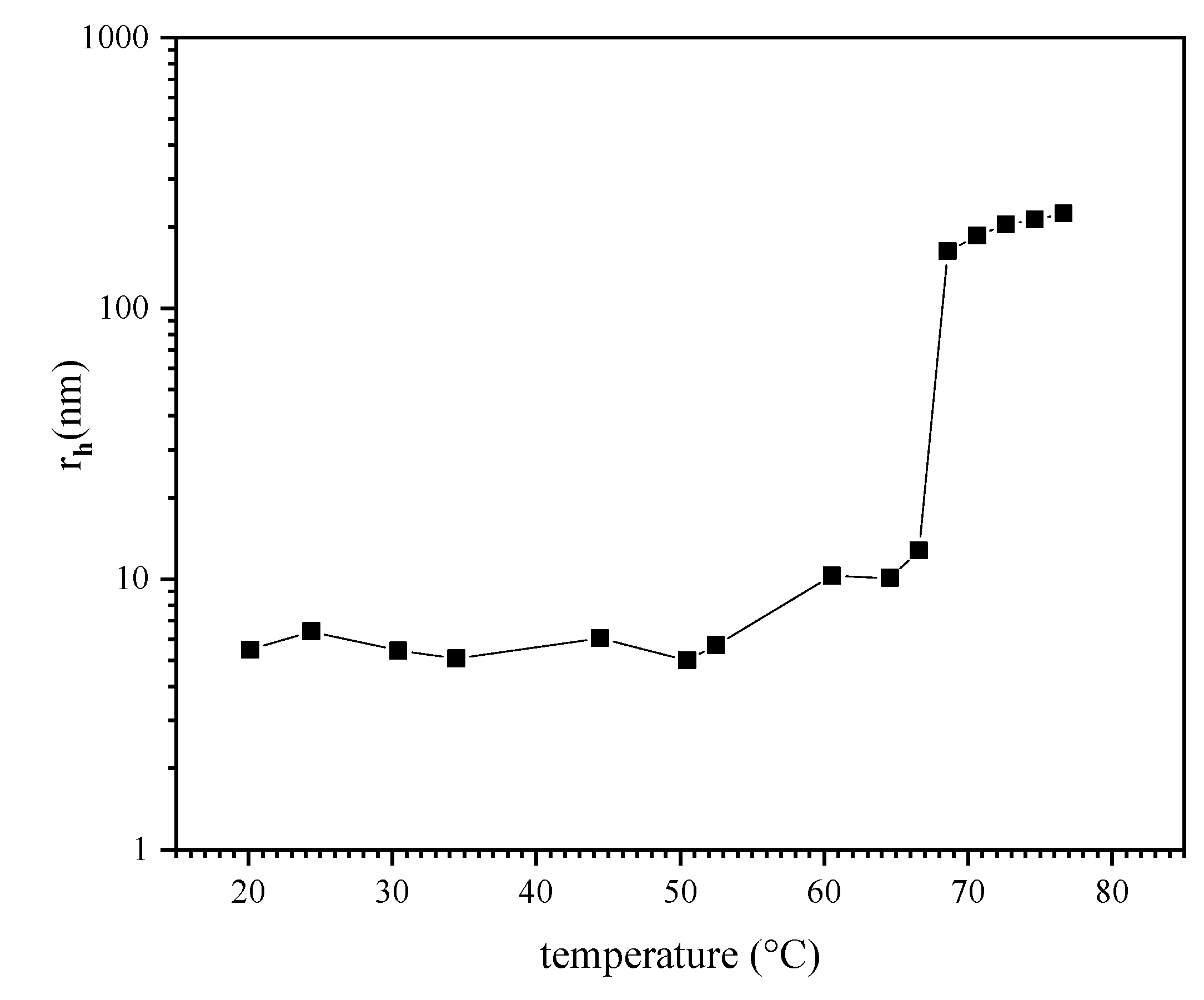
3.1. Phase Separation of P (TEGA-Co-SPA) in Dilute Aqueous Solution
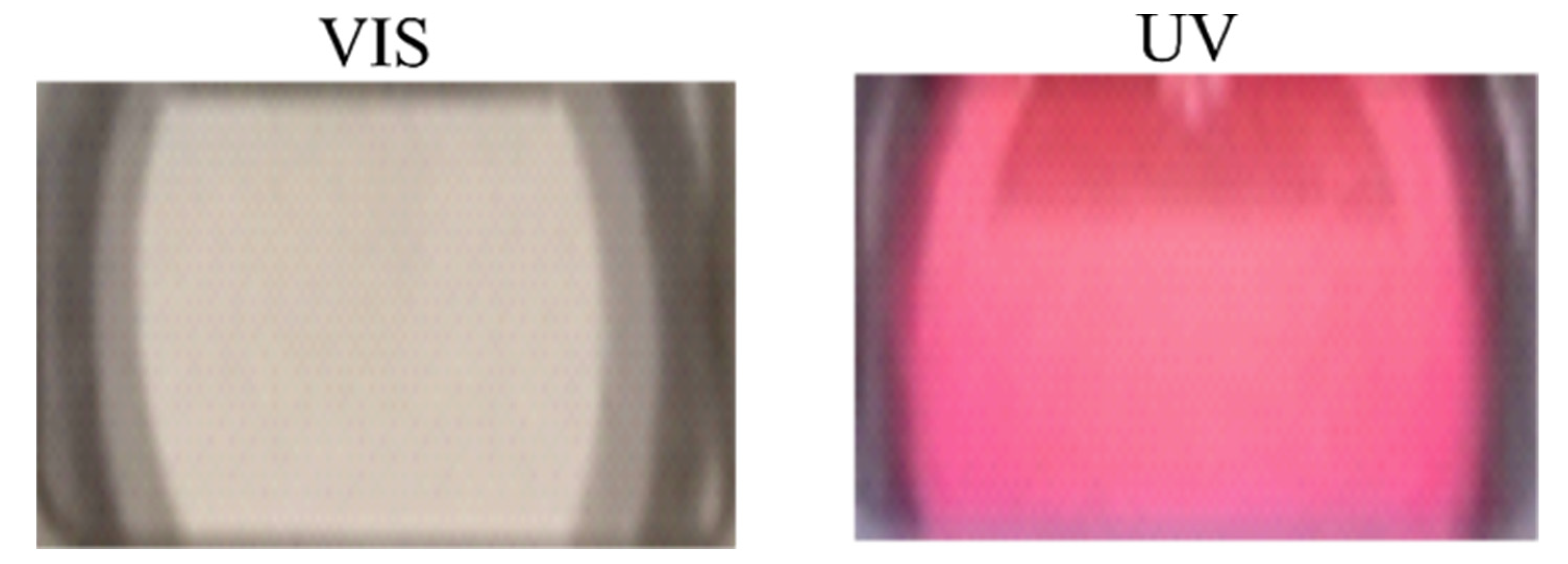
3.2. Effect of UV-Irradiation on the Hydration of P (TEGA-Co-SPA) Films below and above the LCST
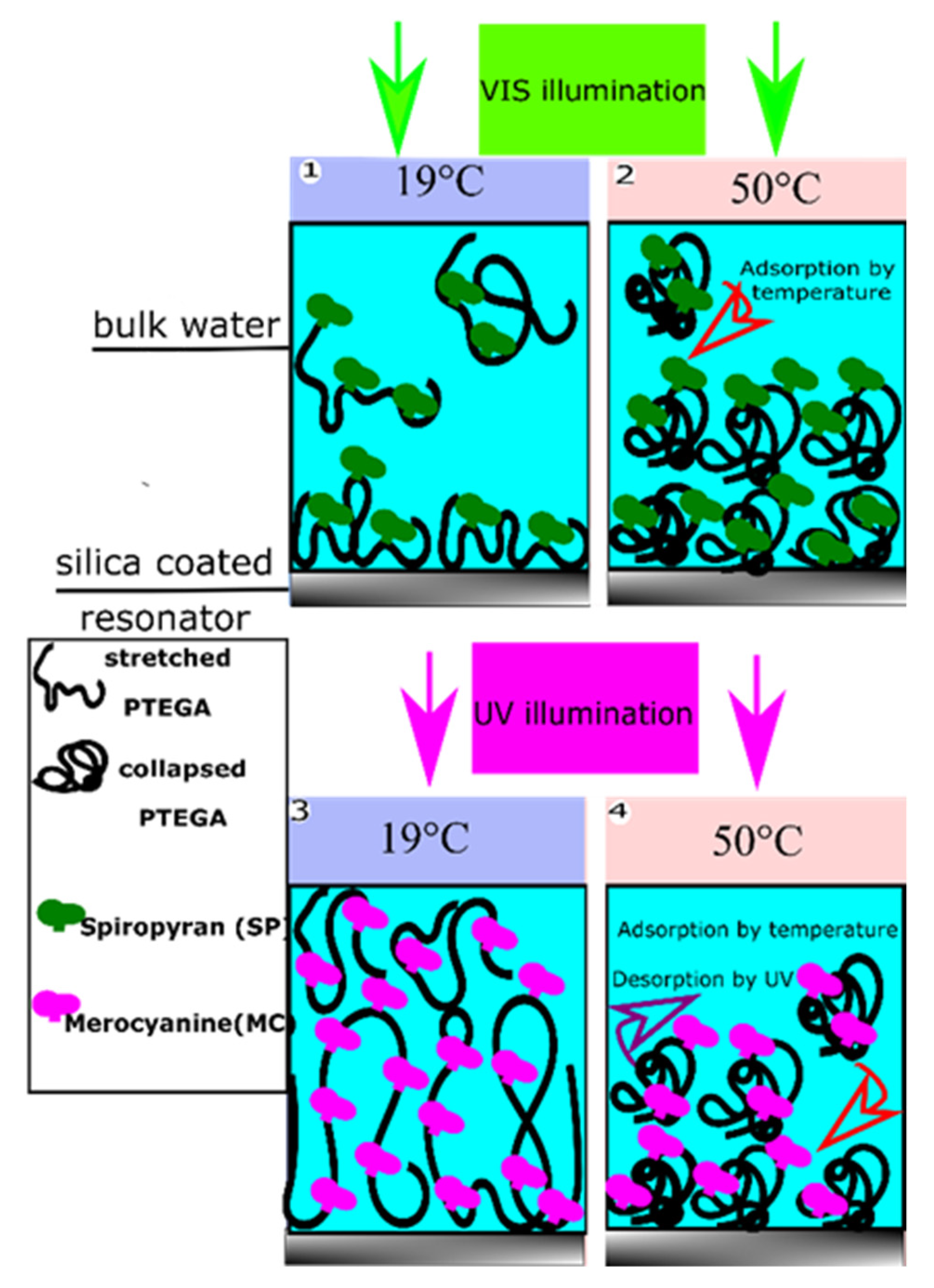
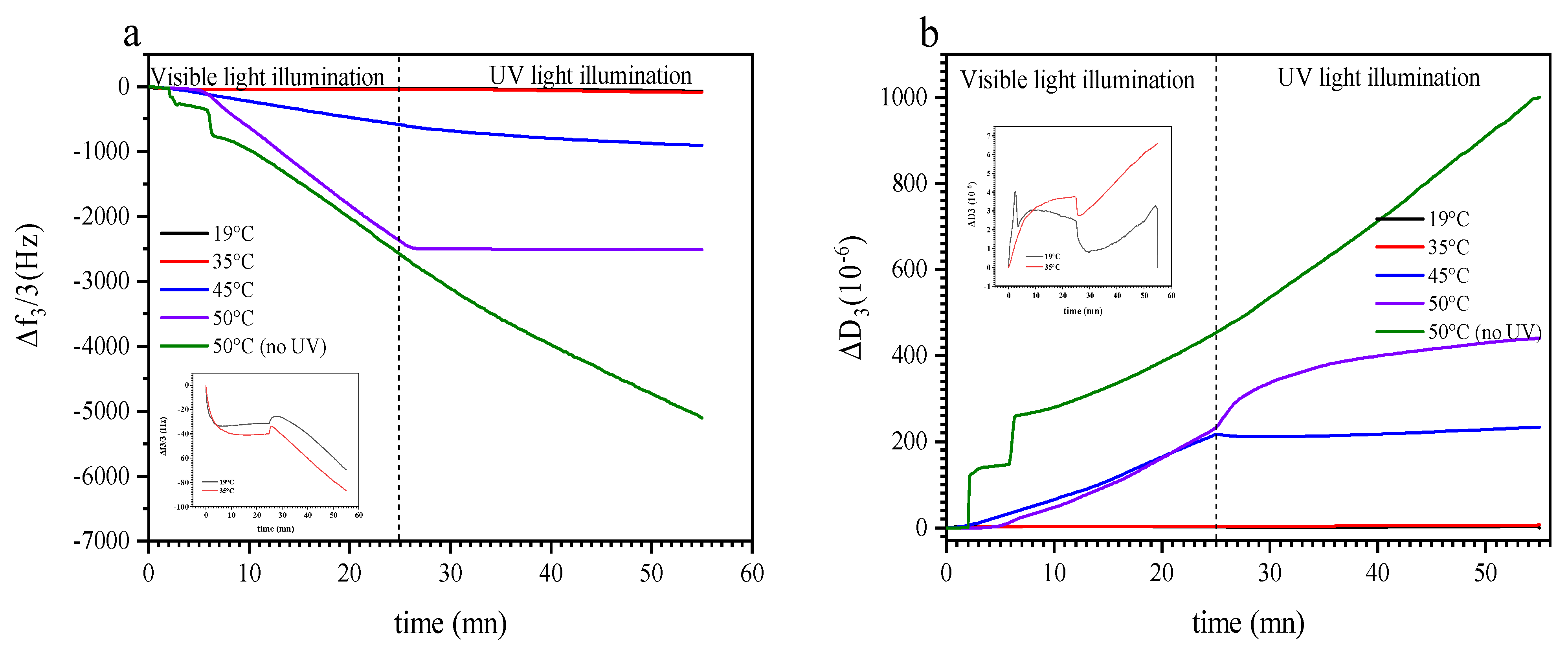
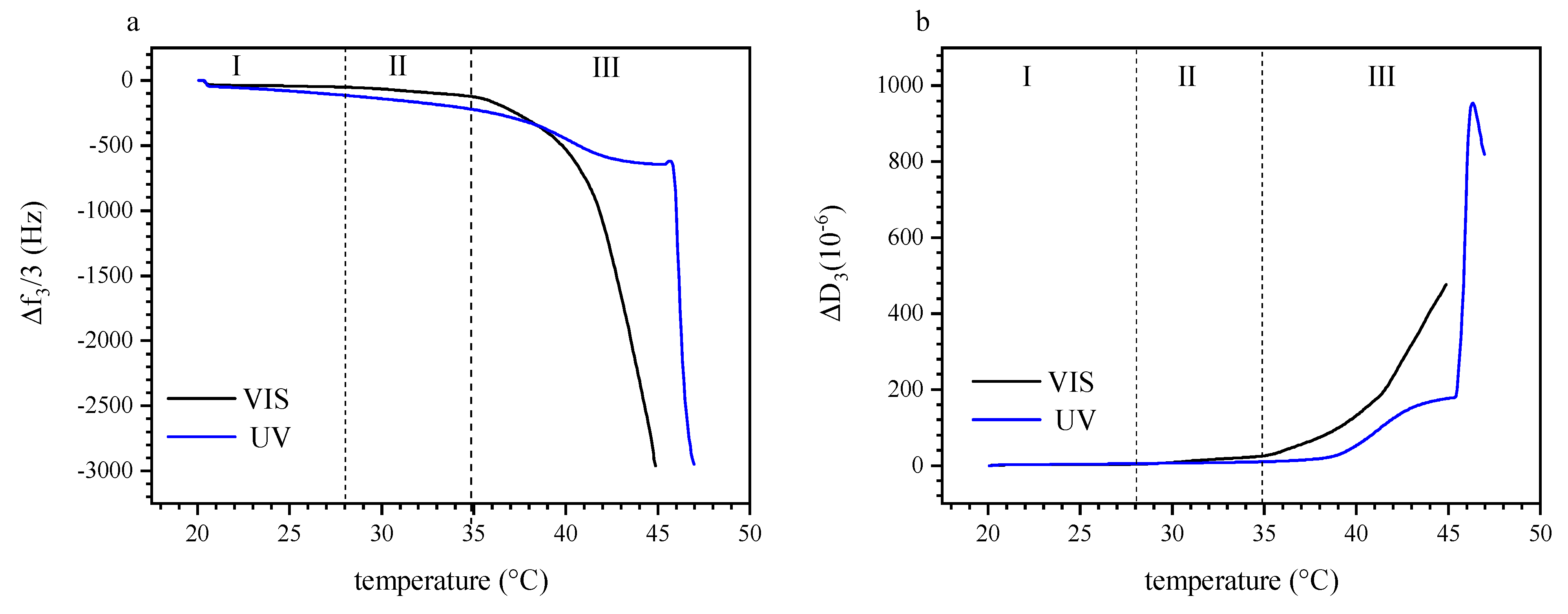
3.3. Dual Temperature and Light Effect on the Build-Up of P (TEGA-co-SPA) Layers on Silica Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirri, G.; Damin, F.; Chiari, M.; Bontempi, E.; Depero, L.E. Characterization of A Polymeric Adsorbed Coating for DNA Microarray Glass Slides. Anal. Chem. 2004, 76, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kim, S.H. Mechanochemistry of Physisorbed Molecules at Tribological Interfaces: Molecular Structure Dependence of Tribochemical Polymerization. Langmuir 2017, 33, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Sen, M.; Zeng, W.; Chen, Z.; Cheung, J.M.; Morimitsu, Y.; Endoh, M.K.; Koga, T.; Fukuto, M.; Yuan, G.; et al. Structure-Induced Switching of Interpolymer Adhesion at a Solid-Polymer Melt Interface. Soft Matter 2018, 14, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Tripp, C.P.; Hair, M.L. Measurement of Polymer Adsorption on Colloidal Silica by in Situ Transmission Fourier Transform Infrared Spectroscopy. Langmuir 1993, 9, 3523–3529. [Google Scholar] [CrossRef]
- Blümmel, J.; Perschmann, N.; Aydin, D.; Drinjakovic, J.; Surrey, T.; Lopez-Garcia, M.; Kessler, H.; Spatz, J.P. Protein Repellent Properties of Covalently Attached PEG Coatings on Nanostructured SiO2-Based Interfaces. Biomaterials 2007, 28, 4739–4747. [Google Scholar] [CrossRef]
- Hu, S.; Ren, X.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Surface-Directed, Graft Polymerization within Microfluidic Channels. Anal. Chem. 2004, 76, 1865–1870. [Google Scholar] [CrossRef]
- Källrot, N.; Dahlqvist, M.; Linse, P. Dynamics of Polymer Adsorption from Bulk Solution onto Planar Surfaces. Macromolecules 2009, 42, 3641–3649. [Google Scholar] [CrossRef]
- Cohen Stuart, M.A.; Fleer, G.J. Adsorbed Polymer Layers in Nonequilibrium Situations. Annu. Rev. Mater. Sci. 1996, 26, 463–500. [Google Scholar] [CrossRef]
- O’Shaughnessy, B.; Vavylonis, D. Irreversible Adsorption from Dilute Polymer Solutions. Eur. Phys. J. E 2003, 11, 213–230. [Google Scholar] [CrossRef]
- Sims, R.A.; Harmer, S.L.; Quinton, J.S. The Role of Physisorption and Chemisorption in the Oscillatory Adsorption of Organosilanes on Aluminium Oxide. Polymers 2019, 11, 410. [Google Scholar] [CrossRef]
- O’Shaughnessy, B.; Vavylonis, D. Non-Equilibrium in Adsorbed Polymer Layers. J. Phys. Condens. Matter 2005, 17. [Google Scholar] [CrossRef]
- Porus, M.; Maroni, P.; Borkovec, M. Structure of Adsorbed Polyelectrolyte Monolayers Investigated by Combining Optical Reflectometry and Piezoelectric Techniques. Langmuir 2012, 28, 5642–5651. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Tomlins, P.; Sahota, T. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Yu, L.; Schlaich, C.; Hou, Y.; Zhang, J.; Noeske, P.L.M.; Haag, R. Photoregulating Antifouling and Bioadhesion Functional Coating Surface Based on Spiropyran. Chem. Eur. J. 2018, 24, 7742–7748. [Google Scholar] [CrossRef] [PubMed]
- Kocak, G.; Tuncer, C.; Bütün, V. PH-Responsive Polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.F.; Zhao, C.S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155. [Google Scholar] [CrossRef]
- Nabiyan, A.; Biehl, P.; Schacher, F.H. Crystallization vs Metal Chelation: Solution Self-Assembly of Dual Responsive Block Copolymers. Macromolecules 2020, 53, 5056–5067. [Google Scholar] [CrossRef]
- Max, J.B.; Nabiyan, A.; Eichhorn, J.; Schacher, F.H. Triple-Responsive Polyampholytic Graft Copolymers as Smart Sensors with Varying Output. Macromol. Rapid Commun. 2020, 2000671, 1–5. [Google Scholar] [CrossRef]
- Wondraczek, L.; Pohnert, G.; Schacher, F.H.; Köhler, A.; Gottschaldt, M.; Schubert, U.S.; Küsel, K.; Brakhage, A.A. Artificial Microbial Arenas: Materials for Observing and Manipulating Microbial Consortia. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. The Light-Controlling of Temperature-Responsivity in Stimuli-Responsive Polymers. Polym. Chem. 2019, 10, 5686–5720. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-Isopropylacrylamide). J. Macromol. Sci. Part Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Sanson, N.; Rieger, J. Synthesis of Nanogels/Microgels by Conventional and Controlled Radical Crosslinking Copolymerization. Polym. Chem. 2010, 1, 965–977. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Chiessi, E.; Zanatta, M.; Orecchini, A.; Zaccarelli, E. Water-Polymer Coupling Induces a Dynamical Transition in Microgels. J. Phys. Chem. Lett. 2019, 10, 870–876. [Google Scholar] [CrossRef]
- Halperin, A.; Krçger, M.; Winnik, F.M. Poly (N-Isopropylacrylamide) Phase Diagrams: Fifty Years of Research Angewandte. Angew. Chem. Int. Ed. 2015, 15342–15367. [Google Scholar] [CrossRef]
- De Oliveira, T.E.; Marques, C.M.; Netz, P.A. Molecular Dynamics Study of the LCST Transition in Aqueous Poly(N-n-Propylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 10100–10107. [Google Scholar] [CrossRef]
- De Solorzano, I.O.; Bejagam, K.K.; An, Y.; Singh, S.K.; Deshmukh, S.A. Solvation Dynamics of N-Substituted Acrylamide Polymers and the Importance for Phase Transition Behavior. Soft Matter 2020, 16, 1582–1593. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.J.H.C.; Van Lankvelt, B.M.; Fijten, M.W.M.; Schubert, U.S. Tuning the LCST of Poly(2-Oxazoline)s by Varying Composition and Molecular Weight: Alternatives to Poly(N-Isopropylacrylamide)? Chem. Commun. 2008, 5758–5760. [Google Scholar] [CrossRef]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-Oxazoline)s: A Comprehensive Overview of Polymer Structures and Their Physical Properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Liu, K.L.; Ni, X.; Li, J. Biodegradable Hyperbranched Amphiphilic Polyurethane Multiblock Copolymers Consisting of Poly(Propylene Glycol), Poly(Ethylene Glycol), and Polycaprolactone as in Situ Thermogels. Biomacromolecules 2012, 13, 3977–3989. [Google Scholar] [CrossRef]
- Su, X.; Tan, M.J.; Li, Z.; Wong, M.; Rajamani, L.; Lingam, G.; Loh, X.J. Recent Progress in Using Biomaterials as Vitreous Substitutes. Biomacromolecules 2015, 16, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)Ethyl Methacrylate and Oligo(Ethylene Glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Hedir, G.G.; Arno, M.C.; Langlais, M.; Husband, J.T.; O’Reilly, R.K.; Dove, A.P. Poly(Oligo(Ethylene Glycol) Vinyl Acetate)s: A Versatile Class of Thermoresponsive and Biocompatible Polymers. Angew. Chem. Int. Ed. 2017, 56, 9178–9182. [Google Scholar] [CrossRef]
- Langer, M.; Brandt, J.; Lederer, A.; Goldmann, A.S.; Schacher, F.H.; Barner-Kowollik, C. Amphiphilic Block Copolymers Featuring a Reversible Hetero Diels-Alder Linkage. Polym. Chem. 2014, 5, 5330–5338. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of Comonomer Hydrophilicity and Ionization on the Lower Critical Solution Temperature of N-Isopropylacrylamide Copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Löwenbein, A.; Katz, W. Über Substituierte spiro-Dibenzopyrane. Ber. Dtsch. Chem. Ges. 1926, 59, 1377–1383. [Google Scholar] [CrossRef]
- Hartley, G. The Cis Form of Azobenene. Nature 1937, 14, 281. [Google Scholar] [CrossRef]
- Irie, M.; Mohri, M. Thermally Irreversible Photochromic Systems. Reversible Photocyclization of Diarylethene Derivatives. J. Org. Chem. 1988, 53, 803–808. [Google Scholar] [CrossRef]
- Grimm, O.; Wendler, F.; Schacher, F.H. Micellization of Photo-Responsive Block Copolymers. Polymers 2017, 9, 396. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-Based Dynamic Materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef]
- Schnurbus, M.; Kabat, M.; Jarek, E.; Krzan, M.; Warszynski, P.; Braunschweig, B. Spiropyran Sulfonates for Photo- And PH-Responsive Air-Water Interfaces and Aqueous Foam. Langmuir 2020, 36, 6871–6879. [Google Scholar] [CrossRef] [PubMed]
- Petriashvili, G.; De Santo, M.P.; Devadze, L.; Zurabishvili, T.; Sepashvili, N.; Gary, R.; Barberi, R. Rewritable Optical Storage with a Spiropyran Doped Liquid Crystal Polymer Film. Macromol. Rapid Commun. 2016, 37, 500–505. [Google Scholar] [CrossRef]
- Dübner, M.; Cadarso, V.J.; Gevrek, T.N.; Sanyal, A.; Spencer, N.D.; Padeste, C. Reversible Light-Switching of Enzymatic Activity on Orthogonally Functionalized Polymer Brushes. ACS Appl. Mater. Interfaces 2017, 9, 9245–9249. [Google Scholar] [CrossRef]
- Grimm, O.; Maßmann, S.C.; Schacher, F.H. Synthesis and Solution Behaviour of Dual Light- and Temperature-Responsive Poly(Triethylene Glycol-:Co-Spiropyran) Copolymers and Block Copolymers. Polym. Chem. 2019, 10, 2674–2685. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic Analysis of Organic Thin Films on Quartz Resonators. Macromol. Chem. Phys. 1999, 200, 501–516. [Google Scholar] [CrossRef]
- Voinova, M.V.; Jonson, M.; Kasemo, B. On Dissipation of Quartz Crystal Microbalance as a Mechanical Spectroscopy Tool. Spectroscopy 2004, 18, 537–544. [Google Scholar] [CrossRef][Green Version]
- Sadman, K.; Wiener, C.G.; Weiss, R.A.; White, C.C.; Shull, K.R.; Vogt, B.D. Quantitative Rheometry of Thin Soft Materials Using the Quartz Crystal Microbalance with Dissipation. Anal. Chem. 2018, 90, 4079–4088. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Zhang, G. Study on Conformation Change of Thermally Sensitive Linear Grafted Poly(N-Isopropylacrylamide) Chains by Quartz Crystal Microbalance. Macromolecules 2004, 37, 6553–6557. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G. Collapse and Swelling of Thermally Sensitive Poly (N-Isopropylacrylamide) Brushes Monitored with a Quartz Crystal Microbalance. J. Phys. Chem. B 2005, 109, 743–747. [Google Scholar] [CrossRef]
- Plunkett, M.A.; Wang, Z.; Rutland, M.W.; Johannsmann, D. Adsorption of PNIPAM Layers on Hydrophobic Gold Surfaces, Measured in Situ by QCM and SPR. Langmuir 2003, 19, 6837–6844. [Google Scholar] [CrossRef]
- Wu, K.; Wu, B.; Wang, P.; Hou, Y.; Zhang, G.; Zhu, D.M. Adsorption Isotherms and Dissipation of Adsorbed Poly(N- Isopropylacrylamide) in Its Swelling and Collapsed States. J. Phys. Chem. B 2007, 111, 8723–8727. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lu, Y.; Chen, T.; Zhang, X.; Du, B. Adsorption of PNIPAmx-PEO20-PPO70-PEO 20-PNIPAmx Pentablock Terpolymer on Gold Surfaces: Effects of Concentration, Temperature, Block Length, and Surface Properties. Phys. Chem. Chem. Phys. 2014, 16, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, D.; Yang, L.; Liu, S. Nanoscale Monolayer Adsorption of Polyelectrolytes at the Solid/Liquid Interface Observed by Quartz Crystal Microbalance. Polym. J. 2017, 49, 543–548. [Google Scholar] [CrossRef]
- Ichi Edahiro, J.; Sumaru, K.; Takagi, T.; Shinbo, T.; Kanamori, T.; Sudoh, M. Analysis of Photo-Induced Hydration of a Photochromic Poly(N-Isopropylacrylamide)—Spiropyran Copolymer Thin Layer by Quartz Crystal Microbalance. Eur. Polym. J. 2008, 44, 300–307. [Google Scholar] [CrossRef]
- Tay, A.; Bendejacq, D.; Monteux, C.; Lequeux, F. How Does Water Wet a Hydrosoluble Substrate? Soft Matter 2011, 7, 6953–6957. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic, Mechanical, and Dielectric Measurements on Complex Samples with the Quartz Crystal Microbalance. Phys. Chem. Chem. Phys. 2008, 10, 4516–4534. [Google Scholar] [CrossRef]
- Benkoski, J.J.; Jesorka, A.; Kasemo, B.; Höök, F. Light-Activated Desorption of Photoactive Polyelectrolytes from Supported Lipid Bilayers. Macromolecules 2005, 38, 3852–3860. [Google Scholar] [CrossRef]
- Heeb, R.; Bielecki, R.M.; Lee, S.; Spencer, N.D. Room-Temperature, Aqueous-Phase Fabrication of Poly(Methacrylic Acid) Brushes by UV-LED-Induced, Controlled Radical Polymerization with High Selectivity for Surface-Bound Species. Macromolecules 2009, 42, 9124–9132. [Google Scholar] [CrossRef]
- Hook, F.F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A Comparative Study of Protein Adsorption on Titanium Oxide Surfaces Using in Situ Ellipsometry, Optical Waveguide Lightmode Spectroscopy, and Quartz Crystal Microbalance/Dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Zhu, L.; Wei, Y.; Du, X.; Zhu, D.M. Viscoelastic Signature of Physisorbed Macromolecules at the Solid-Liquid Interface. J. Colloid Interface Sci. 2012, 383, 208–214. [Google Scholar] [CrossRef]
- Duarte, A.A.; Abegão, L.M.G.; Ribeiro, J.H.F.; Lourenço, J.P.; Ribeiro, P.A.; Raposo, M. Study of in Situ Adsorption Kinetics of Polyelectrolytes and Liposomes Using Quartz Crystal Microbalance: Influence of Experimental Layout. Rev. Sci. Instrum. 2015, 86. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung von Schwingquarzen Zur Wägung Dünner Schichten Und Zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in Coupled Water, Viscoelastic Properties, and Film Thickness of a Mefp-1 Protein Film during Adsorption and Cross-Linking: A Quartz Crystal Microbalance with Dissipation Monitoring, Ellipsometry, and Surface Plasmon Resonance Study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, K.; Bund, A.; Johannsmann, D. Acoustic Second Harmonic Generation from Rough Surfaces under Shear Excitation in Liquids. Langmuir 2004, 20, 10346–10350. [Google Scholar] [CrossRef]
- Wehner, S.; Wondraczek, K.; Johannsmann, D.; Bund, A. Roughness-Induced Acoustic Second-Harmonic Generation during Electrochemical Metal Deposition on the Quartz-Crystal Microbalance. Langmuir 2004, 20, 2356–2360. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Fluid Mechanics: Landau and Lifshitz: Course of Theoretical Physics; Pergamon: Oxford, UK, 1987. [Google Scholar]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a Quartz Microbalance in Contact with Liquid. Anal. Chem. 1985. [Google Scholar] [CrossRef]
- Lutz, J.F.; Weichenhan, K.; Akdemir, Ö.; Hoth, A. About the Phase Transitions in Aqueous Solutions of Thermoresponsive Copolymers and Hydrogels Based on 2-(2-Methoxyethoxy)Ethyl Methacrylate and Oligo(Ethylene Glycol) Methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Lessard, D.G.; Ousalem, M.; Zhu, X.X.; Eisenberg, A.; Carreau, P.J. Study of the Phase Transition of Poly(n,n-Diethylacrylamide) in Water by Rheology and Dynamic Light Scattering. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1627–1637. [Google Scholar] [CrossRef]
- Kujawa, P.; Aseyev, V.; Tenhu, H.; Winnik, F.M. Temperature-Sensitive Properties of Poly(N-Isopropylacrylamide) Mesoglobules Formed in Dilute Aqueous Solutions Heated above Their Demixing Point. Macromolecules 2006, 39, 7686–7693. [Google Scholar] [CrossRef]
- Israelachvili, J. Commentary The Different Faces of Poly (Ethylene Glycol). Proc. Natl. Acad. Sci. USA 1997, 94, 8378–8379. [Google Scholar] [CrossRef]
- Begum, R.; Matsuura, H. Conformational Properties of Short Poly(Oxyethylene) Chains in Water Studied by IR Spectroscopy. J. Chem. Soc. Faraday Trans. 1997, 93, 3839–3848. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
- Judah, H.L.; Liu, P.; Zarbakhsh, A.; Resmini, M. Influence of Buffers, Ionic Strength, and PH on the Volume Phase Transition Behavior of Acrylamide-Based Nanogels. Polymers 2020, 12, 2590. [Google Scholar] [CrossRef]
- Chevallier, E.; Mamane, A.; Stone, H.A.; Tribet, C.; Lequeux, F.; Monteux, C. Pumping-out Photo-Surfactants from an Air-Water Interface Using Light. Soft Matter 2011, 7, 7866–7874. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Brzezinski, P.; Kasemo, B. Energy Dissipation Kinetics for Protein and Antibody-Antigen Adsorption under Shear Oscillation on a Quartz Crystal Microbalance. Langmuir 1998, 14, 729–734. [Google Scholar] [CrossRef]
- Johannsmann, D.; Reviakine, I.; Richter, R.P. Dissipation in Films of Adsorbed Nanospheres Studied by Quartz Crystal Microbalance (QCM). Anal. Chem. 2009, 81, 8167–8176. [Google Scholar] [CrossRef]
- Cohen Stuart, M.A.; Waajen, F.H.W.H.; Cosgrove, T.; Vincent, B.; Crowley, T.L. Hydrodynamic Thickness of Adsorbed Polymer Layers. Macromolecules 1984, 17, 1825–1830. [Google Scholar] [CrossRef]
- Plunkett, M.A.; Claesson, P.M.; Rutland, M.W. Adsorption of a Cationic Polyelectrolyte Followed by Surfactant-Induced Swelling, Studied with a Quartz Crystal Microbalance. Langmuir 2002, 18, 1274–1280. [Google Scholar] [CrossRef]
- Domack, A.; Prucker, O.; Rühe, J.; Johannsmann, D. Swelling of a Polymer Brush Probed with a Quartz Crystal Resonator. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1997, 56, 680–689. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Shaffer, J.S.; Adriani, P.M. On the Existence of Quasi-Two-Dimensional Glasslike Structures at Strongly Interacting Polymer-Solid Interfaces. Macromolecules 1991, 24, 5226–5229. [Google Scholar] [CrossRef]
- Kremer, K. Glassy States of Adsorbed Flexible Polymers and Spread Polymer “Monolayers”. J. Phys. 1986. [Google Scholar] [CrossRef]
- Raviv, U.; Klein, J.; Witten, T.A. The Polymer Mat: Arrested Rebound of a Compressed Polymer Layer. Eur. Phys. J. E 2002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.W.; Napper, D.H. Conformational Transitions of Poly(N-Isopropylacrylamide) Chains Loopily Absorbed at the Surfaces of Poly(N-Tert-Butylacrylamide) Latex Particles in Water. J. Phys. Chem. B 1997, 101, 3155–3160. [Google Scholar] [CrossRef]
- Zhu, P.W.; Napper, D.H. Effects of Thermal History on the Dynamics of Relaxation of Poly([Formula Presented]-Isopropylacrylamide) Adsorbed at Latex Interfaces in Water. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1998, 57, 3101–3106. [Google Scholar] [CrossRef]
- Cahn, J.W. Critical Point Wetting. J. Chem. Phys. 1977, 66, 3667–3672. [Google Scholar] [CrossRef]
- Kawata, Y.; Yamamoto, T.; Kihara, H.; Yamamura, Y.; Saito, K.; Ohno, K. Unusual Photoresponses in the Upper Critical Solution Temperature of Polymer Solutions Mediated by Changes in Intermolecular Interactions in an Azo-Doped Liquid Crystalline Solvent. Phys. Chem. Chem. Phys. 2018, 20, 5850–5855. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.L.; Richards, R.W. Polymers at Surfaces and Interfaces; Cambridge University Press: New York, NY, USA, 1999. [Google Scholar]
- Wiener, C.G.; Weiss, R.A.; Vogt, B.D. Overcoming Confinement Limited Swelling in Hydrogel Thin Films Using Supramolecular Interactions. Soft Matter 2014. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R. Poly(N-Isopropylacrylamide) (PNIPAM) Is Never Hydrophobic. J. Colloid Interface Sci. 2010. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Miled, A.; Nabiyan, A.; Wondraczek, K.; Schacher, F.H.; Wondraczek, L. Controlling Growth of Poly (Triethylene Glycol Acrylate-Co-Spiropyran Acrylate) Copolymer Liquid Films on a Hydrophilic Surface by Light and Temperature. Polymers 2021, 13, 1633. https://doi.org/10.3390/polym13101633
Ben-Miled A, Nabiyan A, Wondraczek K, Schacher FH, Wondraczek L. Controlling Growth of Poly (Triethylene Glycol Acrylate-Co-Spiropyran Acrylate) Copolymer Liquid Films on a Hydrophilic Surface by Light and Temperature. Polymers. 2021; 13(10):1633. https://doi.org/10.3390/polym13101633
Chicago/Turabian StyleBen-Miled, Aziz, Afshin Nabiyan, Katrin Wondraczek, Felix H. Schacher, and Lothar Wondraczek. 2021. "Controlling Growth of Poly (Triethylene Glycol Acrylate-Co-Spiropyran Acrylate) Copolymer Liquid Films on a Hydrophilic Surface by Light and Temperature" Polymers 13, no. 10: 1633. https://doi.org/10.3390/polym13101633
APA StyleBen-Miled, A., Nabiyan, A., Wondraczek, K., Schacher, F. H., & Wondraczek, L. (2021). Controlling Growth of Poly (Triethylene Glycol Acrylate-Co-Spiropyran Acrylate) Copolymer Liquid Films on a Hydrophilic Surface by Light and Temperature. Polymers, 13(10), 1633. https://doi.org/10.3390/polym13101633







